Effects of Trimethylamine Concentrations in Hatching Eggs on Chick Quality in Dwarf Hens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Feeding Management
2.2. Collection of Hatching Eggs
2.3. Determination of the TMA Content of the Egg Yolk
2.4. Hatching
2.5. Hatchability and Chick Scoring
2.6. Assessment of Chick Growth Performance
2.7. Classification Based on Yolk TMA Concentration
2.8. Correlation Analysis Between TMA Content and Chick Development Indicators
2.9. Statistical Analysis
3. Results
3.1. TMA Content of the Egg Yolk
3.2. Pasgar Scores of Chicks
3.3. Growth Curve and Mortality Rate of Chicks
3.4. Comparison of Chick Quality Between High- and Low-TMA Groups
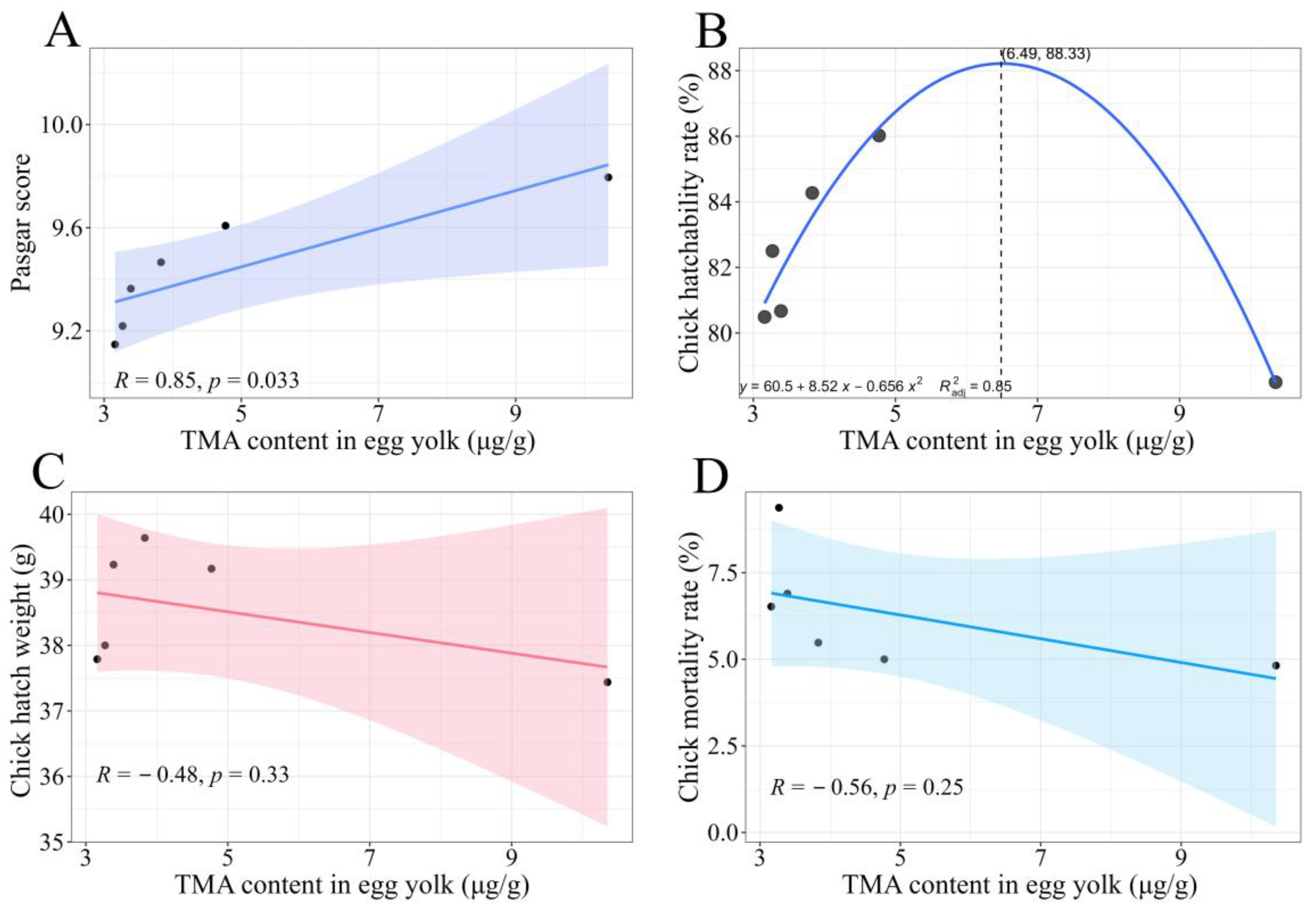
3.5. Results of Correlation Between TMA Content and Chick Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMA | Trimethylamine |
| TMAO | Trimethylamine-N-oxide |
References
- Da Silva Oliveira, G.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Effects of Sanitizers on Microbiological Control of Hatching Eggshells and Poultry Health during Embryogenesis and Early Stages after Hatching in the Last Decade. Animals 2022, 12, 2826. [Google Scholar] [CrossRef]
- Da Silva Oliveira, G.; McManus, C.; Vale, I.R.R.; dos Santos, V.M. Obtaining Microbiologically Safe Hatching Eggs from Hatcheries: Using Essential Oils for Integrated Sanitization Strategies in Hatching Eggs, Poultry Houses and Poultry. Pathogens 2024, 13, 260. [Google Scholar] [CrossRef]
- Olsen, R.; Kudirkiene, E.; Thøfner, I.; Pors, S.; Karlskov-Mortensen, P.; Li, L.; Papasolomontos, S.; Angastiniotou, C.; Christensen, J. Impact of Egg Disinfection of Hatching Eggs on the Eggshell Microbiome and Bacterial Load. Poult. Sci. 2017, 96, 3901–3911. [Google Scholar] [CrossRef]
- Melo, E.F.; Clímaco, W.L.S.; Triginelli, M.V.; Vaz, D.P.; De Souza, M.R.; Baião, N.C.; Pompeu, M.A.; Lara, L.J.C. An Evaluation of Alternative Methods for Sanitizing Hatching Eggs. Poult. Sci. 2019, 98, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Réhault-Godbert, S.; Hincke, M.; Guabiraba, R.; Guyot, N.; Gautron, J. Innate Defenses of the Avian Egg. Avian Immunol. 2022, 365–386. [Google Scholar] [CrossRef]
- Heier, B.T.; Jarp, J. An Epidemiological Study of the Hatchability in Broiler Breeder Flocks. Poult. Sci. 2001, 80, 1132–1138. [Google Scholar] [CrossRef]
