Prevalence and Diversity of Staphylococcus aureus in Bulk Tank Milk from Community-Based Alpine Dairy Pastures in Tyrol, Austria
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
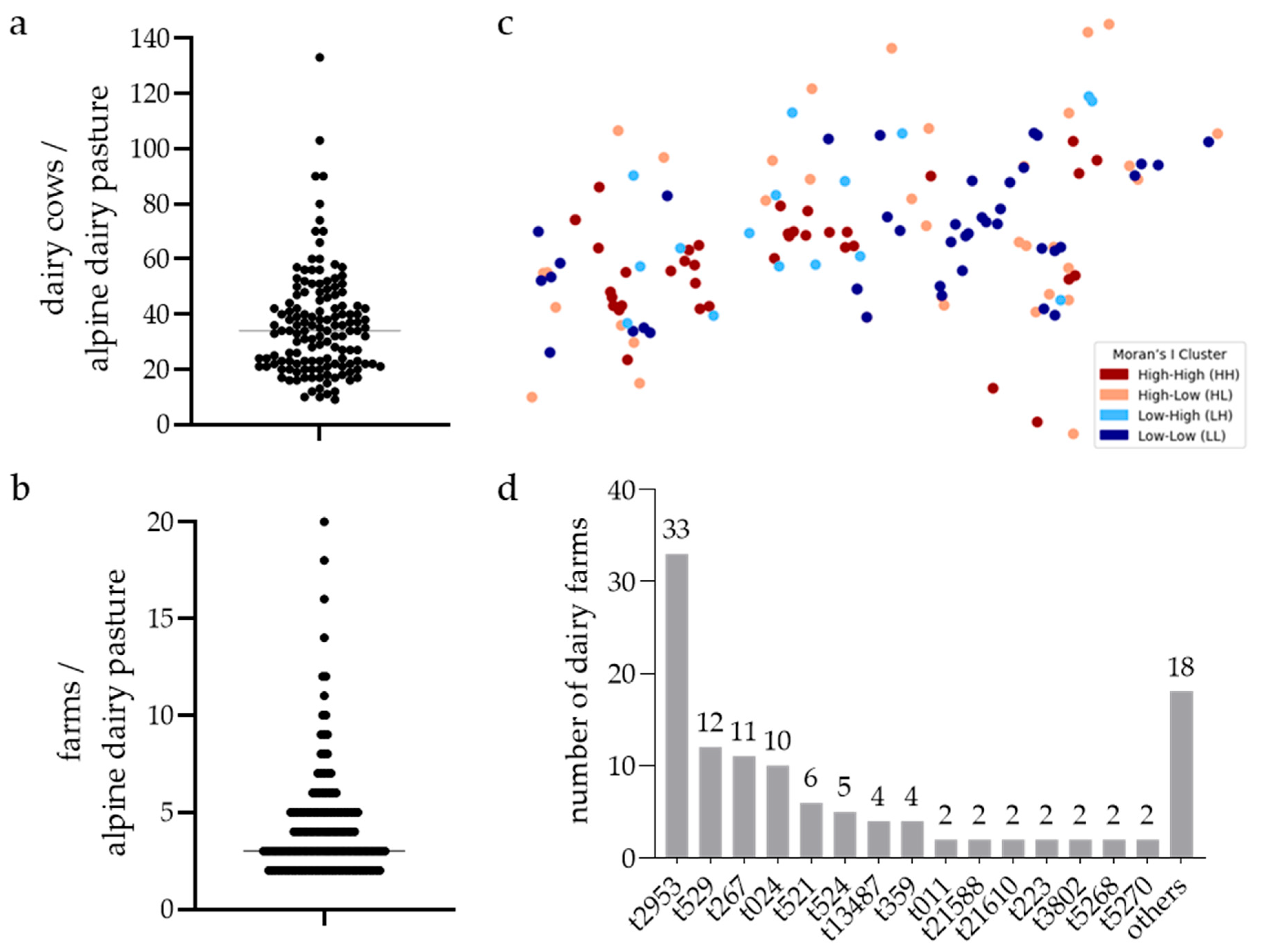
2.1. Sampling
2.2. Bacterial Isolation and Identification
2.3. Molecular Subtyping and Microarray
2.4. Antimicrobial Susceptibility Testing
2.5. Moran’s I Spatial Autocorrelation
3. Results
3.1. Prevalence of S. aureus in BTM Samples of Pastures
3.2. Diversity of S. aureus Isolates in BTM Samples of Pastures
3.3. DNA Microarray-Based Genotyping, MLST, and Antimicrobial Susceptibility Testing of Selected Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BTM | bulk tank milk |
| CC | clonal complex |
| CC8bov | bovine-adapted CC8 variant |
| GTB | genotype B |
| IMI | intramammary infection |
| MALDI-TOF-MS | matrix-assisted laser desorption ionisation–time-of-flight mass spectrometry |
| MLST | multi-locus sequence typing |
References
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Boss, R.; Cosandey, A.; Luini, M.; Artursson, K.; Bardiau, M.; Breitenwieser, F.; Hehenberger, E.; Lam, T.; Mansfeld, M.; Michel, A.; et al. Bovine Staphylococcus aureus: Subtyping, evolution, and zoonotic transfer. J. Dairy Sci. 2016, 99, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Resch, G.; François, P.; Morisset, D.; Stojanov, M.; Bonetti, E.J.; Schrenzel, J.; Sakwinska, O.; Moreillon, P. Human-to-bovine jump of Staphylococcus aureus CC8 is associated with the loss of a β-hemolysin converting prophage and the acquisition of a new staphylococcal cassette chromosome. PLoS ONE 2013, 8, e58187. [Google Scholar] [CrossRef] [PubMed]
- Sakwinska, O.; Giddey, M.; Moreillon, M.; Morisset, D.; Waldvogel, A.; Moreillon, P. Staphylococcus aureus host range and human-bovine host shift. Appl. Environ. Microbiol. 2011, 77, 5908–5915. [Google Scholar] [CrossRef] [PubMed]
- Kummel, J.; Stessl, B.; Gonano, M.; Walcher, G.; Bereuter, O.; Fricker, M.; Grunert, T.; Wagner, M.; Ehling-Schulz, M. Staphylococcus aureus Entrance into the Dairy Chain: Tracking, S. aureus from Dairy Cow to Cheese. Front. Microbiol. 2016, 7, 1603. [Google Scholar] [CrossRef] [PubMed]
