1. Introduction
In ruminant diets, protein plays a crucial role, not only in reducing the cost of production but also in influencing nitrogen (N) emissions to the environment. Owing to the activity of rumen microbes, protein metabolism in ruminants differs from monogastrics in that proteins pass through the rumen where they may be degraded and resynthesized with varying efficiency [
1]. Based on their susceptibility to ruminal breakdown, dietary proteins are classified into rumen degradable protein (RDP) and rumen undegradable protein (RUP) [
2]. RDP is enzymatically degraded by microbes into peptides, amino acids, and ammonia (NH
3). The NH
3 is then converted into microbial protein (MCP), which, together with RUP and a small fraction of endogenous protein, progresses to the intestinal tract for enzymatic digestion and absorption as amino acids and peptides [
3]. In this case, metabolizable protein (MP), consisting of RUP, MCP, and minimal endogenous protein [
2], more accurately reflects true protein utilization in ruminants compared to crude protein content alone. Notably, MCP supplies 50–80% of the absorbable true protein available to the host animal [
4]. Feeding high-protein diets is a common practice to maximize animal productivity, while overfeeding protein can reduce N efficiency and increase N excretion [
5]. In a study on cows, feed efficiency increased, but N use efficiency (g milk N/100 g N intake) demonstrated a linear decline (up to 5.4 percentage units), whereas urinary N excretion (g/100 g N intake) increased linearly with progressively higher MP supply levels (85%, 100%, and 115% of requirement) [
6]. Importantly, research has demonstrated that optimizing dietary MP levels can improve N utilization and reduce nitrogen excretion and consequent environmental pollution [
7].
On the other hand, ruminants maintain diverse and complex ruminal microbiota, which play crucial roles in converting fiber-rich plants and nonprotein nitrogen into short-chain fatty acids and microbial proteins [
8,
9]. A complex interrelationship exists among dietary nutrients, the rumen microbial composition, fermentation function, and the physiological activities of ruminants [
10]. For example, the ruminal microbiota in weaned lambs are sensitive to dietary protein.
Prevotella,
RC9_gut_group,
Succinivibrionaceae_uncultured, and
Veillonellaceae_uncultured were the predominant bacterial genera in the rumen of lambs fed a high-protein diet (15% of DM), whereas