- Mazanko, M.S.; Gorlov, I.F.; Prazdnova, E.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Chistyakov, V.A.; Tutelyan, A.V.; Komarova, Z.B.; Mosolova, N.I.; et al. Bacillus Probiotic Supplementations Improve Laying Performance, Egg Quality, Hatching of Laying Hens, and Sperm Quality of Roosters. Probiotics Antimicrob. Proteins 2018, 10, 367–373. [Google Scholar] [CrossRef]
- Wlazlo, L.; Drabik, K.; Al-Shammari, K.I.A.; Batkowska, J.; Nowakowicz-Debek, B.; Gryzińska, M. Use of Reactive Oxygen Species (Ozone, Hydrogen Peroxide) for Disinfection of Hatching Eggs. Poult. Sci. 2020, 99, 2478–2484. [Google Scholar] [CrossRef]
- Motola, G.; Hafez, H.M.; Brüggemann-Schwarze, S. Assessment of Three Alternative Methods for Bacterial Disinfection of Hatching Eggs in Comparison with Conventional Approach in Commercial Broiler Hatcheries. PLoS ONE 2023, 18, e0283699. [Google Scholar] [CrossRef]
- Cadirci, S. Disinfection of Hatching Eggs by Formaldehyde Fumigation—A Review. Eur. Poult. Sci. 2009, 73, 116–123. [Google Scholar] [CrossRef]
- Wang, J.; Yue, H.Y.; Xia, Z.Q.; Wu, S.G.; Zhang, H.J.; Ji, F.; Xu, L.; Qi, G.H. Effect of Dietary Choline Supplementation under Different Flavin-Containing Monooxygenase 3 Genotypes on Trimethylamine Metabolism in Laying Hens. Poult. Sci. 2012, 91, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.Q.; Cheam, G.; Wang, Y. Understanding Choline Bioavailability and Utilization: First Step Toward Personalizing Choline Nutrition. J. Agric. Food Chem. 2021, 69, 10774–10789. [Google Scholar] [CrossRef] [PubMed]
- Honkatukia, M.; Reese, K.; Preisinger, R.; Tuiskula-Haavisto, M.; Weigend, S.; Roito, J.; Mäki-Tanila, A.; Vilkki, J. Fishy Taint in Chicken Eggs Is Associated with a Substitution within a Conserved Motif of the FMO3 Gene. Genomics 2005, 86, 225–232. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Song, J.; Shi, X.; Chen, X.; Yang, F.; Pi, J.; Zhang, H.; Xu, G.; Zheng, J. Analysis of Fishy Taint in Duck Eggs Reveals the Causative Constituent of the Fishy Odor and Factors Affecting the Perception Ability of This Odor. Poult. Sci. 2019, 98, 5198–5207. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Li, X.; He, Z.; Chen, X.; Song, J.; Zeng, L.; Liang, Q.; Li, J.; Xu, G.; et al. Antibacterial Properties of TMA against Escherichia Coli and Effect of Temperature and Storage Duration on TMA Content, Lysozyme Activity and Content in Eggs. Foods 2022, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, J.; Shi, X.; Huang, M.; Liu, L.; Yi, G.; Yang, N.; Xu, G.; Zheng, J. FMO3 Deficiency of Duck Leads to Decreased Lipid Deposition and Increased Antibacterial Activity. J. Anim. Sci. Biotechnol. 2022, 13, 119. [Google Scholar] [CrossRef]