- Schabauer, A.; Pinior, B.; Gruber, C.M.; Firth, C.L.; Käsbohrer, A.; Wagner, M.; Rychli, K.; Obritzhauser, W. The relationship between clinical signs and microbiological species, spa type, and antimicrobial resistance in bovine mastitis cases in Austria. Vet. Microbiol. 2018, 227, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Kuhnert, P.; Frey, J.; Miserez, R.; Kirchhofer, M.; Kaufmann, T.; Steiner, A.; Graber, H.U. Bovine Staphylococcus aureus: Association of virulence genes, genotypes and clinical outcome. Res. Vet. Sci. 2008, 85, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Graber, H.U.; Naskova, J.; Studer, E.; Kaufmann, T.; Kirchhofer, M.; Brechbuhl, M.; Schaeren, W.; Steiner, A.; Fournier, C. Mastitis-related subtypes of bovine Staphylococcus aureus are characterized by different clinical properties. J. Dairy Sci. 2009, 92, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Sesso, L.; Vanzetti, T.; Weber, J.; Vaccani, M.; Riva Scettrini, P.; Sartori, C.; Ivanovic, I.; Romanò, A.; Bodmer, M.; Bacciarini, L.N.; et al. District-wide herd sanitation and eradication of intramammary Staphylococcus aureus genotype B infection in dairy herds in Ticino, Switzerland. J. Dairy Sci. 2024, 107, 8299–8312. [Google Scholar] [CrossRef] [PubMed]
- van den Borne, B.H.P.; van Schaik, G.; Lam, T.J.G.M.; Nielen, M. Therapeutic effects of antimicrobial treatment during lactation of recently acquired bovine subclinical mastitis: Two linked randomized field trials. J. Dairy Sci. 2010, 93, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Voelk, V.; Graber, H.U.; van den Borne, B.H.; Sartori, C.; Steiner, A.; Bodmer, M.; Haerdi-Landerer, M.C. A longitudinal study investigating the prevalence of Staphylococcus aureus genotype B in seasonally communal dairy herds. J. Dairy Sci. 2014, 97, 4184–4192. [Google Scholar] [CrossRef] [PubMed]
- Hummerjohann, J.; Naskova, J.; Baumgartner, A.; Graber, H.U. Enterotoxin-producing Staphylococcus aureus genotype B as a major contaminant in Swiss raw milk cheese. J. Dairy Sci. 2014, 97, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Austrian Federal Ministry of Agriculture, Forestry, Environment and Water Management. INVEKOS Referenzflächen Österreich 2018. Available online: https://geoportal.inspire.gv.at/metadatensuche/FhHKXRQ9/api/records/6d1524bc-f610-486a-be54-ba5c956af068 (accessed on 10 July 2025).
