Optimizing Essential Oil Mixtures: Synergistic Effects on Cattle Rumen Fermentation and Methane Emission
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Ethics Statement
2.2. Experiment 1: Optimizing Fermentation Characteristics Through Targeted EO Synergy Analysis
2.2.1. Experimental Treatments and Incubation Substrate
2.2.2. In Vitro System
2.2.3. Rumen Fluid Collection
2.2.4. The in Vitro Procedure for Evaluating Rumen Microbial Fermentation
2.2.5. Sample Collection
2.3. Experiment 2 (Optimizing Total Gas and CH4 Output Using Targeted EO Combinations)
2.3.1. Experimental Treatments and Incubation Substrate
2.3.2. The in Vitro Procedure for Evaluating Total Gas and CH4 Production and Kinetics
2.3.3. Gas Measurements and Analysis
2.3.4. Statistical Analysis
Experiment 1
Experiment 2
3. Results
3.1. Experiment 1 (Screening the Best Combination for an Optimum Rumen Fermentation)
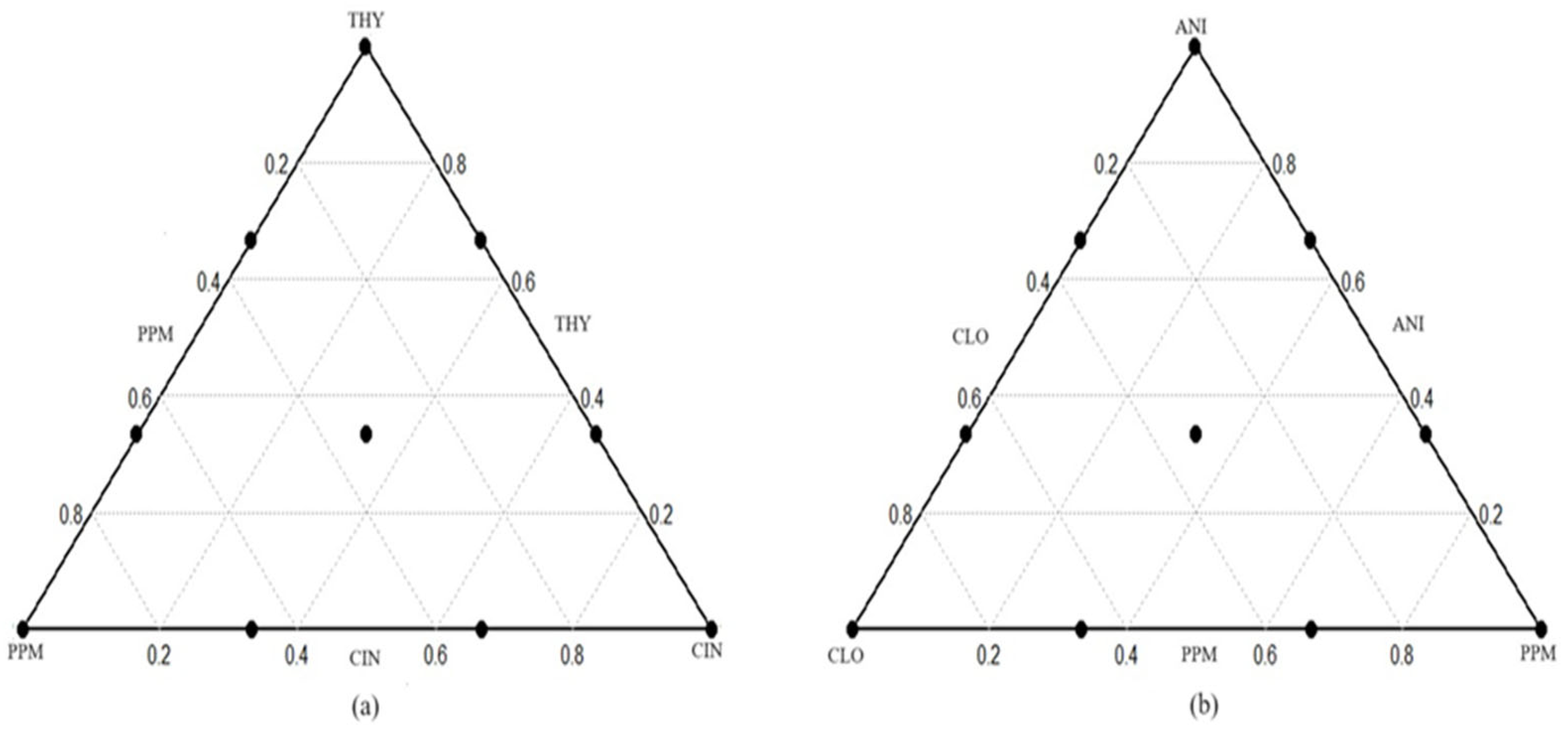
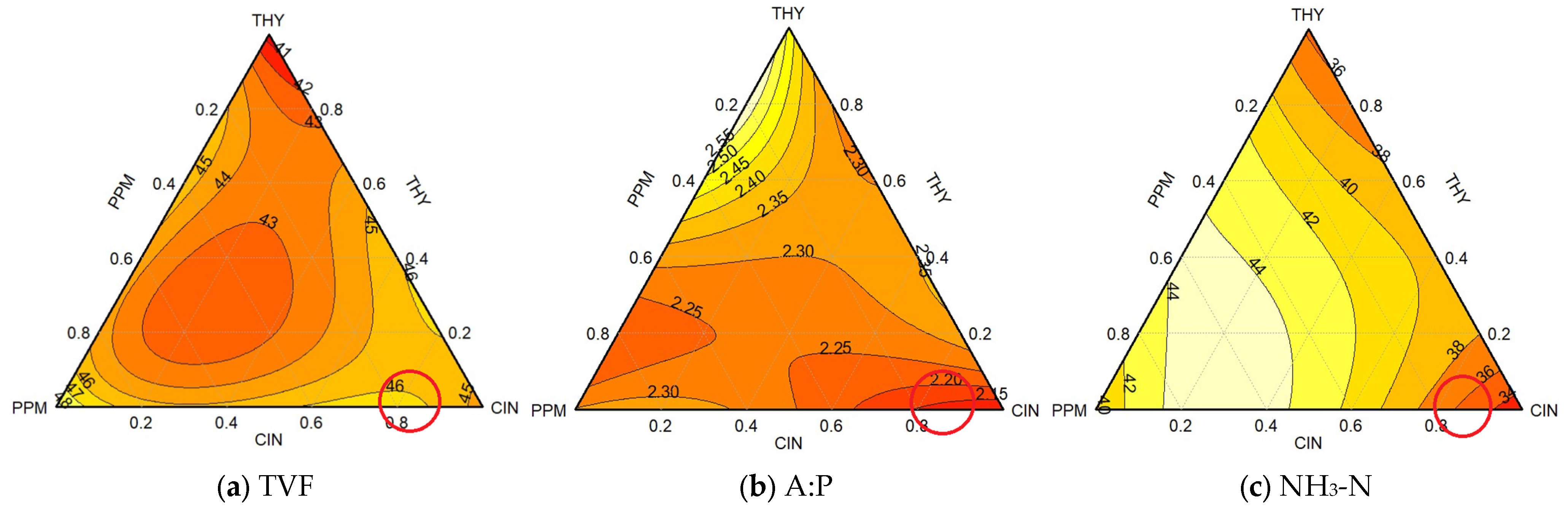
3.1.1. Combination Triad 1 (Different Proportions of THY, PPM, and CIN EOs)
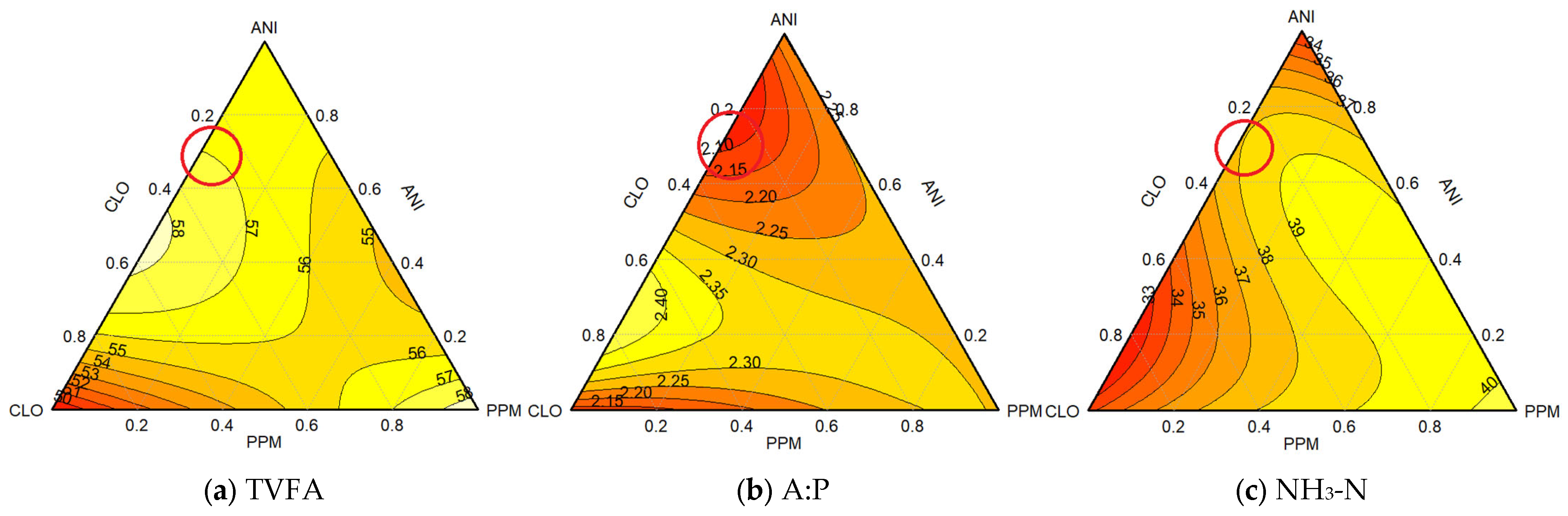
3.1.2. Combination Triad 2 (Different Proportions of ANI, CLO, and PPM EOs)
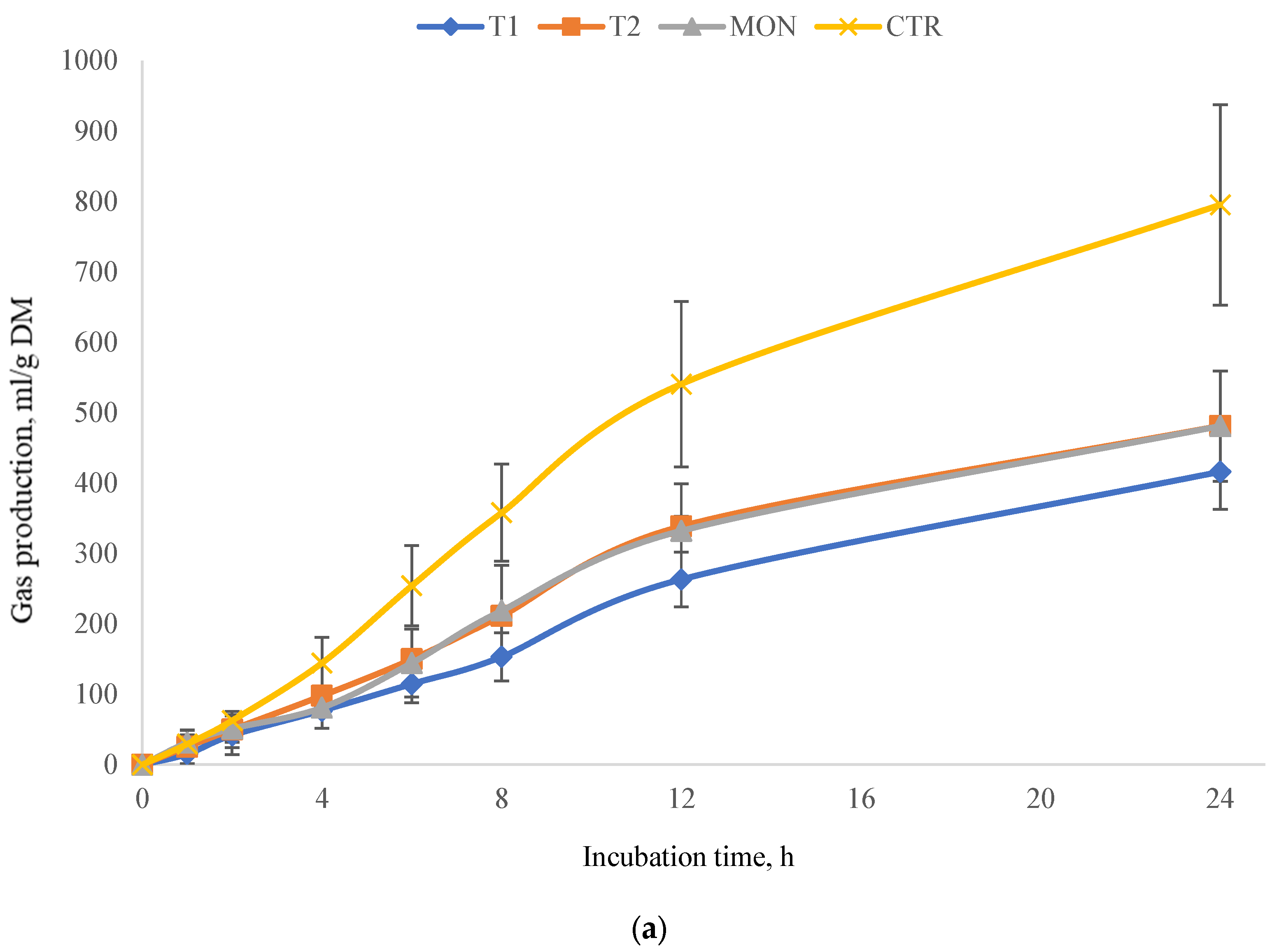
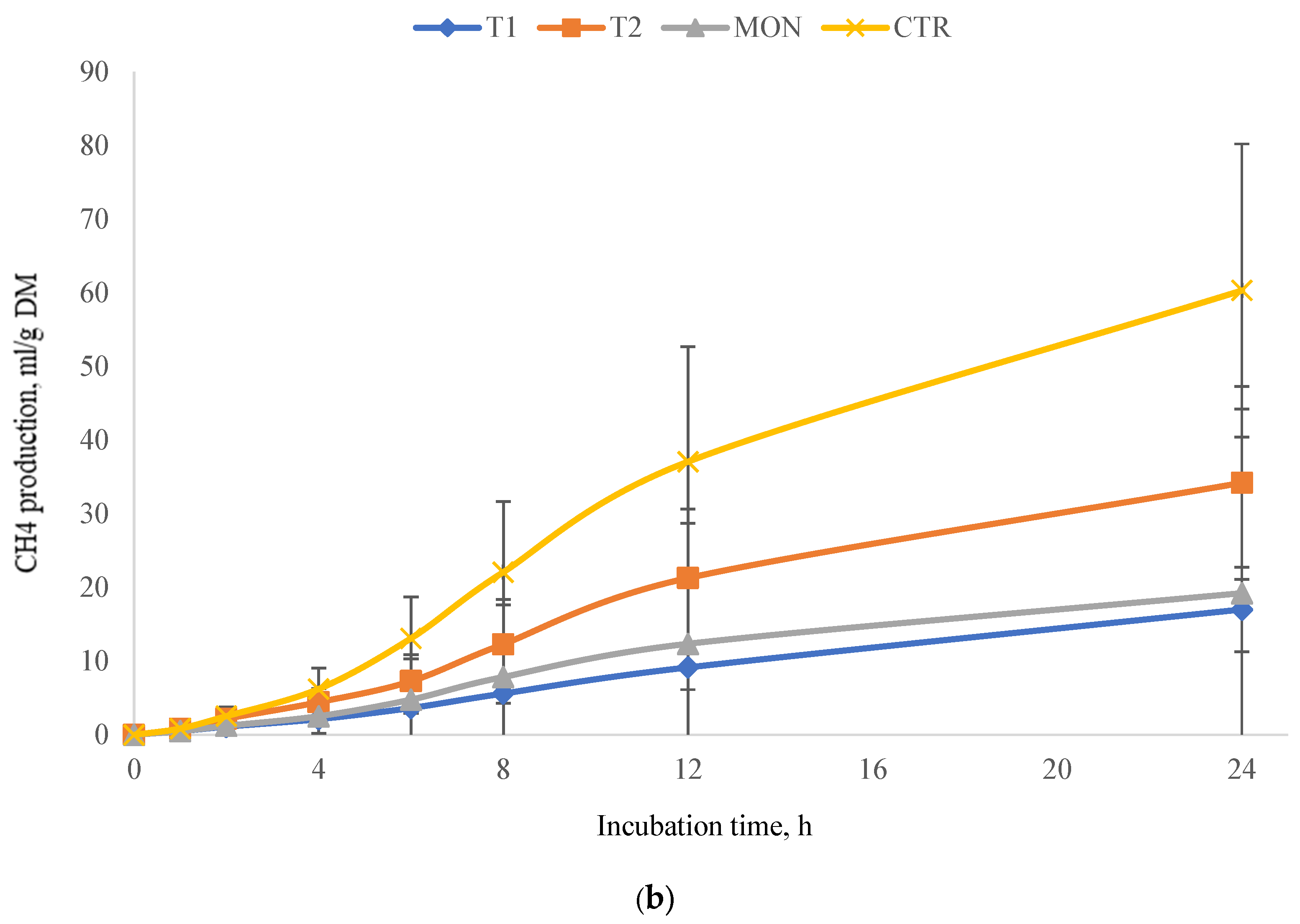
3.2. Experiment 2 (Total Gas and Methane Production)
4. Discussion
4.1. Experiment 1 (Rumen Microbial Fermentation)
4.1.1. Individual Efficacy: Baseline Performance of Essential Oils
4.1.2. Enhanced Efficacy: Synergistic Outcomes of Essential Oil Combinations
4.2. Experiment 2 (Total Gas and Methane Production)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GHG | Greenhouse gas |
| EO | Essential oil |
| EOB | Essential oil blend |
| VFA | Volatile fatty acid |
| TVFA | Total VFAs |
| A:P | Acetate-to-propionate ratio |
| NH3-N | Ammonia-N |
| HABP | Hyper-ammonia-producing bacteria |
| CH4 | Methane |
| SCD | Simplex centroid design |
References
- Borrel, G.; Fadhlaoui, K.; Ben Hania, W.; Gaci, N.; Pehau-Arnaudet, G.; Chaudhary, P.P.; Vandekerckove, P.; Ballet, N.; Alric, M.; O’toole, P.W. Methanomethylophilus Alvi Gen. Nov., Sp. Nov., a Novel Hydrogenotrophic Methyl-Reducing Methanogenic Archaea of the Order Methanomassiliicoccales Isolated from the Human Gut and Proposal of the Novel Family Methanomethylophilaceae Fam. Nov. Microorganisms 2023, 11, 2794. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane Production by Ruminants: Its Contribution to Global Warming. In Annales de Zootechnie; EDP Sciences: London, UK, 2000; Volume 49, pp. 231–253. [Google Scholar] [CrossRef]