- Boerjan, M.L. Programs for Single Stage Incubation and Chick Quality. Avian Poult. Biol. Rev. 2002, 13, 237. [Google Scholar]
- Mo, F.; Zheng, J.; Wang, P.; Lian, L.; Yi, G.; Xu, G.; Yang, N. Quail FMO3 Gene Cloning, Tissue Expression Profiling, Polymorphism Detection and Association Analysis with Fishy Taint in Eggs. PLoS ONE 2013, 8, e81416. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Yuan, G.; Chen, X.; Guo, Y.; Yang, N.; Pi, J.; Zhang, H.; Zheng, J. Fishy Odor and TMA Content Levels in Duck Egg Yolks. J. Food Sci. 2018, 83, 39–45. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Wang, Y.-J.; Zhang, A.; Ding, Y.-J.; Zhang, X.-N.; Jia, Q.-J.; Zhu, Y.-P.; Li, Y.-Y.; Lv, S.-C.; Zhang, J.-P. TMA/TMAO in Hypertension: Novel Horizons and Potential Therapies. J. Cardiovasc. Transl. Res. 2021, 14, 1117–1124. [Google Scholar] [CrossRef]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The Relationship between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Wolino, K.S.; de F. Cardozo, L.F.M.; de Oliveira Leal, V.; Mafra, D.; Stockler-Pinto, M.B. Can Diet Modulate Trimethylamine N-Oxide (TMAO) Production? What Do We Know so Far? Eur. J. Nutr. 2021, 60, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.K.; Classen, H.L.; Buchanan, F.C. Fishy-Egg Tainting Is Recessively Inherited When Brown-Shelled Layers Are Fed Canola Meal. Poult. Sci. 2009, 88, 714–721. [Google Scholar] [CrossRef]
- Almas, K.; Al-Lafi, T.R. The Natural Toothbrush. World Health Forum 1995, 16, 206–210. [Google Scholar]
- Jones, S.E.; Ho, L.; Rees, C.A.; Hill, J.E.; Nodwell, J.R.; Elliot, M.A. Streptomyces Exploration Is Triggered by Fungal Interactions and Volatile Signals. eLife 2017, 6, e21738. [Google Scholar] [CrossRef]
- Aygun, A.; Sert, D.; Copur, G. Effects of Propolis on Eggshell Microbial Activity, Hatchability, and Chick Performance in Japanese Quail (Coturnix coturnix japonica) Eggs. Poult. Sci. 2012, 91, 1018–1025. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, Q.; Lan, F.; Li, J.; Yang, N.; Sun, C. Microbial Composition of Egg Component and Its Association with Hatchability of Laying Hens. Front. Microbiol. 2022, 13, 943097. [Google Scholar] [CrossRef]
- Tebrün, W.; Motola, G.; Hafez, M.H.; Bachmeier, J.; Schmidt, V.; Renfert, K.; Reichelt, C.; Brüggemann-Schwarze, S.; Pees, M. Preliminary Study: Health and Performance Assessment in Broiler Chicks Following Application of Six Different Hatching Egg Disinfection Protocols. PLoS ONE 2020, 15, e0232825. [Google Scholar] [CrossRef]
