Recovery of Male Siamese Fighting Fish (Betta splendens) After Overland Shipping
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics of Animals
2.2. Fish Preparation
2.3. Fish Packaging and Transportation
2.4. Recovery Time Study and Specimen Collection
2.5. Water Quality Parameters
2.6. Bubble-Nest Creation
2.7. Skin Coloration
2.8. Determination of Digestive Enzyme Activities
2.9. Muscle Quality Determination
2.9.1. Muscle Protein Synthesis Capacity
2.9.2. Muscle Myosin and Actin Contents
2.10. Analysis of Whole-Body Composition
2.11. Statistical Analysis
3. Results
3.1. Survival, Morphometrics, and Growth Recovery
3.2. Bubble-Nest Creation Ability
3.3. Body Coloration
3.4. Digestive Enzyme Activities
3.5. Muscle Quality
3.6. Whole-Body Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichak, M.R.; Barber, J.R.; Kwon, Y.M.; Francis, K.X.; Bendesky, A. Care and use of Siamese fighting fish (Betta splendens) for research. Comp. Med. 2022, 72, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sermwatanakul, A. Capacitating the local farmers to enhance global marketing of Thailand’s national aquatic animal, the Siamese fighting fish. Fish People 2019, 17, 42–48. [Google Scholar]
- Cerreta, A.J.; Lewbart, G.A.; Harrison, T.M. A novel approach to ornamental fish transportation for the aquarium hobbyist. J. Surv. Fish. Sci. 2020, 7, 105–112. [Google Scholar] [CrossRef]
- Thongprajukaew, K.; Takaeh, S.; Esor, N.; Saekhow, S.; Malawa, S.; Nuntapong, N.; Hahor, W.; Choodum, A. Optimal water volume for transportation of male Siamese fighting fish (Betta splendens). Aquac. Rep. 2023, 28, 101430. [Google Scholar] [CrossRef]
- Iwata, E.; Masamoto, K.; Kuga, H.; Ogino, M. Timing of isolation from an enriched environment determines the level of aggressive behavior and sexual maturity in Siamese fighting fish (Betta splendens). BMC Zool. 2021, 6, 15. [Google Scholar] [CrossRef]
- Pattanasiri, T.; Taparhudee, W.; Suppakul, P. Anaesthetic efficacy of clove oil-coated LDPE bag on improving water quality and survival in the Siamese fighting fish, Betta splendens, during transportation. Aquac. Int. 2017, 25, 197–209. [Google Scholar] [CrossRef]