S24-7_norank and
Ruminococcus showed significantly higher relative abundance in lambs receiving a low-protein diet (11.7% of DM) [
10].
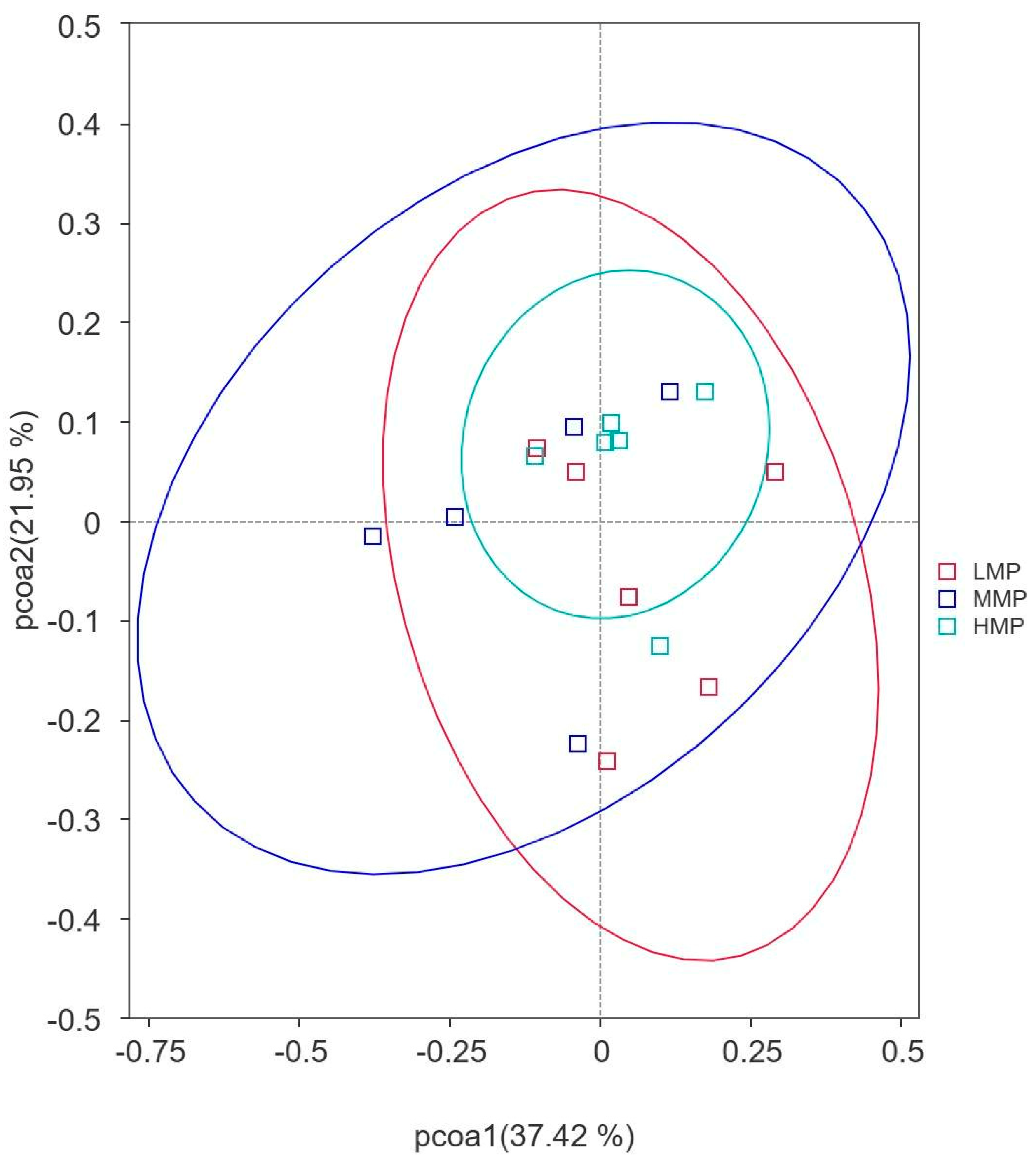
Therefore, three experimental diets with varying MP levels (low MP [LMP], moderate MP [MMP], and high MP [HMP]) in our study were conducted in Hu lambs, a locally protected Chinese breed, to investigate their effects on N utilization efficiency, plasma amino acid (AA) profiles, rumen fermentation characteristics, and ruminal microbiota composition. Especially, our study offers a detailed analysis of how dietary MP influences the composition, diversity, and functional dynamics of the ruminal microbiota in lambs. We also hypothesized that (1) optimizing dietary MP levels may enhance N utilization efficiency and minimize N excretion to the environment and that (2) increasing dietary MP levels would induce changes in the composition of the ruminal microbiota.
4. Discussion
Nitrogen is a vital nutrient that plays a critical role in the growth and productivity of ruminants, but its inefficient utilization leads to excessive excretion and environmental pollution [
9]. The use of the MP system, rather than the CP system, aligns more closely with the actual protein needs of Hu lambs and plays an important role in reducing N emission, thereby promoting environmental sustainability in intensive livestock production. The protein level in feed should match the physiological demands of the animal to ensure adequate protein utilization and absorption [
20]. The growth rate and energy efficiency can be improved by providing sufficient MP to meet the requirements for genetic growth potential under optimal energy intake [
21]. In the current study, the ADG of Hu lambs increased with increasing dietary MP levels, aligning with previous research findings that ADG is closely associated with dietary N, rumen fermentable carbohydrates, and the intestinal MP supply [
22]. Nevertheless, no change in DMI was observed despite the increase in MP supply, a finding that is consistent with studies on dairy cows [
23,
24]. When dietary MP levels align with the metabolic demands of Hu lambs, N retention improves, reducing waste excretion. This could explain why higher MP diets in our study enhanced ADG without increasing DMI, a potential metabolic adaptation where improved protein utilization optimized growth efficiency rather than stimulating feed consumption.