- Meisinger, B.D. Investigation of the Impacts of Hatchery Practices on Intestinal Microflora of Late-Stage Embryos and Early Post-Hatch Chicks. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2022. [Google Scholar]
- Chousalkar, K.K.; Khan, S.; McWhorter, A.R. Microbial Quality, Safety and Storage of Eggs. Curr. Opin. Food Sci. 2021, 38, 91–95. [Google Scholar] [CrossRef]
- Maatjens, C.M.; van Roovert-Reijrink, I.A.M.; Engel, B.; Van der Pol, C.W.; Kemp, B.; Van den Brand, H. Temperature during the Last Week of Incubation. I. Effects on Hatching Pattern and Broiler Chicken Embryonic Organ Development. Poult. Sci. 2016, 95, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.S.; Liebhart, D.; Hess, C.; Hess, M.; Paudel, S. Bacterial Infection in Chicken Embryos and Consequences of Yolk Sac Constitution for Embryo Survival. Vet. Pathol. 2021, 58, 71–79. [Google Scholar] [CrossRef]
- King’Ori, A.M. Review of the Factors That Influence Egg Fertility and Hatchability in Poultry. Int. J. Poult. Sci. 2011, 10, 483–492. [Google Scholar] [CrossRef]
- Julian, R.J. Production and Growth Related Disorders and Other Metabolic Diseases of Poultry—A Review. Vet. J. 2005, 169, 350–369. [Google Scholar] [CrossRef]
- Yerpes, M.; Llonch, P.; Manteca, X. Factors Associated with Cumulative First-Week Mortality in Broiler Chicks. Animals 2020, 10, 310. [Google Scholar] [CrossRef]
- Gadde, U.; Rathinam, T.; Lillehoj, H.S. Passive Immunization with Hyperimmune Egg-Yolk IgY as Prophylaxis and Therapy for Poultry Diseases—A Review. Anim. Health Res. Rev. 2015, 16, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S. Egg Yolk Antibody-IgY. In Handbook of Egg Science and Technology; CRC Press: Boca Raton, FL, USA, 2023; pp. 495–566. [Google Scholar]
- Hatta, H.; Ozeki, M.; Tsuda, K. Egg Yolk Antibody IgY and Its Application. In Hen Eggs; CRC press: Boca Raton, FL, USA, 2018; pp. 151–178. [Google Scholar]



| Items | Items | ||
|---|---|---|---|
| Ingredients (%) | Nutrient levels 3 | ||
| Corn | 62.50 | Metabolic energy (MJ/Kg) | 11.42 |
| Soybean meal | 26.20 | Total methionine | 0.42 |
| Soy oil | 1.00 | Crude protein | 16.04 |
| Limestone | 9.58 | Total lysine | 0.81 |
| Sodium chloride | 0.30 | Total threonine | 0.60 |
| Choline chloride | 0.10 | Ca | 3.64 |
| Vitamin premix 1 | 0.02 | Available phosphorus | 0.35 |
| Trace mineral premix 2 | 0.30 |
| Criterion | Ideal Condition (Score = 10) | Scoring Rule |
|---|---|---|
| Vitality | Alert and responsive | 1 point deducted for reduced activity or unresponsiveness |