- Hristov, A.; Oh, J.; Firkins, J.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.; Adesogan, A.; Yang, W.; Lee, C.; et al. Special Topics—Mitigation of Methane and Nitrous Oxide Emissions from Animal Operations: I. A Review of Enteric Methane Mitigation Options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Beauchemin, K.; Kreuzer, M.; O’mara, F.; McAllister, T. Nutritional Management for Enteric Methane Abatement: A Review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Chhabra, A.; Manjunath, K.; Panigrahy, S.; Parihar, J. Spatial Pattern of Methane Emissions from Indian Livestock. Curr. Sci. 2009, 96, 683–689. Available online: http://sa.indiaenvironmentportal.org.in/files/Spatial%20pattern%20of%20methane%20emissions.pdf (accessed on 11 February 2023).
- Pinares-Patiño, C.; Waghorn, G.; Machmüller, A.; Vlaming, B.; Molano, G.; Cavanagh, A.; Clark, H. Methane Emissions and Digestive Physiology of Non-Lactating Dairy Cows Fed Pasture Forage. Can. J. Anim. Sci. 2007, 87, 601–613. [Google Scholar] [CrossRef]
- Boadi, D.; Wittenberg, K. Methane Production from Dairy and Beef Heifers Fed Forages Differing in Nutrient Density Using the Sulphur Hexafluoride (SF6) Tracer Gas Technique. Can. J. Anim. Sci. 2002, 82, 201–206. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Li, M.M.; Zhang, G.G.; Sun, X.Z.; Dong, S.T.; Hoskin, S.O. Studies on Methane Emissions from Pastoral Farming in New Zea-land. J. Integr. Agric. 2014, 13, 365–377. [Google Scholar] [CrossRef]
- Ingale, S.; Lokhande, A.; Zadbuke, S. Nutritional Strategies to Mitigate Greenhouse Gases Emission from Livestock Agriculture: A Review. Livest. Res. Int. 2013, 1, 34–45. [Google Scholar]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; Available online: http://www.fao.org/docrep/018/i3437e/i3437e00.htm (accessed on 12 April 2023).
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-Analysis of the Relationship between Dietary Tannin Level and Methane Formation in Ruminants from in Vivo and in Vitro Experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Nkonya, E.M. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. Available online: https://www.ipcc.ch/report/2019-refinement-to-the-2006-ipcc-guidelines-for-national-greenhouse-gas-inventories/ (accessed on 16 April 2023).