Previous studies have demonstrated that high-protein diets significantly increase urinary N excretion while exerting limited effects on fecal or milk N outputs [
25,
26]. As urinary N constitutes the predominant route of N excretion, it serves as a robust biomarker for assessing animal N utilization efficiency. Urinary urea-N is rapidly converted into ammonium and volatilized as ammonia, contributing to environmental pollution [
27]. Consequently, optimizing dietary MP levels is crucial for mitigating environmental issues while maintaining animal productivity. Elevated dietary CP levels proportionally increase N intake in ruminants [
28,
29]; this positive correlation between dietary MP levels and N retention was similarly demonstrated in our current study. However, while CP reduction may decrease N excretion, it concurrently imposes growth performance limitations [
30]. Notably, a previous study has documented that apparent N digestibility is greater in animals under low dietary protein [
31], a finding corroborated by Ouellet and Chiquette’s report [
32] of improved N digestibility despite reduced MP intake in dairy cattle. Furthermore, the N retention rate from high dietary protein intake was higher [
28,
33].
The excretion of PD exhibited significant dependence on dietary CP levels [
14]. Rumen microorganisms utilize N and carbon sources to synthesize MCP, with the MCP quantity increasing as PD levels rise [
34]. Both PD excretion and MCP synthesis were shown to be positively correlated with increasing dietary CP content [
35], as elevated protein provision enhances N substrate availability for microbial proliferation, consequently increasing both RDP and RUP quantities. Notably, our results revealed that the actual MP synthesized by female Hu lambs was lower than that predicted by the NRC standards [
15], likely due to the young age of the animals used in this study and their underdeveloped rumen, as well as a lower efficiency of dietary CP utilization by rumen microorganisms than that in adult sheep.
The MP requirements are fundamentally determined by the efficiency of various physiological processes, including maintenance, lactation, growth, and pregnancy [
36,
37]. Lee et al. [
38] reported that decreasing MP supply while maintaining balanced AA profiles in dairy diets decreased the urinary N and urea-N excretion, suggesting that the AA balance might be more important than the MP supply. Modifying dairy cow diets to lower total protein content while maintaining adequate AA balance can effectively mitigate N excretion’s environmental impact, lower feed costs, and sustain milk production [
39]. Räisänen et al. [
40] found that when dairy cows were fed an MP-adequate diet, the supply of dHis did not influence milk true protein concentration or yield, while milk fat and energy-corrected milk yields were optimized at dHis supply of 69 g/d or 2.65% of MP. These findings prompted our investigation of plasma AA profiles to assess AA utilization efficiency. AA requirements are primarily governed by two key factors: protein retention capacity and nitrogen excretion patterns [
41]. Our results revealed that dietary MP deficiency in the LMP group significantly reduced plasma concentrations of several critical AAs, including Arg, Lys, Met, Phe, Thr, Asp, and Pro, as well as total essential AAs and non-essential AAs compared to the MMP and HMP groups. Lys, Met, and Thr, as limiting AAs, are critical for the growth, physiology, and reproductive performance of calves [
42]. The current findings demonstrated a positive association between growth performance and plasma concentrations of these limiting AAs, with the MMP and HMP groups showing higher levels than the LMP group. Lambs fed the LMP diet had the lowest plasma Pro concentration. This might be related to the lowest Lys digestible flow with the MP diets, as Lys serves as an important precursor for de novo Pro synthesis [
43]. Interestingly, the plasma AA profiles observed between the MMP and HMP groups suggested that AA absorption and utilization reached saturation at MMP levels, indicating that the dietary MP provision in the MMP group likely represents the optimal threshold for these growing lambs.