| Navel closure | Complete closure, well healed | 1 point deducted for incomplete closure or inflammation |
| Leg condition | Normal appearance, no swelling or redness | 1 point deducted for swelling, redness, or deformity |
| Beak development | Proper shape, no deformities | 1 point deducted for any visible deformity |
| Yolk sac absorption | Minimal residual yolk, fully absorbed | 1 point deducted for visible or unabsorbed yolk sac |
| Items | Hatching Rate (%) | Rate of Pasgar Perfect Score (%) | Abnormal Vitality Rate (%) | Abnormal Rate of Legs (%) | Beak AbnorMality Rate (%) | Navel AbnorMality Rate (%) | Belly AbnorMality Rate (%) | |
|---|---|---|---|---|---|---|---|---|
| Control group | C_AA | 80.49 | 47.06 | 20.59 | 0 | 0 | 44.12 | 2.94 |
| C_AT | 80.67 | 62.27 | 3.64 | 0 | 1.82 | 21.82 | 12.73 | |
| C_TT | 84.27 | 78.08 | 2.74 | 0 | 0 | 17.81 | 8.22 | |
| Experiment group | E_AA | 82.50 | 45.50 | 9.38 | 0 | 0 | 34.38 | 6.88 |
| E_AT | 86.02 | 67.09 | 6.33 | 0 | 0 | 14.18 | 6.33 | |
| E_TT | 78.50 | 80.72 | 2.41 | 0 | 0 | 12.05 | 6.02 | |
| Group | TMA Content (μg/g) | Hatching Rate (%) | Pasgar Score | Rate of Pasgar Perfect Score (%) | Abnormal Vitality Rate (%) | Navel Abnormality Rate (%) | Belly AbnorMality Rate (%) |
|---|---|---|---|---|---|---|---|
| Low-TMA | 3.28 ± 0.15 b | 81.22 ± 0.64 | 9.26 ± 0.07 b | 51.61 ± 5.35 b | 11.20 ± 4.98 | 33.44 ± 6.45 | 7.52 ± 2.84 |
| High-TMA | 6.32 ± 0.55 a | 82.93 ± 2.27 | 9.63 ± 0.04 a | 75.30 ± 4.17 a | 3.83 ± 1.26 | 14.68 ± 1.68 | 6.86 ± 0.69 |
| p-value | <0.001 | 0.535 | 0.001 | 0.028 | 0.273 | 0.092 | 0.841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Xuan, L.; Lai, J.; Jiang, C.; Li, J.; Xu, G.; Zheng, J. Effects of Trimethylamine Concentrations in Hatching Eggs on Chick Quality in Dwarf Hens. Animals 2025, 15, 2121. https://doi.org/10.3390/ani15142121
Shi X, Xuan L, Lai J, Jiang C, Li J, Xu G, Zheng J. Effects of Trimethylamine Concentrations in Hatching Eggs on Chick Quality in Dwarf Hens. Animals. 2025; 15(14):2121. https://doi.org/10.3390/ani15142121
Chicago/Turabian StyleShi, Xuefeng, Lin Xuan, Jiahui Lai, Caiyun Jiang, Junying Li, Guiyun Xu, and Jiangxia Zheng. 2025. "Effects of Trimethylamine Concentrations in Hatching Eggs on Chick Quality in Dwarf Hens" Animals 15, no. 14: 2121. https://doi.org/10.3390/ani15142121
APA StyleShi, X., Xuan, L., Lai, J., Jiang, C., Li, J., Xu, G., & Zheng, J. (2025). Effects of Trimethylamine Concentrations in Hatching Eggs on Chick Quality in Dwarf Hens. Animals, 15(14), 2121. https://doi.org/10.3390/ani15142121