- Mester, P.; Schoder, D.; Wagner, M.; Rossmanith, P. Rapid Sample Preparation for Molecular Biological Food Analysis Based on Magnesium Chloride. Food Anal. Methods 2014, 7, 926–934. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, D.; Claus, H.; Witte, W.; Rothganger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Loncaric, I.; Künzel, F.; Licka, T.; Simhofer, H.; Spergser, J.; Rosengarten, R. Identification and characterization of methicillin-resistant Staphylococcus aureus (MRSA) from Austrian companion animals and horses. Vet. Microbiol. 2014, 168, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Slickers, P.; Ehricht, R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol. Med. Microbiol. 2008, 53, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.A.; Carrico, J.A.; Oliveira, D.C.; Ramirez, M.; de Lencastre, H. Analysis of typing methods for epidemiological surveillance of both methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J. Clin. Microbiol. 2008, 46, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Sangvik, M.; Olsen, R.S.; Olsen, K.; Simonsen, G.S.; Furberg, A.-S.; Sollid, J.U.E. Age- and gender-associated Staphylococcus aureus spa types found among nasal carriers in a general population: The Tromso Staph and Skin Study. J. Clin. Microbiol. 2011, 49, 4213–4218. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L.; Fischer, M.; Scholten, H.J.; Unwin, D. Spatial analytical perspectives on GIS. In The Moran Scatterplot as an ESDA Tool to Assess Local Instability in Spatial Association; Taylor and Francis: London, UK, 1996; pp. 111–125. [Google Scholar]
- Nemati, G.; Romanó, A.; Wahl, F.; Berger, T.; Rojo, L.V.; Graber, H.U. Bovine Staphylococcus aureus: A European study of contagiousness and antimicrobial resistance. Front. Vet. Sci. 2023, 10, 1154550. [Google Scholar] [CrossRef] [PubMed]
- Cortimiglia, C.; Luini, M.; Bianchini, V.; Marzagalli, L.; Vezzoli, F.; Avisani, D.; Bertoletti, M.; Ianzano, A.; Franco, A.; Battisti, A. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus clonal complexes in bulk tank milk from dairy cattle herds in Lombardy Region (Northern Italy). Epidemiol. Infect. 2016, 144, 3046–3051. [Google Scholar] [CrossRef] [PubMed]
- Kortstegge, J.; Krömker, V. Prevalence of Contagious Mastitis Pathogens in Bulk Tank Milk from Dairy Farms in Lower Saxony, Germany. Hygiene 2024, 4, 122–134. [Google Scholar] [CrossRef]
- Haran, K.P.; Godden, S.M.; Boxrud, D.; Jawahir, S.; Bender, J.B.; Sreevatsan, S. Prevalence and characterization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, isolated from bulk tank milk from Minnesota dairy farms. J. Clin. Microbiol. 2012, 50, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Olde Riekerink, R.G.; Barkema, H.W.; Scholl, D.T.; Poole, D.E.; Kelton, D.F. Management practices associated with the bulk-milk prevalence of Staphylococcus aureus in Canadian dairy farms. Prev. Vet. Med. 2010, 97, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, A.; Maisano, A.M.; Bianchini, V.; Vezzoli, F.; Romanò, A.; Graber, H.U.; Cremonesi, P.; Zanardi, G.; Cappa, V.; Luini, M. Short communication: Characterization of Staphylococcus aureus from bulk tank milk of dairy cattle in Lombardy (northern Italy). J. Dairy Sci. 2020, 103, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Haveri, M.; Hovinen, M.; Roslof, A.; Pyorala, S. Molecular types and genetic profiles of Staphylococcus aureus strains isolated from bovine intramammary infections and extramammary sites. J. Clin. Microbiol. 