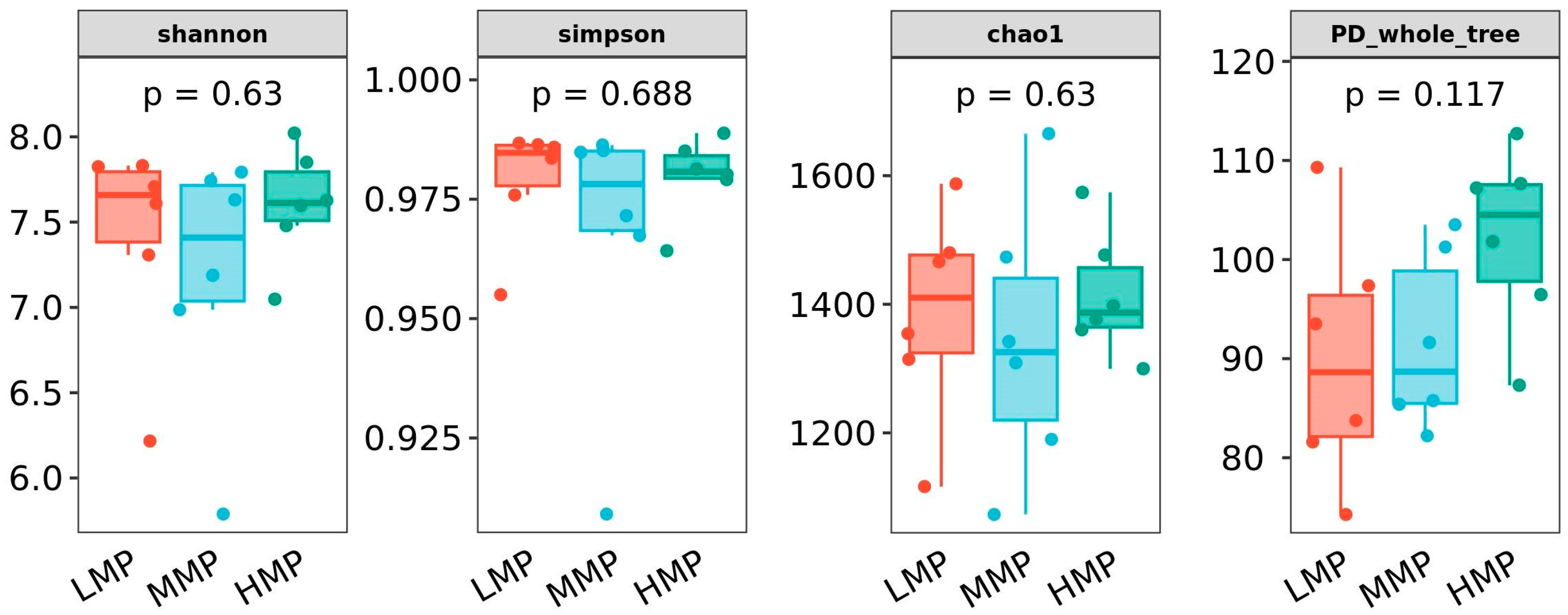
Dietary MP levels did not alter rumen fermentation patterns, as indicated by stable concentrations of total VFAs and individual VFAs, with the exception of iso-butyrate. Iso-butyrate, a degradation product of feed protein in the rumen, increases with increasing MCP synthesis [
44]. He et al. [
45] demonstrated that high-CP diets increased the abundance of iso-butyrate-producing bacteria, consequently promoting iso-butyrate production. Furthermore, supplemental levels of iso-butyric acid were found to increase the effective degradation rates of cellulose and hemicellulose while decreasing the effective degradation rate of crude protein [
46]. This may account for the observed reduction in apparent N digestibility with increasing dietary MP levels in the present study. Ruminal ammonia generally reflects the extent of protein degradation. More protein of the same degradability means more rumen ammonia [
47]. Previous studies have established that elevated dietary crude protein levels lead to increased ruminal NH
3-N concentrations, confirming a direct positive relationship between protein intake and NH
3-N production [
48]. Interestingly, Benchaar et al. [
6] reported that ruminal NH
3 concentration and urinary purine derivatives remained unchanged across different MP supply levels, indicating stable microbial protein synthesis. This stability likely resulted from sufficient RDP provision in their study. Importantly, our findings suggest that the improved animal performance observed with higher MP supply primarily stems from increased contributions of RUP to the metabolizable protein pool.