- Nastoh, N.; Waqas, M.; Çınar, A.; Salman, M. The Impact of Phytogenic Feed Additives on Ruminant Production: A Review. J. Anim. Feed Sci. 2024, 33, 431–453. [Google Scholar] [CrossRef]
- Kholif, A.; Hassan, A.; El Ashry, G.M.; Bakr, M.; El-Zaiat, H.; Olafadehan, O.; Matloup, O.; Sallam, S. Phytogenic Feed Additives Mixture Enhances the Lactational Performance, Feed Utilization and Ruminal Fermentation of Friesian Cows. Anim. Biotechnol. 2021, 32, 708–718. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition; European Commission: Brussels, Belgium, 2003; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003R1831&rid=10 (accessed on 16 April 2023).
- Karatzia, M.A.; Pourliotis, K.; Katsoulos, P.D.; Karatzias, H. Effects of In-Feed Inclusion of Clinoptilolite on Blood Serum Concentrations of Aluminium and Inorganic Phosphorus and on Ruminal pH and Volatile Fatty Acid Concentrations in Dairy Cows. Biol. Trace Elem. Res. 2011, 142, 159–166. [Google Scholar] [CrossRef]
- Singh, A.K.; Ojha, L.; Kumari, P.; Choubey, M.; Chaudhary, S.K. Phytochemicals as Natural Feed Additives for Ruminants. In Feed Additives and Supplements for Ruminants; Springer: Berlin/Heidelberg, Germany, 2024; pp. 167–196. [Google Scholar] [CrossRef]
- Zeller, W.E. Activity, Purification, and Analysis of Condensed Tannins: Current State of Affairs and Future Endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Kholif, A.E. A Review of Effect of Saponins on Ruminal Fermentation, Health and Performance of Ruminants. Vet. Sci. 2023, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Li, S.; Li, D.; Li, X.; Xu, Z.; Liu, D. Effects of Yeast Culture on Growth Performance, Immune function, Anti-oxidant Capacity and Hormonal Profile in Mongolian Ram Lambs. Front. Vet. Sci. 2024, 11, 1424073. [Google Scholar] [CrossRef]
- Bisinotto, R.; Greco, L.; Ribeiro, E.; Martinez, N.; Lima, F.; Staples, C.; Thatcher, W.; Santos, J. Influences of Nutrition and Metabolism on Fertility of Dairy Cows. Anim. Reprod. AR 2018, 9, 260–272. Available online: https://animal-reproduction.org/article/5b5a6057f7783717068b46e5 (accessed on 6 May 2023).