- Monvises, A.; Ruenwongsa, P.; Panijpan, B. A Siamese fighting fish learning unit for cooperative learning among primary students. Int. J. Learn. 2010, 17, 231–246. [Google Scholar] [CrossRef]
- Sampaio, F.D.; Freire, C.A. An overview of stress physiology of fish transport: Changes in water quality as a function of transport duration. Fish Fish. 2016, 17, 1055–1072. [Google Scholar] [CrossRef]
- Sampaio, F.D.; Silva-de-Assis, H.C.; Bettim, F.L.; Fávaro, L.F.; Freire, C.A. Water acidification causes death of marine ornamental fish (Perciformes: Pomacentridae) during transport: Contributing to the conservation of wild populations. Zoologia 2019, 36, e25083. [Google Scholar] [CrossRef]
- Vanderzwalmen, M.; McNeill, J.; Delieuvin, D.; Senes, S.; Sanchez-Lacalle, D.; Mullen, C.; McLellan, I.; Carey, P.; Snellgrove, D.; Foggo, A.; et al. Monitoring water quality changes and ornamental fish behaviour during commercial transport. Aquaculture 2021, 531, 735860. [Google Scholar] [CrossRef]
- Fang, L.; Ruan, G.L.; Guo, K.; Fan, W.H.; Yang, D.Q. Proper duration and intensity of feed deprivation promote a compensatory growth response in the ricefield eel, Monopterus albus. Aquac. Res. 2021, 52, 890–896. [Google Scholar] [CrossRef]
- Morshedi, V.; Kochanian, P.; Ahmadi-Niko, M.; Azodi, M.; Pasha-Zanoosi, H. Compensatory growth response of sailfin molly, Poecilia latipinna (Lesueur, 1821) to starvation and refeeding. Int. J. Aquat. Biol. 2013, 1, 109–115. [Google Scholar] [CrossRef]
- Agues-Barbosa, T.; Andrade, P.V.; Silva, P.F.; de Almeida Moura, C.; Galvão, N.L.; Freire, F.A.; Luchiari, A.C. Variation in nest building, aggression, learning, and steroid hormone levels in Betta splendens. Gen. Comp. Endocrinol. 2022, 323, 114044. [Google Scholar] [CrossRef]
- Rungruangsak-Torrissen, K. Digestive efficiency, growth and qualities of muscle and oocyte in Atlantic salmon (Salmo salar L.) fed on diets with krill meal as an alternative protein source. J. Food Biochem. 2007, 31, 509–540. [Google Scholar] [CrossRef]