2008, 46, 3728–3735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aanensen, D.M.; Feil, E.J.; Holden, M.T.; Dordel, J.; Yeats, C.A.; Fedosejev, A.; Goater, R.; Castillo-Ramírez, S.; Corander, J.; Colijn, C.; et al. Whole-Genome Sequencing for Routine Pathogen Surveillance in Public Health: A Population Snapshot of Invasive Staphylococcus aureus in Europe. mBio 2016, 7, e00444-16. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, M.; Boerhout, E.M.; van Wigcheren, G.F.; Koymans, K.J.; Mols-Vorstermans, T.G.; de Haas, C.J.; Aerts, P.C.; Daemen, I.J.; van Kessel, K.P.; Koets, A.P.; et al. LukMF’ is the major secreted leukocidin of bovine Staphylococcus aureus and is produced in vivo during bovine mastitis. Sci. Rep. 2016, 6, 37759. [Google Scholar] [CrossRef] [PubMed]
- Antók, F.I.; Mayrhofer, R.; Marbach, H.; Masengesho, J.C.; Keinprecht, H.; Nyirimbuga, V.; Fischer, O.; Lepuschitz, S.; Ruppitsch, W.; Ehling-Schulz, M.; et al. Characterization of Antibiotic and Biocide Resistance Genes and Virulence Factors of Staphylococcus Species Associated with Bovine Mastitis in Rwanda. Antibiotics 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Weder, D.; Bridy, C.; Huguenin, M.C.; Robert, L.; Hummerjohann, J.; Stephan, R. Outbreak of staphylococcal food poisoning among children and staff at a Swiss boarding school due to soft cheese made from raw milk. J. Dairy Sci. 2015, 98, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Fretz, R.; Winter, P.; Mann, M.; Hoger, G.; Stoger, A.; Ruppitsch, W.; Ladstatter, J.; Mayer, N.; de Martin, A.; et al. Outbreak of staphylococcal food intoxication after consumption of pasteurized milk products, June 2007, Austria. Wien. Klin. Wochenschr. 2009, 121, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, A.; Tenhagen, B.-A. Risk Factors for the Occurrence of Methicillin-Resistant Staphylococcus aureus in Dairy Herds: An Update. Foodborne Pathog. Dis. 2020, 17, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, I.; Boss, R.; Romanò, A.; Guédon, E.; Le-Loir, Y.; Luini, M.; Graber, H.U. Penicillin resistance in bovine Staphylococcus aureus: Genomic evaluation of the discrepancy between phenotypic and molecular test methods. J. Dairy Sci. 2023, 106, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Karell, J.; Petzl, W.; Gangl, A.; Huber-Schlenstedt, R.; Sorge, U.S. Changes in antimicrobial resistance of Staphylococcus aureus in bovine quarter milk samples from southern Germany between 2012 and 2022. J. Dairy Sci. 2024, 107, 3802–3812. [Google Scholar] [CrossRef] [PubMed]

| Strain-ID (1) | spa | CC | ST | agr Type | Antimicrobial Resistance Profile | Toxins/Superantigens | Leukocidins (4) | |
|---|---|---|---|---|---|---|---|---|
| Phenotype | Genes Detected | |||||||
| 502_1 | t2953 | CC8 | ST8 | agr I | NR | blaZ | sed, sej, ser | lukF/S, lukD/E |
| 69 | t529 | CC151 | ST504 | agr II | NR | egc cluster (3) | lukMF’, lukF/S, lukD/E | |
| 229 | t267 | CC97 | ST352 | agr I | NR | lukMF’, lukF/S, lukD/E | ||
| 304 | t024 | CC8 | ST8 | agr I | NR | blaZ | sea, sed, sej, ser | lukF/S, lukD/E |
| 244 | t521 | CC97 | ST97 | agr I | NR | lukMF’, lukF/S, lukD/E | ||
| 41 | t524 | CC97 | ST71 | agr I | NR | lukF/S, lukD/E | ||
| 17 | t13487 | CC479 | ST1380 | agr II | NR | egc cluster (3) | lukMF’, lukF/S, lukD/E | |