- Qianrige; Roh, S.; Kim, D.-H.; Shishido, T.; Ogura, S.-I. Effects of Rumen Fermentation Characteristics on Stress-Related Hormones and Behavior in Sheep. Animals 2023, 13, 3701. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A Meta-Analysis Describing the Effects of the Essential Oils Blend Agolin Ruminant on Performance, Rumen Fermentation and Methane Emissions in Dairy Cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef]
- Castillejos, L.; Calsamiglia, S.; Ferret, A. Effect of Essential Oil Active Compounds on Rumen Microbial Fermentation and Nutrient Flow in in Vitro Systems. J. Dairy Sci. 2006, 89, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.; McIntosh, F.; Williams, P.; Losa, R.; Wallace, R. Effects of a Specific Blend of Essential Oil Compounds on Rumen Fermentation. Anim. Feed Sci. Technol. 2004, 114, 105–112. [Google Scholar] [CrossRef]
- Abdillah, A.E.; Sarah, D.; Ardian, A.A.; Anas, M.A.; Aprianto, M.A.; Hanim, C.; Kurniawati, A.; Muhlisin; Yusiati, L.M. Effect of Nutmeg Essential Oil (Myristica Fragrans Houtt.) on Methane Production, Rumen Fermentation, and Nutrient Digestibility in Vitro. Sci. Rep. 2024, 14, 3554. [Google Scholar] [CrossRef] [PubMed]
- Benetel, G.; Silva, T.D.S.; Fagundes, G.M.; Welter, K.C.; Melo, F.A.; Lobo, A.A.; Muir, J.P.; Bueno, I.C. Essential Oils as in Vitro Ruminal Fermentation Manipulators to Mitigate Methane Emission by Beef Cattle Grazing Tropical Grasses. Molecules 2022, 27, 2227. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.; Castillejos, L.; Ferret, A. Invited Review: Essential Oils as Modifiers of Rumen Microbial Fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical Evaluation of Essential Oils as Rumen Modifiers in Ruminant Nutrition: A Review. Sci. Total Environ. 2016, 545, 556–568. [Google Scholar] [CrossRef]
- Bryan, D.D.; Classen, H.L. In Vitro Methods of Assessing Protein Quality for Poultry. Animals 2020, 10, 551. [Google Scholar] [CrossRef]
- Ortolani, I.R.; Amanzougarene, Z.; Fondevila, M. In Vitro Estimation of the Effect of Grinding on Rumen Fermentation of Fibrous Feeds. Animals 2020, 10, 732. [Google Scholar] [CrossRef]
- García-González, R.; López, S.; Fernández, M.; Bodas, R.; González, J.S. Screening the Activity of Plants and Spices for Decreasing Ruminal Methane Production in Vitro. Anim. Feed Sci. Technol. 2008, 147, 36–52. [Google Scholar] [CrossRef]
- Durmic, Z.; Hutton, P.; Revell, D.; Emms, J.; Hughes, S.; Vercoe, P. In Vitro Fermentative Traits of Australian Woody Perennial Plant Species That May Be Considered as Potential Sources of Feed for Grazing Ruminants. Anim. Feed Sci. Technol. 2010, 160, 98–109. [Google Scholar] [CrossRef]
- Hatew, B.; Cone, J.; Pellikaan, W.; Podesta, S.; Bannink, A.; Hendriks, W.; Dijkstra, J. Relationship between in Vitro and in Vivo Methane Production Measured Simultaneously with Different Dietary Starch Sources and Starch Levels in Dairy Cattle. Anim. Feed Sci. Technol. 2015, 202, 20–31. [Google Scholar] [CrossRef]
- Goering, H.K. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970. Available online: https://www.govinfo.gov/content/pkg/GOVPUB-A-PURL-gpo24229/pdf/GOVPUB-A-PURL-gpo24229.pdf (accessed on 24 February 2023).
- Kh, M. Estimation of the Energetic Feed VALUE obtained from Chemical Analysis and in Vitro Gas Production Using Rumen Fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Patra, A.K.; Yu, Z. Effects of Gas Composition in Headspace and Bicarbonate Concentrations in Media on Gas and Methane Production, Degradability, and Rumen Fermentation Using in Vitro Gas Production Techniques. J. Dairy Sci. 2013, 96, 4592–4600. [Google Scholar] [CrossRef]
- Bodas, R.; López, S.; Fernandez, M.; García-González, R.; Rodríguez, A.; Wallace, R.; González, J. In Vitro Screening of the Potential of Numerous Plant Species as Antimethanogenic Feed Additives for Ruminants. Anim. Feed Sci. Technol. 2008, 145, 245–258. [Google Scholar] [CrossRef]
- Zhou, F.; Ji, B.; Zhang, H.; Jiang, H.; Yang, Z.; Li, J.; Li, J.; Ren, Y.; Yan, W. Synergistic Effect of Thymol and Carvacrol Combined with Chelators and Organic Acids against Salmonella Typhimurium. J. Food Prot. 2007, 70, 1704–1709. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Plant Extracts Affect in Vitro Rumen Microbial Fermentation. J. Dairy Sci. 2006, 89, 761–771. [Google Scholar] [CrossRef]
- Tilley, J.; Terry, D.R. A Two-Stage Technique for the in Vitro Digestion of Forage Crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A Simple Gas Production Method Using a Pressure Transducer to Determine the Fermentation Kinetics of Ruminant Feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data; Wiley-Interscience: Hoboken, NJ, USA, 1843; Volume 255. [Google Scholar]
- Cardozo, P.; Calsamiglia, S.; Ferret, A.; Kamel, C. Screening for the Effects of Natural Plant Extracts at Different pH on in Vitro Rumen Microbial Fermentation of a High-Concentrate Diet for Beef Cattle. J. Anim. Sci. 2005, 83, 2572–2579. [Google Scholar] [CrossRef]
- McDougall, E. Studies on Ruminant Saliva. 1. The Composition and Output of Sheep’s Saliva. Biochem. J. 1948, 43, 99. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1274641/pdf/biochemj00946-0114.pdf (accessed on 24 February 2023). [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Temmar, R.; Rodríguez-Prado, M.; Forgeard, G.; Rougier, C.; Calsamiglia, S. Interactions among Natural Active Ingredients to Improve the Efficiency of Rumen Fermentation in Vitro. Animals 2021, 11, 1205. [Google Scholar] [CrossRef]
- Mould, F.; Kliem, K.; Morgan, R.; Mauricio, R. In Vitro Microbial Inoculum: A Review of Its Function and Properties. Anim. Feed Sci. Technol. 2005, 123, 31–50. [Google Scholar] [CrossRef]
