Impacts of 360 mg/kg Niacinamide Supplementation in Low-Protein Diets on Energy and Nitrogen Metabolism and Intestinal Microbiota in Growing–Finishing Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use, Care, and Ethical Considerations
2.2. Diets and Experimental Design
2.3. Recording and Sample Collection
2.4. Chemical Analysis and Calculation
2.5. Statistical Analysis
3. Results
3.1. Serum Biochemical Parameters
3.2. Liver Urea Cycle-Related Indicators
3.3. Limiting Enzymes in Liver and Ileal Mucosa Related to Nutrient Metabolism
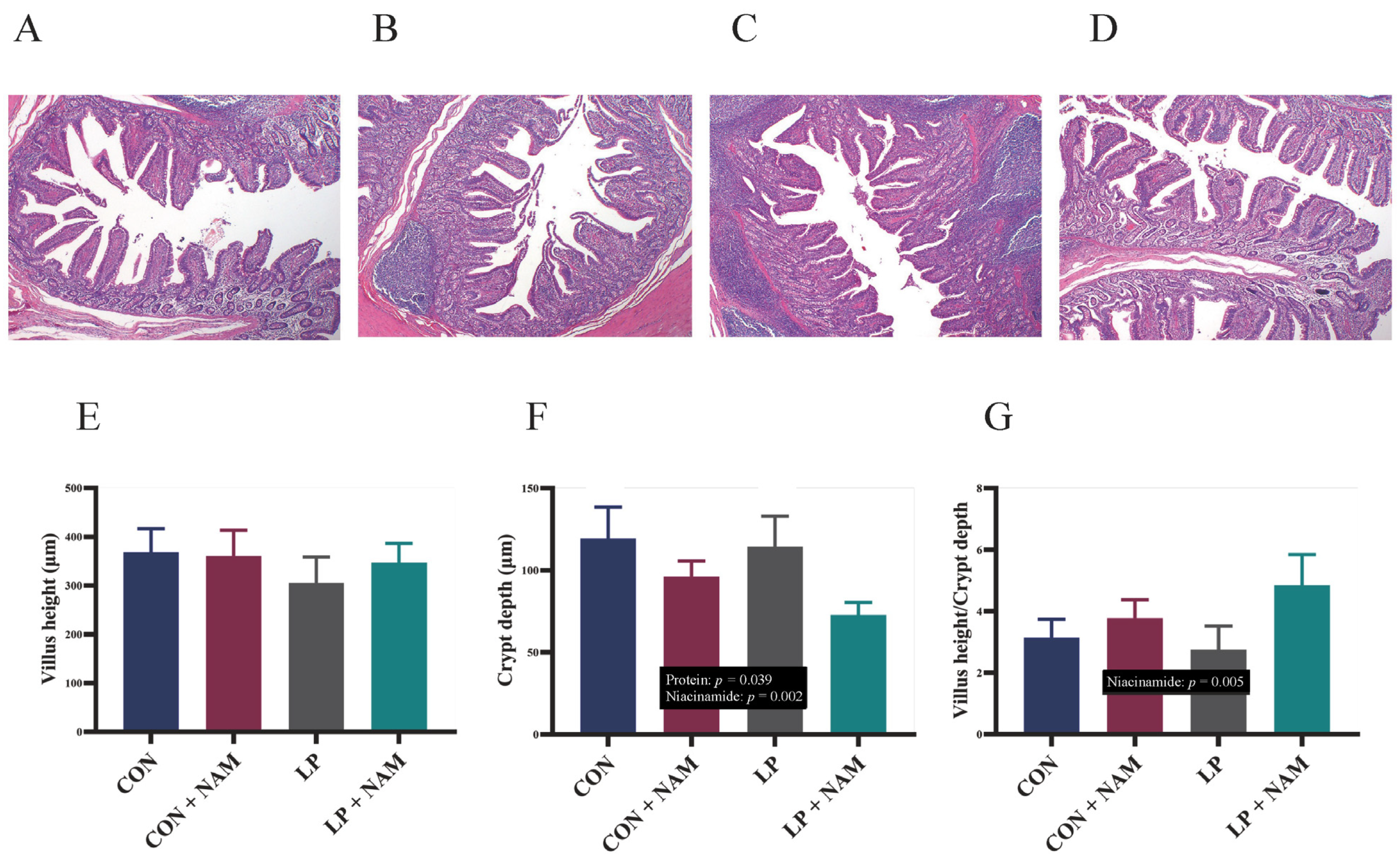
3.4. Ileal Mucosa Morphology
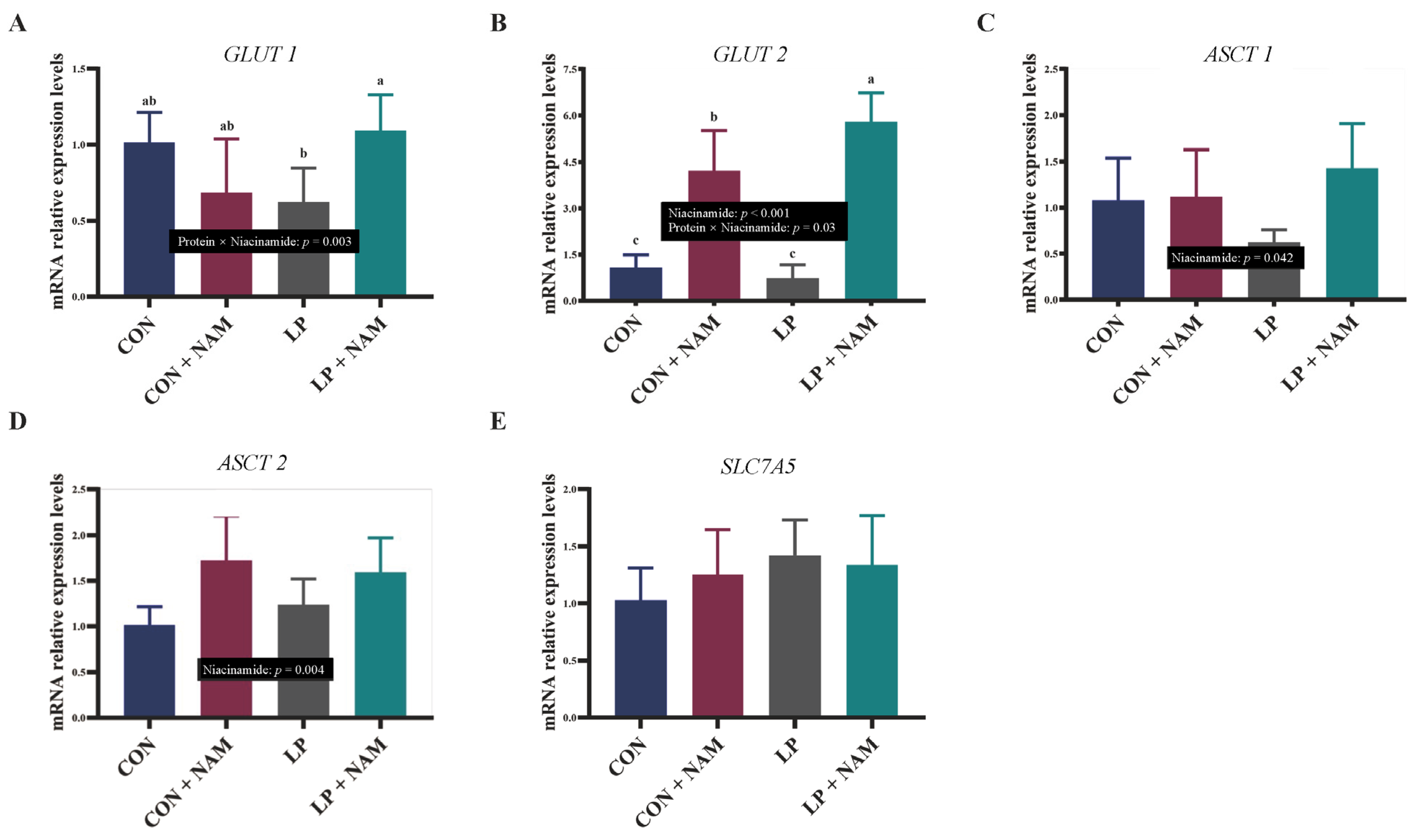
3.5. mRNA Expression of Molecules in the Ileum Mucosa Involved in Glucose and Glutamine Absorption and Transport