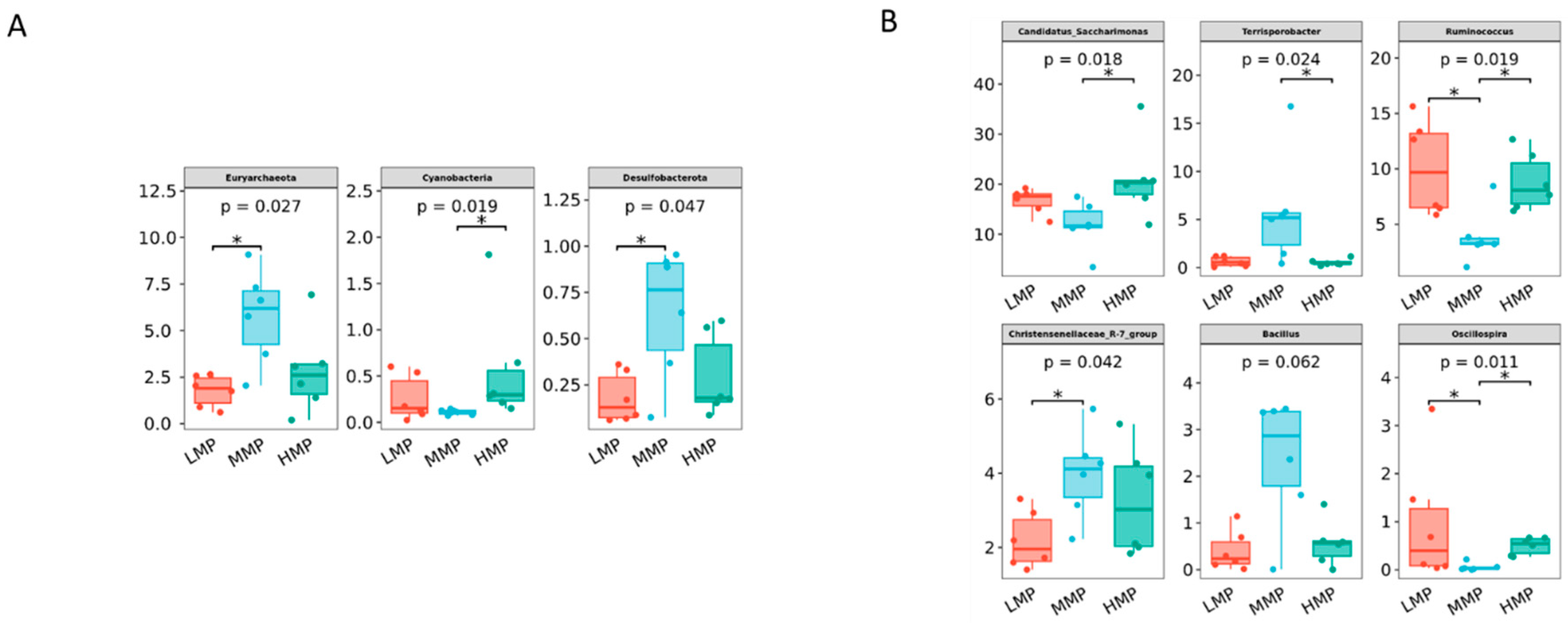
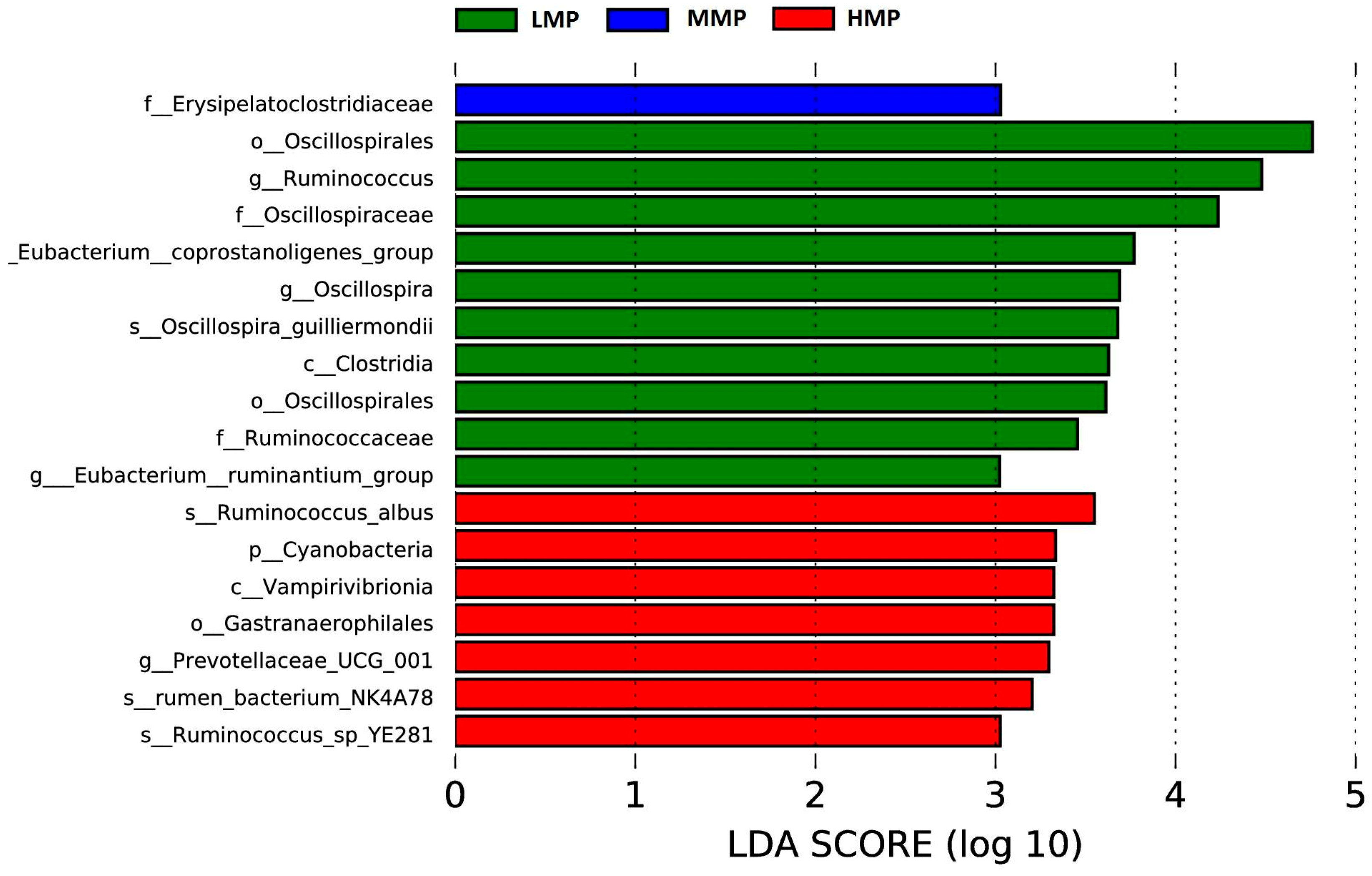
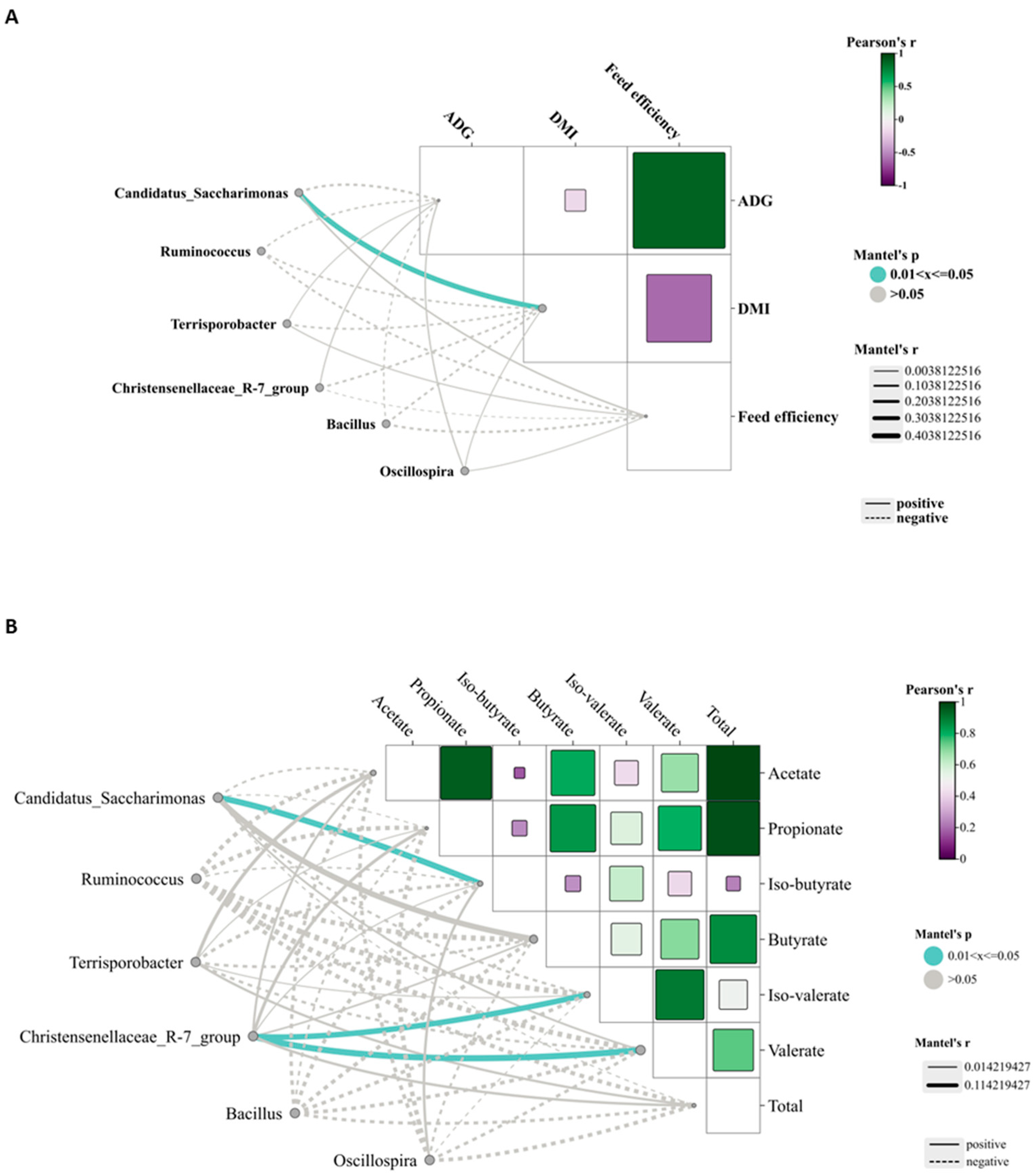
Candidatus_Saccharimonas has emerged as the dominant genus and shows a positive correlation with metabolites participating in amino acid biosynthesis and energy substrate metabolism [
49]. In the HMP group, its relative abundance was the highest, likely attributable to increased protein availability in the rumen, which may enhance its role in protein degradation and amino acid synthesis.
Terrisporobacter, known for its involvement in the in vitro anaerobic fermentation of indigestible rice straw in monogastric animals [
50], exhibited the highest relative abundance in the MMP group, suggesting this group possesses greater ruminal cellulose fermentation capacity.
Ruminococcus is a genus of organic acid-producing bacteria crucial for fiber digestion, that utilize glucose and xylose as primary fermentation products [