| 54 | t359 | CC97 | ST97 | agr I | NR | sea | lukF/S, lukD/E | |
| 174 | t011 | CC398 | ST2199 | agr I | PEN, TET | blaZ, tet(K), tet(M) | lukF/S | |
| 146 | t21588 | CC8 | ST8 | agr I | NR | blaZ | sed, sej, ser | lukF/S, lukD/E |
| 44 | t21610 | CC8 | ST6180 | agr I | NR | sea | lukF/S, lukD/E | |
| 18 | t223 | CC22 | ST5974 | agr I | NR | tst-1 (human), egc cluster (3) | lukF/S | |
| 291 | t3802 | CC8 | ST7509 | agr I | NR | sea | lukF/S, lukD/E | |
| 274 | t5268 | CC8 | ST8 | agr I | NR | blaZ | sea, sed, sej, ser | lukF/S, lukD/E |
| 155 | t5270 | CC8 | ST8 | agr I | NR | blaZ | sea, sed, sej, ser | lukF/S, lukD/E |
| 16 | t002 | CC5 | ST5 | agr II | ERY, CLI (2) | erm(A) | egc cluster (3) | lukF/S, lukD/E |
| 89 | t065 | CC45 | ST45 | agr I | PEN | blaZ | egc cluster (3) | lukF/S |
| 68 | t084 | CC15 | ST15 | agr II | PEN | blaZ | lukF/S, lukD/E | |
| 171 | t127 | CC1 | ST1 | agr III | NR | seh | lukF/S, lukD/E | |
| 502_2 | t1340 | CC5 | ST5 | agr II | NR | lukF/S, lukD/E | ||
| 430 | t179 | CC5 | ST5 | agr II | PEN | blaZ | sed, sej, ser, egc cluster (3) | lukF/S, lukD/E |
| 491 | t18776 | CC151 | ST504 | agr II | NR | egc cluster (3) | lukMF’, lukF/S, lukD/E | |
| 489 | t19341 | CC8 | ST8384 | agr I | NR | blaZ | sea, sed, sej, ser | lukF/S, lukD/E |
| 31 | t208 | CC49 | ST49 | agr II | NR | lukMF’, lukF/S, lukD/E | ||
| 223 | t21587 | CC8 | ST6180 | agr I | NR | blaZ | sea, sed, sej, ser | lukF/S, lukD/E |
| 135 | t21743 | CC8 | ST8 | agr I | NR | blaZ | sea, sed, sej, ser | lukF/S, lukD/E |
| 147 | t21744 | CC479 | ST1380 | agr II | NR | egc cluster (3) | lukMF’, lukF/S, lukD/E | |
| 25 | t21745 | CC8 | ST9273 | agr I | NR | blaZ | sea, sed, sej, ser | lukS, lukD/E |
| 446 | t21746 | CC8 | ST8384 | agr I | NR | blaZ | sea, sed, sej, ser | lukF/S, lukD/E |
| 95 | t21747 | CC8 | ST8 | agr I | NR | blaZ | sed, sej, ser | lukF/S, lukD/E |
| 150 | t527 | CC97 | ST352 | agr I | NR | lukMF’, lukF/S, lukD/E | ||
| 513 | t528 | CC97 | ST71 | agr I | NR | egc cluster (3) | lukF/S, lukD/E | |
| 125 | t843 | CC130 | ST2490 | agr III | NR | lukF/S, lukD | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramezanigardaloud, N.; Loncaric, I.; Mikuni-Mester, P.; Alinaghi, M.; Ehling-Schulz, M.; Khol, J.L.; Grunert, T. Prevalence and Diversity of Staphylococcus aureus in Bulk Tank Milk from Community-Based Alpine Dairy Pastures in Tyrol, Austria. Animals 2025, 15, 2153. https://doi.org/10.3390/ani15142153
Ramezanigardaloud N, Loncaric I, Mikuni-Mester P, Alinaghi M, Ehling-Schulz M, Khol JL, Grunert T. Prevalence and Diversity of Staphylococcus aureus in Bulk Tank Milk from Community-Based Alpine Dairy Pastures in Tyrol, Austria. Animals. 2025; 15(14):2153. https://doi.org/10.3390/ani15142153
Chicago/Turabian StyleRamezanigardaloud, Nasrin, Igor Loncaric, Patrick Mikuni-Mester, Masoumeh Alinaghi, Monika Ehling-Schulz, Johannes Lorenz Khol, and Tom Grunert. 2025. "Prevalence and Diversity of Staphylococcus aureus in Bulk Tank Milk from Community-Based Alpine Dairy Pastures in Tyrol, Austria" Animals 15, no. 14: 2153. https://doi.org/10.3390/ani15142153
APA StyleRamezanigardaloud, N., Loncaric, I., Mikuni-Mester, P., Alinaghi, M., Ehling-Schulz, M., Khol, J. L., & Grunert, T. (2025). Prevalence and Diversity of Staphylococcus aureus in Bulk Tank Milk from Community-Based Alpine Dairy Pastures in Tyrol, Austria. Animals, 15(14), 2153. https://doi.org/10.3390/ani15142153