- Hart, P.J.B.; Reynolds, J.D. Handbook of Fish Biology and Fisheries: Volume 1, Fish Biology; Blackwell Science: Oxford, UK, 2002; p. 413. [Google Scholar]
- Sunde, J.; Taranger, G.L.; Rungruangsak-Torrissen, K. Digestive protease activities and free amino acids in white muscle as indicators for feed conversion efficiency and growth rate in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2001, 25, 335–345. [Google Scholar] [CrossRef]
- D’Elia, K.; Dasen, J.S. Development, functional organization, and evolution of vertebrate axial motor circuits. Neural Dev. 2018, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Gerry, S.P.; Ellerby, D.J. Resolving shifting patterns of muscle energy use in swimming fish. PLoS ONE 2014, 9, e106030. [Google Scholar] [CrossRef]
- Boerrigter, J.G.; Manuel, R.; van den Bos, R.; Roques, J.A.; Spanings, T.; Flik, G.; van de Vis, H.W. Recovery from transportation by road of farmed European eel (Anguilla anguilla). Aquac. Res. 2015, 46, 1248–1260. [Google Scholar] [CrossRef]
- Manuel, R.; Boerrigter, J.; Roques, J.; van der Heul, J.; van den Bos, R.; Flik, G.; van de Vis, H. Stress in African catfish (Clarias gariepinus) following overland transportation. Fish Physiol. Biochem. 2014, 40, 33–44. [Google Scholar] [CrossRef]
- Honryo, T.; Oakada, T.; Kawahara, M.; Kurata, M.; Agawa, Y.; Sawada, Y.; Miyashita, S.; Takii, K.; Ishibashi, Y. Estimated time for recovery from transportation stress and starvation in juvenile Pacific bluefin tuna Thunnus orientalis. Aquaculture 2018, 484, 175–183. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Water Quality and Pond Soil Analyses for Aquaculture; Auburn University: Auburn, AL, USA, 1992; p. 183. [Google Scholar]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998; p. 1085. [Google Scholar]
- Worthington, V. Worthington Enzyme Manual. Enzymes and Related Biochemicals; Worthington Chemical: New Jersey, NJ, USA, 1993; p. 399. [Google Scholar]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbø, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef]