- Blümmel, M.; Steingaβ, H.; Becker, K. The Relationship between in Vitro Gas Production, in Vitro Microbial Biomass Yield and 15N Incorporation and Its Implications for the Prediction of Voluntary Feed Intake of Roughages. Br. J. Nutr. 1997, 77, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Getachew, G.; Blümmel, M.; Makkar, H.; Becker, K. In Vitro Gas Measuring Techniques for Assessment of Nutritional Quality of Feeds: A Review. Anim. Feed Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.; Lopez, S.; Bannink, A. Estimating the Extent of Degradation of Ruminant Feeds from a Description of Their Gas Production Profiles Observed in Vitro: Derivation of Models and Other Mathematical Considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef]
- Foiklang, S.; Wanapat, M.; Norrapoke, T. Effect of Grape Pomace Powder, Mangosteen Peel Powder and Monensin on Nutrient Digestibility, Rumen Fermentation, Nitrogen Balance and Microbial Protein Synthesis in Dairy Steers. Asian-Australas. J. Anim. Sci. 2015, 29, 1416. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and Antimicrobial Activities of Lippia Multiflora Moldenke, Mentha x Piperita L. and Ocimum Basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Patra, A.K. Effects of Essential Oils on Rumen Fermentation, Microbial Ecology and Ruminant Production. Asian J. Anim. Vet. Adv. 2011, 6, 416–428. [Google Scholar] [CrossRef]
- Patra, A.K.; Park, T.; Braun, H.-S.; Geiger, S.; Pieper, R.; Yu, Z.; Aschenbach, J.R. Dietary Bioactive Lipid Compounds Rich in Menthol Alter Interactions among Members of Ruminal Microbiota in Sheep. Front. Microbiol. 2019, 10, 2038. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of Essential Oils on Methane Production and Fermentation by, and Abundance and Diversity of, Rumen Microbial Populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef]
- Farghaly, M.; Abdullah, M. Effect of Dietary Oregano, Rosemary and Peppermint as Feed Additives on Nutrients Digestibility, Rumen Fermentation and Performance of Fattening Sheep. Egypt. J. Nutr. Feeds 2021, 24, 365–376. [Google Scholar] [CrossRef]
- Hosoda, K.; Matsuyama, H.; PARK, W.-Y.; Nishida, T.; Ishida, M. Supplementary Effect of Peppermint (Mentha\times Piperita) on Dry Matter Intake, Digestibility, Ruminal Fermentation and Milk Production in Early Lactating Dairy Cows. Anim. Sci. J. 2006, 77, 503–509. [Google Scholar] [CrossRef]
- Spadini, C.; Iannarelli, M.; Carrillo Heredero, A.M.; Montanaro, S.L.; Mezzasalma, N.; Simoni, M.; Cabassi, C.S. Stability of the Antimicrobial Activity of Selected Essential Oils and Nature Identical Compounds and Their Interaction with Tween 20 against Reference Bacterial Strains of Zootechnical Interest. Ital. J. Anim. Sci. 2024, 23, 189–199. [Google Scholar] [CrossRef]
- Simoni, M.; Temmar, R.; Bignamini, D.A.; Foskolos, A.; Sabbioni, A.; Ablondi, M.; Quarantelli, A.; Righi, F. Effects of the Combination between Selected Phytochemicals and the Carriers Silica and Tween 80 on Dry Matter and Neutral Detergent Fibre Digestibility of Common Feeds. Ital. J. Anim. Sci. 2020, 19, 723–738. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, M.S.; Alagawany, M.; Farag, M.R.; Alqaisi, O.; Aqib, A.I.; Qumar, M.; Siddique, F.; Ramadan, M.F. Clove (Syzygium Aromaticum) and its Phytochemicals in Ruminant Feed: An Updated Review. Rend. Lincei. Sci. Fis. Nat. 2021, 32, 273–285. [Google Scholar] [CrossRef]
- Benchaar, C.; Chaves, A.V.; Fraser, G.R.; Beauchemin, K.A.; McAllister, T.A. Effect of essential oils and their components on invitro rumen microbial fermentation. Can. J. Anim. Sci. 2007, 87, 413–419. [Google Scholar] [CrossRef]
- Castillejos, L.; Calsamiglia, S.; Ferret, A.; Losa, R. Effects of Dose and Adaptation Time of a Specific Blend of Essential Oil Compounds on Rumen Fermentation. Anim. Feed Sci. Technol. 2007, 132, 186–201. [Google Scholar] [CrossRef]
- Chahaardoli, A.; Soroor, M.N.; Foroughi, A. The Effects of Anise (Pimpinella Anisum) Essential Oil and Extract on in Vitro Rumen Fermentation Parameters and Protozoa Population of Sheep. Inter. J. Vet. Sci. 2018, 7, 21–27. Available online: https://www.ijvets.com/pdf-files/Volume-7-no-1-2018/21-27.pdf (accessed on 30 June 2023).
- Hussain, I.; Cheeke, P. Effect of Dietary Yucca Schidigera Extract on Rumen and Blood Profiles of Steers Fed Concentrate-or Roughage-Based Diets. Anim. Feed Sci. Technol. 1995, 51, 231–242. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Molan, A.L.; Meagher, L.P.; Kolb, B.; Foo, L.Y.; Lane, G.A.; Attwood, G.A.; Fraser, K.; Tavendale, M. Variation in Antimicrobial Action of Proanthocyanidins from Dorycnium Rectum against Rumen Bacteria. Phytochemistry 2004, 65, 2485–2497. [Google Scholar] [CrossRef]
- Ribeiro, A.D.B.; Ferraz, M.; Polizel, D.M.; Miszura, A.A.; Gobato, L.G.M.; Barroso, J.P.R.; Susin, I.; Pires, A.V. Thyme Essential Oil for Sheep: Effect on Rumen Fermentation, Nutrient Digestibility, Nitrogen Metabolism, and Growth. Arq. Bras. Med. Veterinária E Zootec. 2019, 71, 2065–2074. [Google Scholar] [CrossRef]
- Benchaar, C. Diet Supplementation with Thyme Oil and Its Main Component Thymol Failed to Favorably Alter Rumen Fermentation, Improve Nutrient Utilization, or Enhance Milk Production in Dairy Cows. J. Dairy Sci. 2021, 104, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Khiaosa-Ard, R.; Metzler-Zebeli, B.; Ahmed, S.; Muro-Reyes, A.; Deckardt, K.; Chizzola, R.; Böhm, J.; Zebeli, Q. Fortification of Dried Distillers Grains plus Solubles with Grape Seed Meal in the Diet Modulates Methane Mitigation and Rumen Microbiota in Rusitec. J. Dairy Sci. 2015, 98, 2611–2626. [Google Scholar] [CrossRef]
- Lin, B.; Lu, Y.; Salem, A.; Wang, J.; Liang, Q.; Liu, J. Effects of Essential Oil Combinations on Sheep Ruminal Fermentation and Digestibility of a Diet with Fumarate Included. Anim. Feed Sci. Technol. 2013, 184, 24–32. [Google Scholar] [CrossRef]
- McIntosh, F.; Williams, P.; Losa, R.; Wallace, R.; Beever, D.; Newbold, C. Effects of Essential Oils on Ruminal Microorganisms and Their Protein Metabolism. Appl. Environ. Microbiol. 2003, 69, 5011–5014. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. A New Perspective on the Use of Plant Secondary Metabolites to Inhibit Methanogenesis in the Rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef]