51]. Theoretically, enhanced glucose metabolism improves the host’s uptake and utilization of nitrogen-containing compounds, implying that elevated protein levels would promote
Ruminococcus growth and dominance. However, the present study observed the lowest
Ruminococcus abundance in the MMP group. Lv et al. [
10] reported that under low-energy diets,
Ruminococcus abundance was lower in high-protein diets than in low-protein diets, whereas no differences were detected in high-energy diets. These findings suggested that dietary protein’s effect on
Ruminococcus abundance is modulated by dietary energy levels. Lambs in the MMP group showed the highest abundance of the
Christensenellaceae_R-7_group, a probiotic commonly found in both the intestinal tract and mucosal lining, which plays a role in AA metabolism [
52]. Furthermore, the
Christensenellaceae_R-7_group belongs to the
Christensenellaceae family, a relatively newly discovered bacterial group associated with host health [
52].
Christensenellaceae also shows a positive correlation with protein catabolism and intestinal metabolites derived from dietary animal proteins [
53,
54]. The
Christensenellaceae_R-7_group, which has been demonstrated in ruminant studies to enhance rumen development and promote nutrient absorption and digestion [
55,
56], showed a significant positive correlation with ruminal valerate concentration in our study. Valerate is recognized as a potent histone deacetylase inhibitor and has demonstrated anti-cancer, anti-diabetic, antihypertensive, anti-inflammatory, and immunomodulatory effects in various studies [
57,
58]. These results indicated that the improved growth performance in MMP-fed lambs is related to the increased efficacy of probiotics in the rumen.