- Areekijseree, M.; Engkagul, A.; Kovitvadhi, U.; Thongpan, A.; Mingmuang, M.; Pakkong, P.; Rungruangsak-Torrissen, K. Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture 2004, 234, 575–587. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; pp. 223–225. [Google Scholar]
- Tamadoni, R.; Nafisi Bahabadi, M.; Morshedi, V.; Bagheri, D.; Torfi Mozanzadeh, M. Effect of short-term fasting and re-feeding on growth, digestive enzyme activities and antioxidant defence in yellowfin seabream, Acanthopagrus latus (Houttuyn, 1782). Aquac. Res. 2020, 51, 1437–1445. [Google Scholar] [CrossRef]
- Morshedi, V.; Kochanian, P.; Bahmani, M.; Yazdani, M.A.; Pourali, H.R.; Ashouri, G.H.; Pasha-Zanoosi, H. Cyclical short-term starvation and refeeding provokes compensatory growth in sub-yearling Siberian sturgeon, Acipenser baerii Brandt, 1869. Anim. Feed Sci. Technol. 2017, 232, 207–214. [Google Scholar] [CrossRef]
- Naghshpour, S.; Bozorgnia, A.; Hossenifard, M.; Javadian, S.R. The effect of short-term period starvation and re-feeding on growth indices and blood factors in sub-yearling beluga (Huso huso). J. Anim. Environ. 2021, 13, 165–174. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Omoregi, E.; Martin, T.; Kukuri, L.; Shilombwelwa, L. Compensatory growth response in Oreochromis mossambicus submitted to short-term cycles of feed deprivation and refeeding. Turk. J. Fish. Aquat. Sci. 2018, 18, 161–166. [Google Scholar] [CrossRef]
- Adakli, A.; Taşbozan, O. The effects of different cycles of starvation and refeeding on growth and body composition on European sea bass (Dicentrarchus labrax). Turk. J. Fish. Aquat. Sci. 2015, 15, 419–427. [Google Scholar] [CrossRef]
- Fu, S.J.; Xie, X.J.; Cao, Z.D. Effect of fasting on resting metabolic rate and postprandial metabolic response in Silurus meridionalis. J. Fish Biol. 2005, 67, 279–285. [Google Scholar] [CrossRef]
- Gallardo-Collí, A.; Pérez-Fuentes, M.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P. Compensatory growth of Nile tilapia Oreochromis niloticus, L. subjected to cyclic periods of feed restriction and feeding in a biofloc system. Aquac. Res. 2020, 51, 1813–1823. [Google Scholar] [CrossRef]