- Chaves, A.; Stanford, K.; Dugan, M.; Gibson, L.; McAllister, T.; Van Herk, F.; Benchaar, C. Effects of Cinnamaldehyde, Garlic and Juniper Berry Essential Oils on Rumen Fermentation, Blood Metabolites, Growth Performance, and Carcass Characteristics of Growing Lambs. Livest. Sci. 2008, 117, 215–224. [Google Scholar] [CrossRef]
- Abdallah Sallam, S.M.; Mohamed Abdelgaleil, S.A.; da Silva Bueno, I.C.; Abdelwahab Nasser, M.E.; Araujo, R.C.; Abdalla, A.L. Effect of Some Essential Oils on in Vitro Methane Emission. Arch. Anim. Nutr. 2011, 65, 203–214. [Google Scholar] [CrossRef]
- Alabi, J.O.; Okedoyin, D.O.; Anotaenwere, C.C.; Wuaku, M.; Gray, D.; Adelusi, O.O.; Ike, K.A.; Olagunju, L.K.; Dele, P.A.; Anele, U.Y. Essential Oil Blends with or without Fumaric Acid Influenced in Vitro Rumen Fermentation, Greenhouse Gas Emission, and Volatile Fatty Acids Production of a Total Mixed Ration. Ruminants 2023, 3, 373–384. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Miranda-Romero, L.A.; Mendoza-Martínez, G.D.; Santiago-Figueroa, I. A Meta-Analysis of Essential Oils Use for Beef Cattle Feed: Rumen Fermentation, Blood Metabolites, Meat Quality, Performance and, Environmental and Economic Impact. Fermentation 2022, 8, 254. [Google Scholar] [CrossRef]
- Wallace, R.J.; McEwan, N.R.; McIntosh, F.M.; Teferedegne, B.; Newbold, C.J. Natural Products as Manipulators of Rumen Fermentation. Asian-Australas. J. Anim. Sci. 2002, 15, 1458–1468. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Vasta, V.; Makkar, H.P.; Mele, M.; Priolo, A. Ruminal Biohydrogenation as Affected by Tannins in Vitro. Br. J. Nutr. 2008, 102, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, P.; Calsamiglia, S.; Ferret, A.; Kamel, C. Effects of Alfalfa Extract, Anise, Capsicum, and a Mixture of Cinnamaldehyde and Eugenol on Ruminal Fermentation and Protein Degradation in Beef Heifers Fed a High-Concentrate Diet. J. Anim. Sci. 2006, 84, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Fandiño, I.; Calsamiglia, S.; Ferret, A.; Blanch, M. Anise and Capsicum as Alternatives to Monensin to Modify Rumen Fermentation in Beef Heifers Fed a High Concentrate Diet. Anim. Feed Sci. Technol. 2008, 145, 409–417. [Google Scholar] [CrossRef]
- Bokharaeian, M.; Ghoorchi, T.; Toghdory, A.; Esfahani, I.J. The Dose-Dependent Role of Sage, Clove, and Pine Essential Oils in Modulating Ruminal Fermentation and Biohydrogenation of Polyunsaturated Fatty Acids: A Promising Strategy to Reduce Methane Emissions and Enhance the Nutritional Profile of Ruminant Products. Appl. Sci. 2023, 13, 11605. [Google Scholar] [CrossRef]
- Molho-Ortiz, A.A.; Romero-Pérez, A.; Ramírez-Bribiesca, E.; Márquez-Mota, C.C.; Castrejón-Pineda, F.A.; Corona, L. Effect of Essential Oils and Aqueous Extracts of Plants on in Vitro Rumen Fermentation and Methane Production. J. Anim. Behav. Biometeorol. 2021, 10, e2210. [Google Scholar] [CrossRef]
- Brice, R.M.; Dele, P.A.; Ike, K.A.; Shaw, Y.A.; Olagunju, L.K.; Orimaye, O.E.; Subedi, K.; Anele, U.Y. Effects of Essential Oil Blends on in Vitro Apparent and Truly Degradable Dry Matter, Efficiency of Microbial Production, Total Short-Chain Fatty Acids and Greenhouse Gas Emissions of Two Dairy Cow Diets. Animals 2022, 12, 2185. [Google Scholar] [CrossRef]
- Lin, B.; Lu, Y.; Wang, J.; Liang, Q.; Liu, J. The Effects of Combined Essential Oils Along with Fumarate on Rumen Fermentation and Methane Production in Vitro. J. Anim. Feed Sci. 2012, 21, 198–210. Available online: http://www.cas.zju.edu.cn/_upload/article/files/35/91/74307abf443899f74cc59328072c/51a09fe7-a83c-41dd-8e54-479f366b5081.pdf (accessed on 30 June 2023). [CrossRef]
- Temmar, R. The Effects of Feed Additives, Essential Oils or Guanidinoacetic Acid, on Cattle Rumen Microbial Fermentation in in Vitro Studies. Ph.D. Thesis, Autonomous University of Barcelona, Barcelona, Spain, 2022. Available online: https://ddd.uab.cat/record/275989 (accessed on 14 July 2023).
- El-Azrak, K.M.; Morsy, A.; Soltan, S.; Sallam, S.M.A.; Samak, M.; El-Komy, A.; Abdalla, A.L. Effect of Specific Blend of Essential Oils on in Vitro Rumen Fermentation and Degradability. In Proceedings of the 22nd Reunión Latinoamericana de Producción Animal, Montevideo, Uruguay, 24–26 October 2011. [Google Scholar]
- Wang, R.; Wang, R.; Yang, B. Extraction of Essential Oils from Five Cinnamon Leaves and Identification of Their Volatile Compound Compositions. Innov. Food Sci. Emerg. Technol. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Macheboeuf, D.; Morgavi, D.; Papon, Y.; Mousset, J.-L.; Arturo-Schaan, M. Dose–Response Effects of Essential Oils on in Vitro Fermentation Activity of the Rumen Microbial Population. Anim. Feed Sci. Technol. 2008, 145, 335–350. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A Review of Plant-Derived Essential Oils in Ruminant Nutrition and Production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Screening for Effects of Plant Extracts and Active Compounds of Plants on Dairy Cattle Rumen Microbial Fermentation in a Continuous Culture System. Anim. Feed Sci. Technol. 2005, 123, 597–613. [Google Scholar] [CrossRef]
- Kamra, D.N.; Pawar, M.; Singh, B. Effect of Plant Secondary Metabolites on Rumen Methanogens and Methane Emissions by Ruminants. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 351–370. [Google Scholar] [CrossRef]
- Wanapat, M.; Cherdthong, A.; Phesatcha, K.; Kang, S. Dietary Sources and Their Effects on Animal Production and Environmental Sustainability. Anim. Nutr. 2015, 1, 96–103. [Google Scholar] [CrossRef]
- Abeer, M.; Ahlam, R.; Marwa, H. Impact of Anise, Clove, and Thyme Essential Oils as Feed Supplements on the Productive Performance and Digestion of Barki Ewes. Aust. J. Basic Appl. Sci. 2019, 13, 1–13. [Google Scholar] [CrossRef]
- Gray, D.; Dele, P.A.; Alabi, J.O.; Adelusi, O.O.; Wuaku, M.; Okedoyin, D.O.; Anotaenwere, C.C.; Ike, K.A.; Oderinwale, O.A.; Kholif, A.E.; et al. Comparative Properties of Anise, Clove, Oregano and Peppermint Essential Oils Used Individually or Combined on Nutrient Digestibility and Greenhouse Gas Emissions in Concentrate-and Fiber-Based Diets. Agric. Conspec. Sci. 2025, 90, 71–81. Available online: https://hrcak.srce.hr/file/476712 (accessed on 1 April 2025).