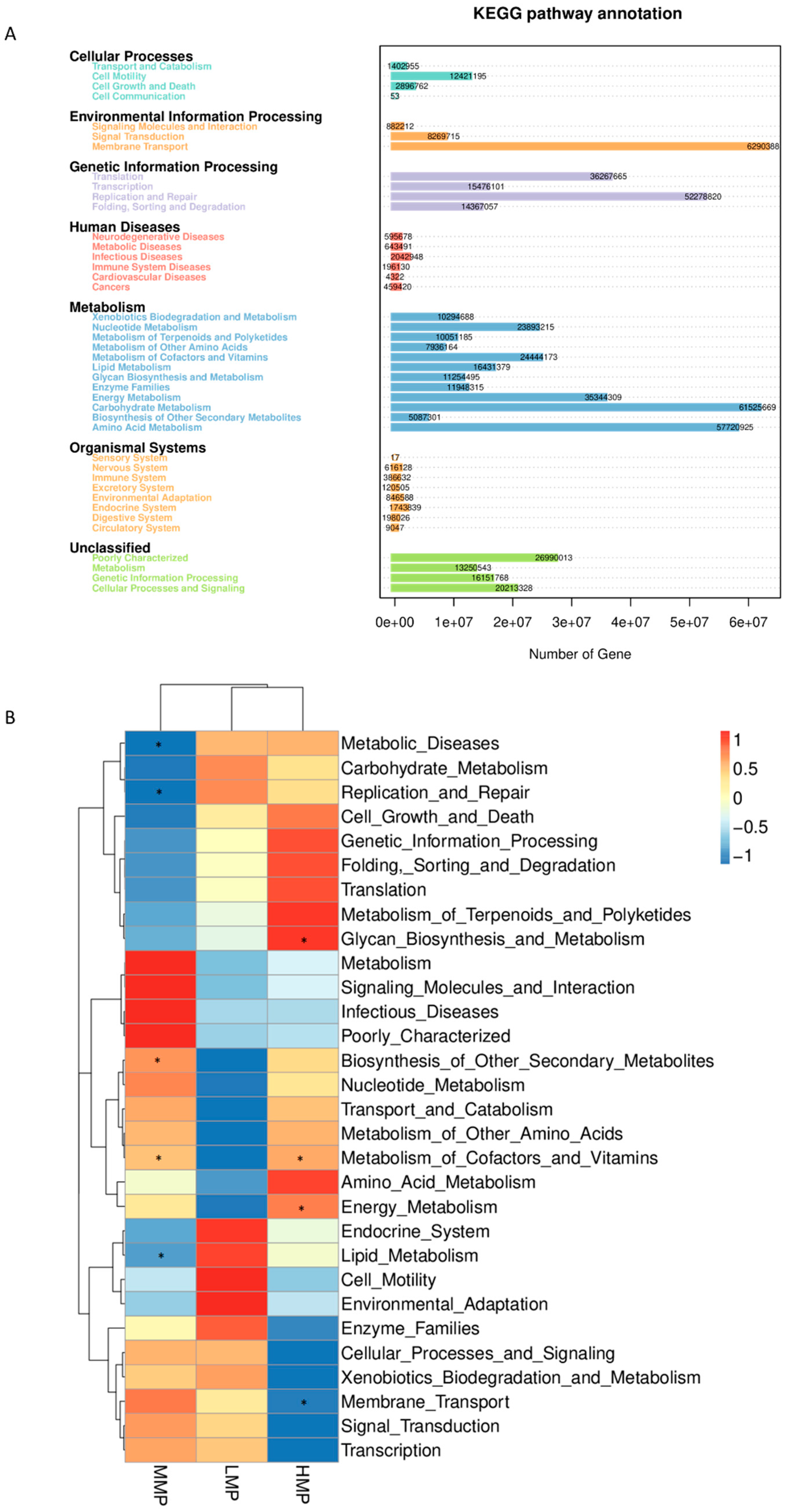
Microbial functional analysis revealed that “membrane transport”, “carbohydrate metabolism”, “amino acid metabolism”, “replication and repair”, “translation”, and “energy metabolism” were the most predominant predicted functions. These findings are consistent with those reported by Yang et al. [
59], demonstrating that the core functions of the rumen microbiota remain relatively stable. Nevertheless, dietary MP levels were found to influence specific metabolic pathways, particularly lipid metabolism. Gebeyew et al. [
60] reported that an increased abundance of
Candidatus_Saccharimonas was associated with alterations in lipid metabolism. In the current study, the MMP group exhibited the lowest abundance of
Candidatus_Saccharimonas along with reduced lipid metabolism, suggesting that moderate MP levels may regulate fat metabolism and decrease fatty acid absorption. Unexpectedly, the function of amino acid metabolism was not affected by the dietary MP levels. This could be attributed to the inherent characteristics of rumen microbiota, as the functional roles of non-classified bacteria may have been undervalued [
60]. As noted by Ma et al. [
61], PICRUSt has certain limitations in predicting microbial functions, underscoring the need for further research using metagenomic and/or metabolomic approaches to better understand the impact of MP levels on rumen functionality.