- Bautista, N.M.; Pothini, T.; Meng, K.; Burggren, W.W. Behavioral consequences of dietary exposure to crude oil extracts in the Siamese fighting fish (Betta splendens). Aquat. Toxicol. 2019, 207, 34–42. [Google Scholar] [CrossRef]
- HedayatiRad, M.; Nematollahi, M.A.; Forsatkar, M.N.; Brown, C. Prozac impacts lateralization of aggression in male Siamese fighting fish. Ecotoxicol. Environ. Saf. 2017, 140, 84–88. [Google Scholar] [CrossRef]
- Nascimento, L.D.S.; Reis, S.M.; Ferreira, P.D.M.F.; Kanashiro, M.Y.; Salaro, A.L.; Zuanon, J.A.S. Effects of Curcuma longa rhizome on growth, skin pigmentation, and stress tolerance after transport of Trichogaster labiosa. Rev. Bras. Zootec. 2019, 48, e20160282. [Google Scholar] [CrossRef]
- Thongprajukaew, K.; Kovitvadhi, U.; Kovitvadhi, S.; Engkagul, A.; Rungruangsak-Torrissen, K. Evaluation of growth performance and nutritional quality of diets using enzymatic markers and in vitro digestibility in Siamese fighting fish (Betta splendens Regan, 1910). Afr. J. Biotechnol. 2013, 12, 1689–1702. [Google Scholar] [CrossRef]
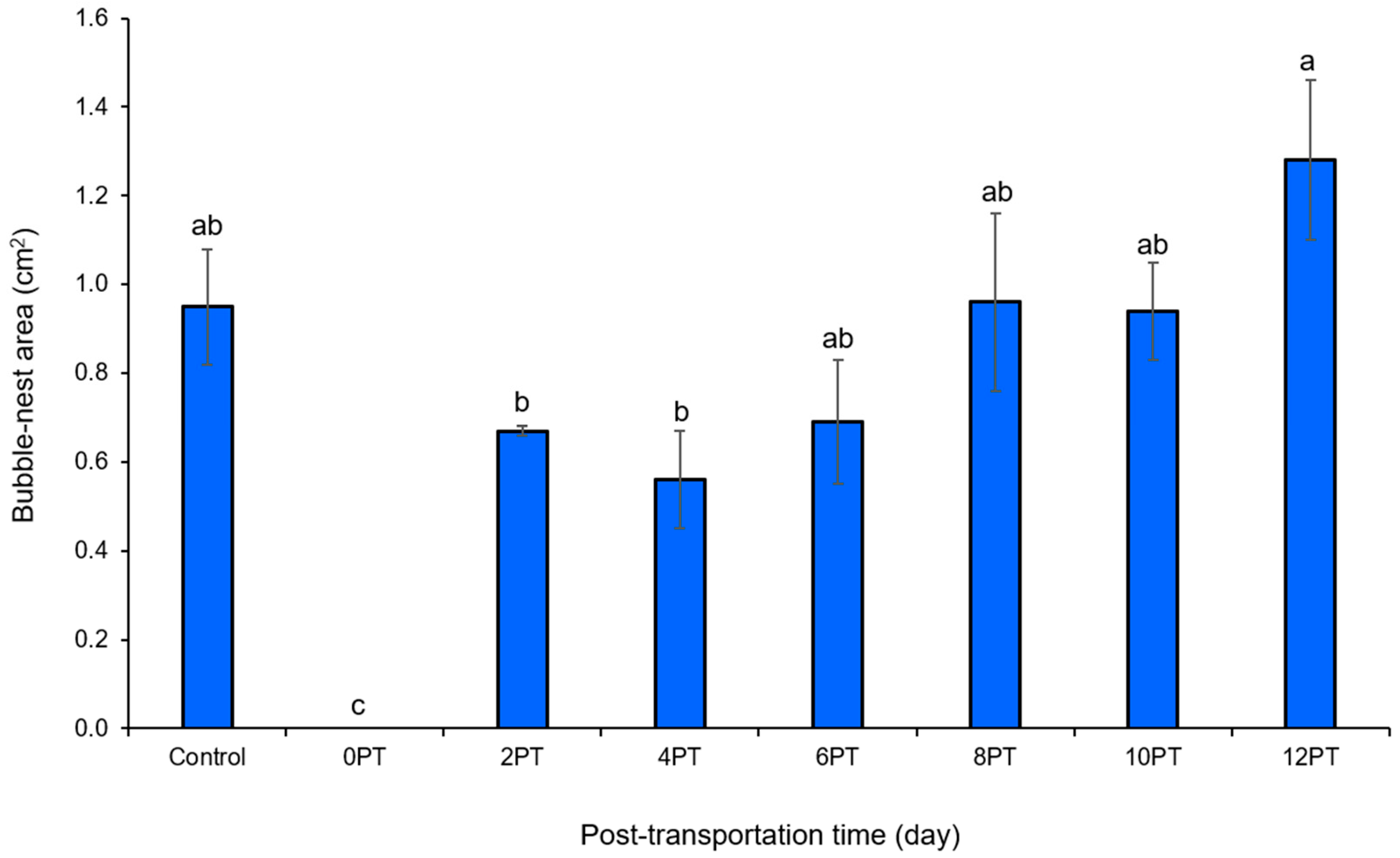
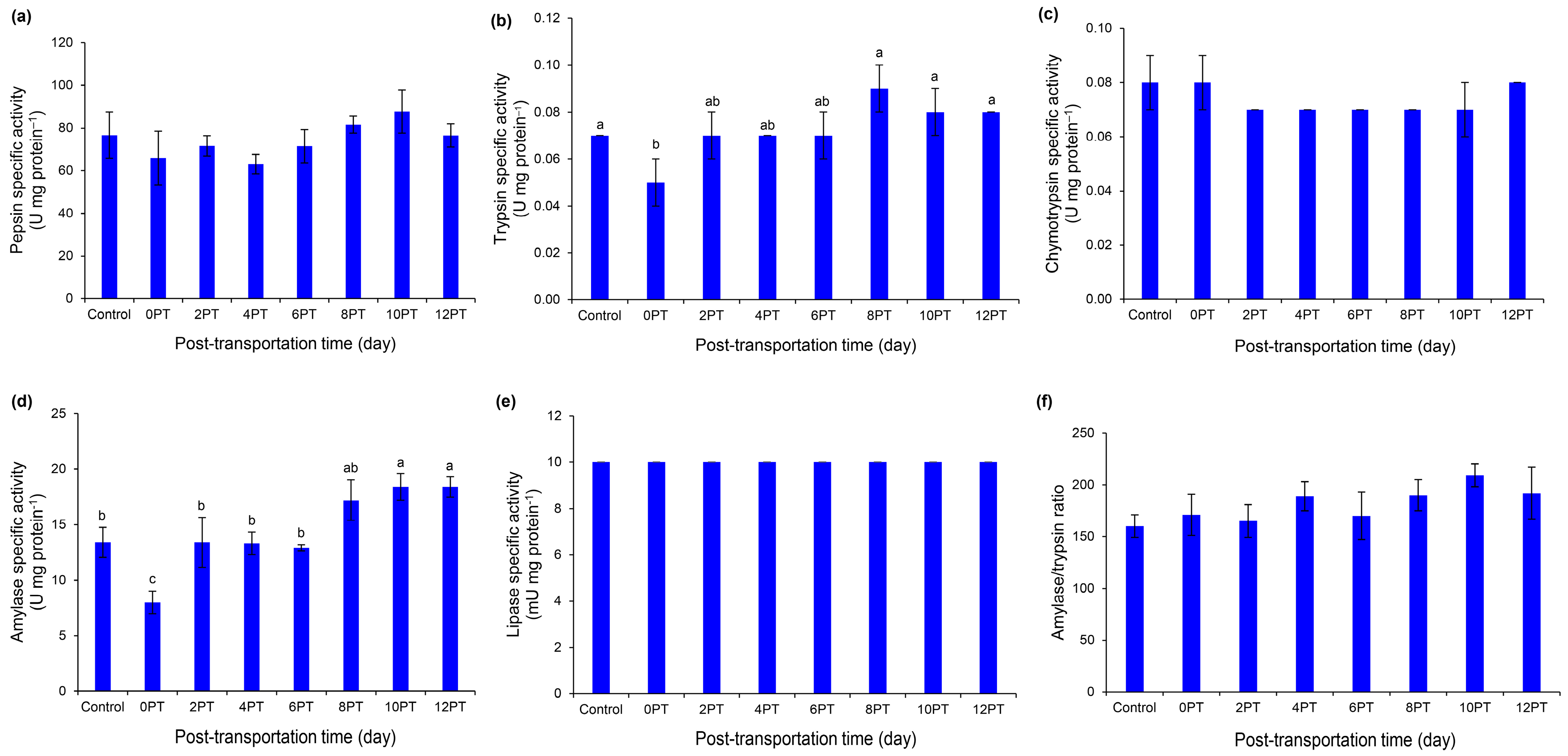
| Parameter | Control | 0 PT | 2 PT | 4 PT | 6 PT | 8 PT | 10 PT | 12 PT | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Survival (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | − |
| IBW (g) | 1.57 ± 0.05 | 1.55 ± 0.04 | 1.57 ± 0.06 | 1.58 ± 0.06 | 1.55 ± 0.04 | 1.57 ± 0.06 | 1.55 ± 0.04 | 1.57 ± 0.05 | 0.999 |
| SL (cm) | 3.27 ± 0.04 b | 3.40 ± 0.03 b | 3.26 ± 0.07 b | 3.40 ± 0.04 b | 3.42 ± 0.04 b | 3.74 ± 0.08 a | 3.82 ± 0.08 a | 3.85 ± 0.05 a | <0.001 |
| TL (cm) | 5.73 ± 0.11 bc | 5.67 ± 0.06 bc | 5.52 ± 0.12 c | 5.81 ± 0.07 b | 5.87 ± 0.07 b | 6.33 ± 0.08 a | 6.18 ± 0.11 a | 6.19 ± 0.11 a | <0.001 |
| VSI (g) | 2.88 ± 0.25 | 2.75 ± 0.33 | 2.99 ± 0.43 | 3.26 ± 0.32 | 3.09 ± 0.18 | 3.51 ± 0.30 | 2.93 ± 0.34 | 3.63 ± 0.48 | 0.561 |