- Benchaar, C.; Greathead, H. Essential Oils and Opportunities to Mitigate Enteric Methane Emissions from Ruminants. Anim. Feed Sci. Technol. 2011, 166, 338–355. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Peiren, N.; Cone, J.W.; Zweifel, B.; Fievez, V.; De Campeneere, S. In Vivo and in Vitro Effects of a Blend of Essential Oils on Rumen Methane Mitigation. Livest. Sci. 2015, 180, 134–142. [Google Scholar] [CrossRef]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen Methanogens and Mitigation of Methane Emission by Anti-Methanogenic Compounds and Substances. J. Anim. Sci. Biotechnol. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Essential Oils Affect Populations of Some Rumen Bacteria in Vitro as Revealed by Microarray (RumenBactArray) Analysis. Front. Microbiol. 2015, 6, 297. [Google Scholar] [CrossRef]
- Deitmers, J.-H.; Gresner, N.; Südekum, K.-H. Opportunities and Limitations of a Standardisation of the Rumen Simulation Technique (RUSITEC) for Analyses of Ruminal Nutrient Degradation and Fermentation and on Microbial Community Characteristics. Anim. Feed Sci. Technol. 2022, 289, 115325. [Google Scholar] [CrossRef]
- Scicutella, F.; Foggi, G.; Daghio, M.; Mannelli, F.; Viti, C.; Mele, M.; Buccioni, A. A Review of in Vitro Approaches as Tools for Studying Rumen Fermentation and Ecology: Effectiveness Compared to in Vivo Outcomes. Ital. J. Anim. Sci. 2025, 24, 589–608. [Google Scholar] [CrossRef]
- Jouany, J.; Lassalas, B. Gas Pressure inside a Rumen in Vitro System Stimulates the Use of Hydrogen. Reprod. Nutr. Dev. 2002, 42, S64. [Google Scholar]
| Product | Main Active Compound (Purity, %) 1 |
|---|---|
| Anise oil (ANI) | Trans-anethole (95) |
| Thyme oil (THY) | Thymol and carvacrol (35) |
| Cinnamon leaf oil (CIN) | Eugenol (80); cinnamic aldehyde (2.5) |
| Peppermint oil (PPM) | Menthol (50) |
| Clove leaf oil (CLO) | Eugenol (84.9) |
| Treatment 1 | Proportion of the Main Active Compound in the Mixture, % | |||||
|---|---|---|---|---|---|---|
| Triad 1 (%) | Triad 2 (%) | |||||
| THY | PPM | CIN | ANI | CLO | PPM | |
| T1 (100-0-0%) | 100 | 0 | 0 | 100 | 0 | 0 |
| T2 (67-33-0%) | 67 | 33 | 0 | 67 | 33 | 0 |
| T3 (33-67-0%) | 33 | 67 | 0 | 33 | 67 | 0 |
| T4 (0-100-0%) | 0 | 100 | 0 | 0 | 100 | 0 |
| T5 (67-0-33%) | 67 | 0 | 33 | 67 | 0 | 33 |
| T6 (33-33-33%) | 33 | 33 | 33 | 33 | 33 | 33 |
| T7 (0-67-33%) | 0 | 67 | 33 | 0 | 67 | 33 |
| T8 (33-0-67%) | 33 | 0 | 67 | 33 | 0 | 67 |
| T9 (0-33-67%) | 0 | 33 | 67 | 0 | 33 | 67 |
| T10 (0-0-100%) | 0 | 0 | 100 | 0 | 0 | 100 |
| EO Treatment | Proportion of the Main Active Compound of Each EO Treatment | Dose, mg/L | ||||
|---|---|---|---|---|---|---|
| PPM | CIN | ANI | CLO | MON | ||
| T 1 | 20 | 80 | – | – | – | 400 |
| T 2 | – | – | 80 | 20 | – | 400 |
| Monensin | – | – | – | – | 100 | 12.5 |
| Combination of EO in the Mixture | TVFAs 1 (mM) | Acetate % | Propionate % | Butyrate % | A:P Ratio 2 | NH3-N 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | |
| THY | 40.5 | 3.70 | <0.01 | 45.5 | 1.01 | <0.01 | 18.4 | 0.92 | <0.01 | 29.6 | 0.99 | <0.01 | 2.38 | 0.09 | <0.01 | 35.6 | 1.94 | <0.01 |
| PPM | 48.4 | 3.70 | <0.01 | 44.7 | 1.01 | <0.01 | 19.4 | 0.92 | <0.01 | 27.4 | 0.99 | <0.01 | 2.30 | 0.09 | <0.01 | 35.7 | 1.94 | <0.01 |
| CIN | 44.5 | 3.70 | <0.01 | 45.0 | 1.01 | <0.01 | 21.4 | 0.92 | <0.01 | 26.1 | 0.99 | <0.01 | 2.10 | 0.09 | <0.01 | 32.7 | 1.94 | <0.01 |
| THY + PPM | 2.86 | 16.7 | 0.87 | 3.46 | 4.60 | 0.46 | 1.04 | 4.20 | 0.80 | −4.56 | 4.47 | 0.33 | 0.05 | 0.44 | 0.91 | 25.7 | 8.80 | <0.01 |
| THY + CIN | 11.4 | 16.7 | 0.51 | −0.99 | 4.60 | 0.83 | −2.03 | 4.20 | 0.63 | 0.84 | 4.47 | 0.85 | 0.11 | 0.44 | 0.80 | 19.9 | 8.80 | 0.02 |
| PPM + CIN | −2.86 | 16.7 | 0.87 | 7.71 | 4.60 | 0.12 | 1.54 | 4.20 | 0.72 | −3.93 | 4.47 | 0.40 | 0.20 | 0.44 | 0.65 | 28.4 | 8.80 | <0.01 |
| THY + PPM + CIN | −83.6 | 124 | 0.51 | 55.3 | 34.0 | 0.13 | 42.2 | 31.1 | 0.20 | −4.90 | 3.90 | 0.20 | −1.55 | 3.30 | 0.64 | −3.40 | 5.10 | 0.60 |
| Statistical Values | ||||||||||||||||||
| RSD | 5.23 | 1.44 | 1.32 | 1.39 | 0.14 | 6.74 | ||||||||||||
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.97 | ||||||||||||
| Adjusted R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.97 | ||||||||||||
| Predicted R2 | 0.97 | 0.99 | 0.99 | 0.99 | 0.99 | 0.96 | ||||||||||||
| PRESS | 1093 | 82.9 | 69.1 | 78.1 | 0.78 | 5955 | ||||||||||||
| Combination of EO in the Mixture | TVFAs 1 (mM) | Acetate % | Propionate % | Butyrate % | A:P Ratio 2 | NH3-N 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | Coef | SEM | p-Value | |
| ANI | 56.2 | 2.42 | <0.01 | 46.9 | 1.26 | <0.01 | 20.9 | 1.46 | <0.01 | 20.9 | 1.02 | <0.01 | 2.26 | 0.19 | <0.01 | 32.7 | 2.51 | <0.01 |
| CLO | 48.9 | 2.42 | <0.01 | 46.2 | 1.26 | <0.01 | 21.9 | 1.46 | <0.01 | 22.7 | 1.02 | <0.01 | 2.13 | 0.19 | <0.01 | 33.7 | 2.51 | <0.01 |
| PPM | 58.8 | 2.42 | <0.01 | 45.4 | 1.26 | <0.01 | 19.9 | 1.46 | <0.01 | 22.9 | 1.02 | <0.01 | 2.30 | 0.19 | <0.01 | 40.6 | 2.51 | <0.01 |
| ANI–CLO | 22.5 | 10.9 | 0.07 | 4.38 | 5.70 | 0.46 | −0.47 | 6.61 | 0.95 | −1.10 | 4.61 | 0.81 | 0.33 | 0.85 | 0.71 | 10.6 | 11.3 | 0.35 |
| ANI–PPM | −9.80 | 10.9 | 0.39 | −3.21 | 5.70 | 0.59 | −0.99 | 6.61 | 0.88 | 0.83 | 4.61 | 0.85 | −0.05 | 0.85 | 0.95 | 9.85 | 11.3 | 0.39 |