| CF (g cm−3) | 4.52 ± 0.14 a | 3.33 ± 0.07 cd | 4.10 ± 0.25 ab | 3.71 ± 0.14 bc | 3.86 ± 0.13 b | 3.09 ± 0.21 d | 3.10 ± 0.23 d | 2.99 ± 0.10 d | <0.001 |
| FBW (g) | − | 1.31 ± 0.04 d | 1.37 ± 0.05 d | 1.44 ± 0.04 cd | 1.53 ± 0.04 bc | 1.57 ± 0.06 ab | 1.65 ± 0.04 ab | 1.70 ± 0.04 a | <0.001 |
| WL (%) | − | 15.0 ± 1.5 a | 12.6 ± 1.9 a | 4.39 ± 0.89 b | 4.05 ± 0.91 b | −3.36 ± 0.98 c | −4.68 ± 0.96 c | −6.99 ± 0.98 c | <0.001 |
| Parameter | Control | 0 PT | 2 PT | 4 PT | 6 PT | 8 PT | 10 PT | 12 PT | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| L* | 24.4 ± 0.9 bcd | 28.5 ± 0.7 a | 26.4 ± 0.7 b | 25.6 ± 0.6 bc | 23.6 ± 0.5 cd | 24.7 ± 0.6 bcd | 24.4 ± 0.8 bcd | 23.3 ± 0.8 d | <0.001 |
| a* | 12.5 ± 0.7 a | 7.61 ± 0.51 c | 9.97 ± 0.47 b | 9.37 ± 0.64 b | 10.4 ± 0.5 b | 11.0 ± 0.6 ab | 9.25 ± 0.48 b | 9.81 ± 0.60 b | <0.001 |
| b* | 4.37 ± 0.81 | 2.96 ± 0.53 | 3.57 ± 0.37 | 2.54 ± 0.56 | 3.16 ± 0.67 | 4.27 ± 0.84 | 2.80 ± 0.41 | 3.42 ± 0.53 | 0.425 |
| C* | 13.6 ± 0.7 a | 8.54 ± 0.30 d | 10.7 ± 0.5 c | 9.99 ± 0.57 cd | 11.2 ± 0.5 bc | 12.4 ± 0.7 ab | 9.83 ± 0.41 cd | 10.6 ± 0.5 c | <0.001 |
| h* | 0.33 ± 0.06 | 0.39 ± 0.08 | 0.34 ± 0.03 | 0.29 ± 0.06 | 0.29 ± 0.06 | 0.37 ± 0.08 | 0.30 ± 0.05 | 0.35 ± 0.06 | 0.941 |
| a*/b* | 2.81 ± 0.40 | 3.17 ± 0.61 | 3.24 ± 0.38 | 3.90 ± 0.76 | 4.09 ± 0.67 | 2.48 ± 0.44 | 3.58 ± 0.47 | 3.78 ± 0.77 | 0.527 |
| Parameter | Control | 0 PT | 2 PT | 4 PT | 6 PT | 8 PT | 10 PT | 12 PT | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Protein synthesis capacity | |||||||||
| RNA (μg g−1) | 3500 ± 157 bc | 3116 ± 291 c | 3351 ± 148 bc | 4255 ± 300 a | 3658 ± 22 abc | 3897 ± 237 ab | 3318 ± 26 bc | 3464 ± 124 bc | 0.017 |
| Protein (mg g−1) | 197 ± 23 | 190 ± 6 | 200 ± 12 | 223 ± 22 | 235 ± 12 | 166 ± 7 | 214 ± 28 | 193 ± 8 | 0.202 |
| RNA/protein ratio (μg mg−1) | 18.5 ± 3.1 | 16.4 ± 1.7 | 16.9 ± 1.7 | 19.8 ± 3.6 | 15.7 ± 0.8 | 23.5 ± 0.6 | 16.1 ± 2.1 | 18.1 ± 1.4 | 0.237 |
| Muscle protein | |||||||||
| ΔH Myosin (J g−1) | 1.34 ± 0.06 | 1.53 ± 0.28 | 1.02 ± 0.39 | 1.33 ± 0.05 | 1.37 ± 0.17 | 1.44 ± 0.22 | 1.43 ± 0.14 | 1.41 ± 0.13 | 0.817 |
| ΔH Actin (J g−1) | 0.37 ± 0.02 | 0.27 ± 0.04 | 0.26 ± 0.06 | 0.37 ± 0.01 | 0.34 ± 0.02 | 0.35 ± 0.08 | 0.29 ± 0.05 | 0.35 ± 0.01 | 0.401 |
| ƩΔH (J g−1) | 1.71 ± 0.04 | 1.80 ± 0.32 | 1.29 ± 0.42 | 1.70 ± 0.04 | 1.71 ± 0.19 | 1.79 ± 0.30 | 1.72 ± 0.15 | 1.76 ± 0.14 | 0.833 |
| ΔH Actin/myosin | 0.28 ± 0.03 | 0.18 ± 0.03 | 0.29 ± 0.08 | 0.28 ± 0.01 | 0.25 ± 0.03 | 0.24 ± 0.03 | 0.20 ± 0.04 | 0.25 ± 0.02 | 0.400 |
| Parameter | Control | 0 PT | 2 PT | 4 PT | 6 PT | 8 PT | 10 PT | 12 PT | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Moisture | 73.9 ± 1.0 | 74.5 ± 0.3 | 73.7 ± 0.6 | 74.9 ± 0.8 | 75.5 ± 1.1 | 73.7 ± 0.2 | 74.0 ± 1.0 | 74.7 ± 0.2 | 0.677 |
| Crude protein | 14.7 ± 0.5 | 15.3 ± 0.4 | 14.9 ± 0.2 | 14.7 ± 0.1 | 14.3 ± 0.3 | 15.0 ± 0.1 | 14.9 ± 0.4 | 14.6 ± 0.2 | 0.429 |
| Crude lipid | 3.18 ± 0.31 | 2.49 ± 0.37 | 3.16 ± 0.71 | 2.86 ± 0.34 | 2.55 ± 0.77 | 3.42 ± 0.19 | 3.08 ± 0.57 | 2.46 ± 0.20 | 0.766 |
| Crude ash | 5.34 ± 0.50 | 4.36 ± 0.13 | 4.74 ± 0.15 | 4.23 ± 0.11 | 4.81 ± 0.09 | 4.46 ± 0.23 | 4.48 ± 0.28 | 5.03 ± 0.48 | 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongprajukaew, K.; Malawa, S.; Poolthajit, S.; Nuntapong, N.; Hahor, W. Recovery of Male Siamese Fighting Fish (Betta splendens) After Overland Shipping. Animals 2025, 15, 2156. https://doi.org/10.3390/ani15142156
Thongprajukaew K, Malawa S, Poolthajit S, Nuntapong N, Hahor W. Recovery of Male Siamese Fighting Fish (Betta splendens) After Overland Shipping. Animals. 2025; 15(14):2156. https://doi.org/10.3390/ani15142156
Chicago/Turabian StyleThongprajukaew, Karun, Saowalak Malawa, Sukanya Poolthajit, Nutt Nuntapong, and Waraporn Hahor. 2025. "Recovery of Male Siamese Fighting Fish (Betta splendens) After Overland Shipping" Animals 15, no. 14: 2156. https://doi.org/10.3390/ani15142156
APA StyleThongprajukaew, K., Malawa, S., Poolthajit, S., Nuntapong, N., & Hahor, W. (2025). Recovery of Male Siamese Fighting Fish (Betta splendens) After Overland Shipping. Animals, 15(14), 2156. https://doi.org/10.3390/ani15142156