| CLO–PPM | 3.13 | 10.9 | 0.78 | 1.35 | 5.70 | 0.82 | −0.16 | 6.61 | 0.98 | 0.92 | 4.61 | 0.84 | 0.03 | 0.85 | 0.97 | 5.55 | 11.3 | 0.63 |
| ANI–CLO–PPM | 4.97 | 80.9 | 0.95 | 33.6 | 42.1 | 0.44 | 11.4 | 48.9 | 0.82 | −4.92 | 3.40 | 0.88 | 1.05 | 6.31 | 0.87 | 2.48 | 83.9 | 0.98 |
| Statistical Values | ||||||||||||||||||
| RSD | 3.42 | 1.78 | 2.07 | 1,44 | 0.27 | 8.69 | ||||||||||||
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.95 | ||||||||||||
| Adjusted R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.95 | ||||||||||||
| Predicted R2 | 0.99 | 0.99 | 0.98 | 0.99 | 0.97 | 0.94 | ||||||||||||
| PRESS | 468 | 127 | 171 | 83.0 | 2.85 | 9893 | ||||||||||||
| EO Proportions (%) | Triad 1 Treatment | TVFAs (mM) | Acetate (%) | Propionate (%) | Butyrate (%) | A:P | NH3-N mg/100 mL |
|---|---|---|---|---|---|---|---|
| THY100 | T1 | 40.5 | 45.5 | 18.4 | 29.6 * | 2.38 | 35.6 |
| THY67 + PPM33 | T2 | 45.7 | 46.5 | 18.4 | 27.8 | 2.54 | 42.7 |
| THY33 + PPM67 | T3 | 44.4 | 45.2 | 19.9 | 27.1 | 2.27 | 43.9 |
| PPM100 | T4 | 48.4 * | 44.7 | 19.4 | 27.4 | 2.30 | 35.7 |
| PPM67 + CIN33 | T7 | 45.2 | 47.5 | 20.6 * | 26.6 | 2.31 | 45.0 |
| PPM33 + CIN67 | T9 | 46.4 * | 45.6 | 20.9 * | 25.2 | 2.19 * | 39.9 |
| CIN100 | T10 | 44.5 | 45.0 | 21.4 * | 26.1 | 2.10 * | 32.7 * |
| THY33 + CIN67 | T8 | 45.1 | 45.7 | 19.4 | 27.8 * | 2.35 | 39.3 |
| THY67 + CIN33 | T5 | 43.8 | 44.3 | 19.5 | 28.3 * | 2.28 | 37.8 |
| THY33 + PPM33 + CIN33 | T6 | 42.2 | 47.7 | 21.1 * | 25.1 | 2.26 | 42.5 |
| Control | CTR | 40.4 | 47.2 | 19.8 | 22.9 | 2.40 | 36.4 |
| SEM | 2.50 | 1.23 | 0.79 | 0.92 | 0.09 | 1.88 | |
| p-Value | 0.05 | 0.41 | <0.01 | <0.01 | <0.01 | <0.01 |
| EO Combination | Observed Trends | ||
|---|---|---|---|
| TVFAs 1 (mM) | A:P Ratio 2 | NH3-N 3 mg/100 mL | |
| PPM 100% | 48 | 2.30 | 40 |
| CIN 80% + PPM 20% 4 | 46 | 2.20 | 36 |
| CIN 90% + THY 10% | 45 | 2.25 | 34 |
| EO Proportions (%) | Triad 2 Treatment | TVFAs (mM) | Acetate (%) | Propionate (%) | Butyrate (%) | A:P | NH3-N mg/100 mL |
|---|---|---|---|---|---|---|---|
| ANI100 | T1 | 56.2 | 46.9 | 20.8 | 20.9 | 2.26 | 32.7 * |
| ANI67 + CLO33 | T2 | 57.2 + | 46.0 | 21.9 * | 22.6 + | 2.11 * | 37.7 |
| ANI33 + CLO67 | T3 | 57.8 + | 49.2 | 20.6 | 20.7 | 2.42 | 33.3 * |
| CLO100 | T4 | 48.9 | 46.2 | 21.9 * | 22.7 + | 2.13 * | 33.7 * |
| CLO67 + PPM33 | T7 | 53.1 | 46.1 | 21.3 * | 23.1 + | 2.17 * | 37.6 |
| CLO33 + PPM67 | T9 | 55.9 | 46.2 | 20.4 | 22.9 + | 2.27 | 39.2 |
| PPM100 | T10 | 58.8 + | 45.4 | 19.9 | 22.9 + | 2.30 | 40.6 |
| ANI33 + PPM67 | T8 | 54.8 | 45.0 | 19.9 | 22.6 + | 2.28 | 39.3 |
| ANI67 + PPM33 | T5 | 55.9 | 45.9 | 20.4 | 21.7 | 2.25 | 38.3 |
| ANI33 + CLO33 + PPM33 | T6 | 55.9 | 47.2 | 20.9 | 21.9 | 2.27 | 38.2 |
| Control | CTR | 55.2 | 50.1 | 20.4 | 19.2 | 2.50 | 38.1 |
| SEM | 2.05 | 1.57 | 0.45 | 0.98 | 0.12 | 2.23 | |
| p-Value | 0.06 | 0.32 | <0.01 | 0.10 | 0.04 | 0.05 |
| EO Combination | Observed Trends | ||
|---|---|---|---|
| TVFAs 1 (mM) | A:P Ratio 2 | NH3-N 3 mg/100 mL | |
| CLO 50% + ANI 50% | 58 | 2.30 | 36 |
| PPM 90% + CLO 10% | 58 | 2.30 | 40 |
| ANI 80% + CLO 20% 4 | 57 | 2.10 | 38 |
| Gas Kinetic Constants | CTR | MON 1 | T1 2 | T2 3 | SEM 4 | p-Value |
|---|---|---|---|---|---|---|
| a, mL | 0.022 | 0.016 | 0.018 | 0.020 | 0.0025 | 0.30 |
| b, h−1 | 0.65 | 0.65 | 0.56 | 0.64 | 0.036 | 0.29 |
| c, h | 0.18 a | 0.18 a | 0.15 b | 0.18 a | 0.006 | <0.01 |
| Item | CTR | MON 1 | T1 2 | T2 3 | SEM 4 | p-Value |
|---|---|---|---|---|---|---|
| Cumulative total gas (mL/24 h) | 795.2 a | 481.0 b | 416.1 c | 481.5 b | 0.03 | <0.01 |
| Cumulative CH4 (mL/24 h) | 60.4 a | 19.2 c | 17.1 c | 34.2 b | 0.01 | 0.01 |
| Ratio CH4/total gas | 0.07 a | 0.04 c | 0.04 c | 0.07 a | 0.01 | 0.05 |
| pH | 6.57 a | 6.60 b | 6.61 b | 6.64 c | 0.014 | 0.01 |
| EO Proportion | Treatment | Observed CH4 (mL/24 h) | Predicted CH4 1 (mL/24 h) | Difference 2 |
|---|---|---|---|---|
| CIN 80% + PPM 20% | T1 | 17.1 | 25.4 | −8.30 |
| ANI 80% + CLO 20% | T2 | 34.2 | 29.9 | 4.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, M.; Rodríguez-Prado, M.; Simoni, M.; Martín-Orúe, S.M.; Pérez, J.F.; Calsamiglia, S. Optimizing Essential Oil Mixtures: Synergistic Effects on Cattle Rumen Fermentation and Methane Emission. Animals 2025, 15, 2105. https://doi.org/10.3390/ani15142105
Nasir M, Rodríguez-Prado M, Simoni M, Martín-Orúe SM, Pérez JF, Calsamiglia S. Optimizing Essential Oil Mixtures: Synergistic Effects on Cattle Rumen Fermentation and Methane Emission. Animals. 2025; 15(14):2105. https://doi.org/10.3390/ani15142105
Chicago/Turabian StyleNasir, Memoona, María Rodríguez-Prado, Marica Simoni, Susana M. Martín-Orúe, José Francisco Pérez, and Sergio Calsamiglia. 2025. "Optimizing Essential Oil Mixtures: Synergistic Effects on Cattle Rumen Fermentation and Methane Emission" Animals 15, no. 14: 2105. https://doi.org/10.3390/ani15142105
APA StyleNasir, M., Rodríguez-Prado, M., Simoni, M., Martín-Orúe, S. M., Pérez, J. F., & Calsamiglia, S. (2025). Optimizing Essential Oil Mixtures: Synergistic Effects on Cattle Rumen Fermentation and Methane Emission. Animals, 15(14), 2105. https://doi.org/10.3390/ani15142105