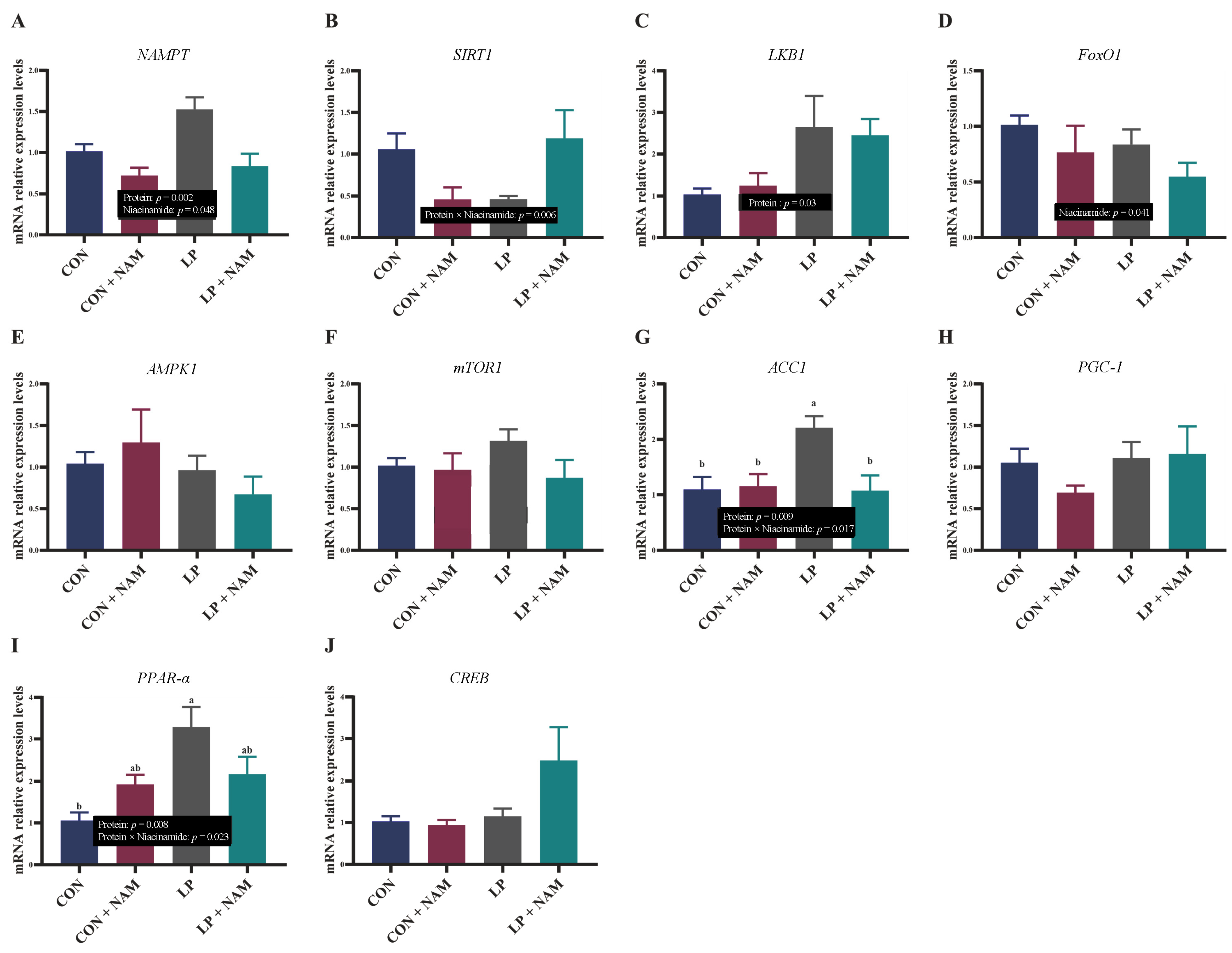
3.6. mRNA Expression of Molecules in the Liver Involved in NAD+ Metabolism
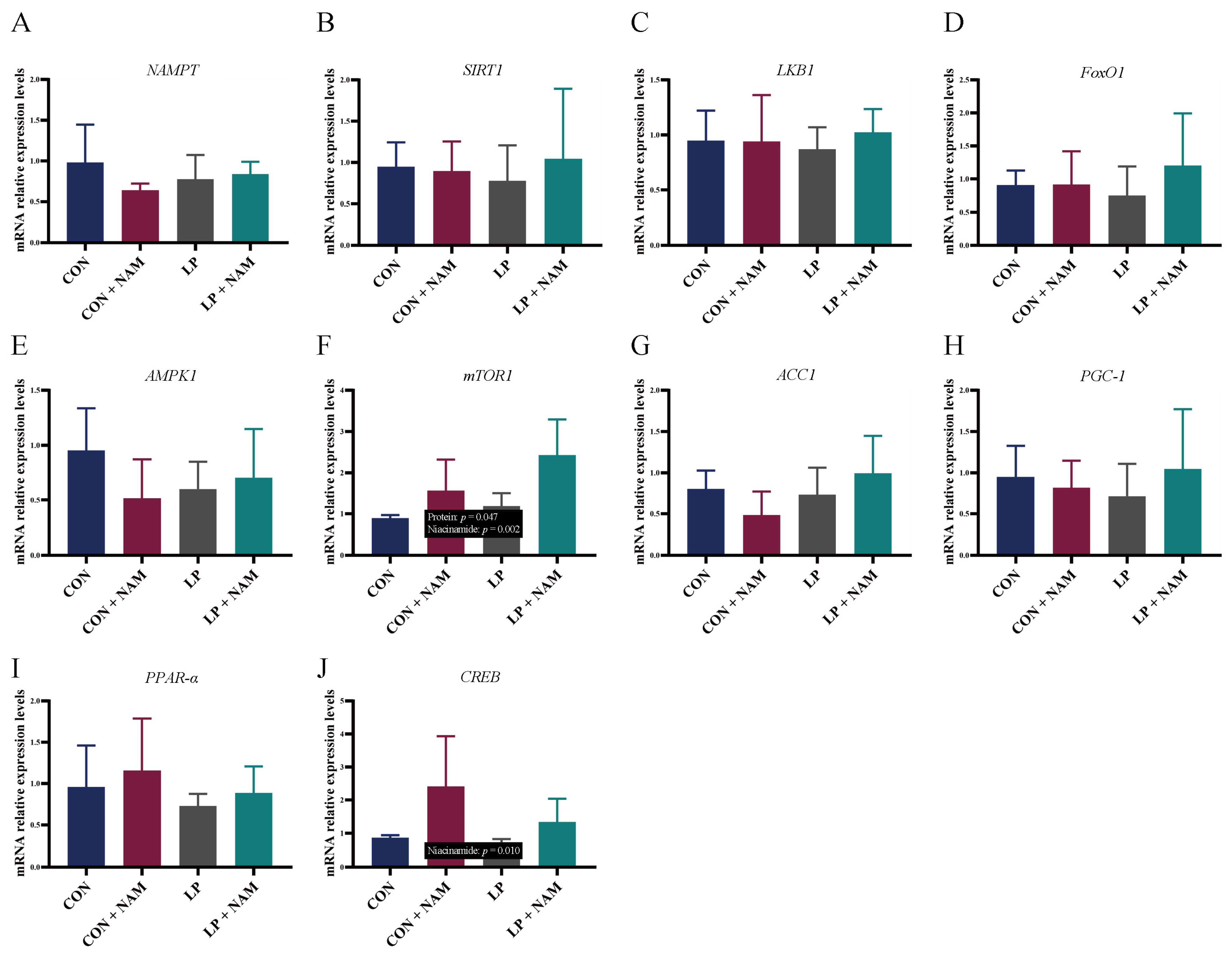
3.7. mRNA Expression of Genes in the Ileum Mucosa Involved in NAD+ Metabolism
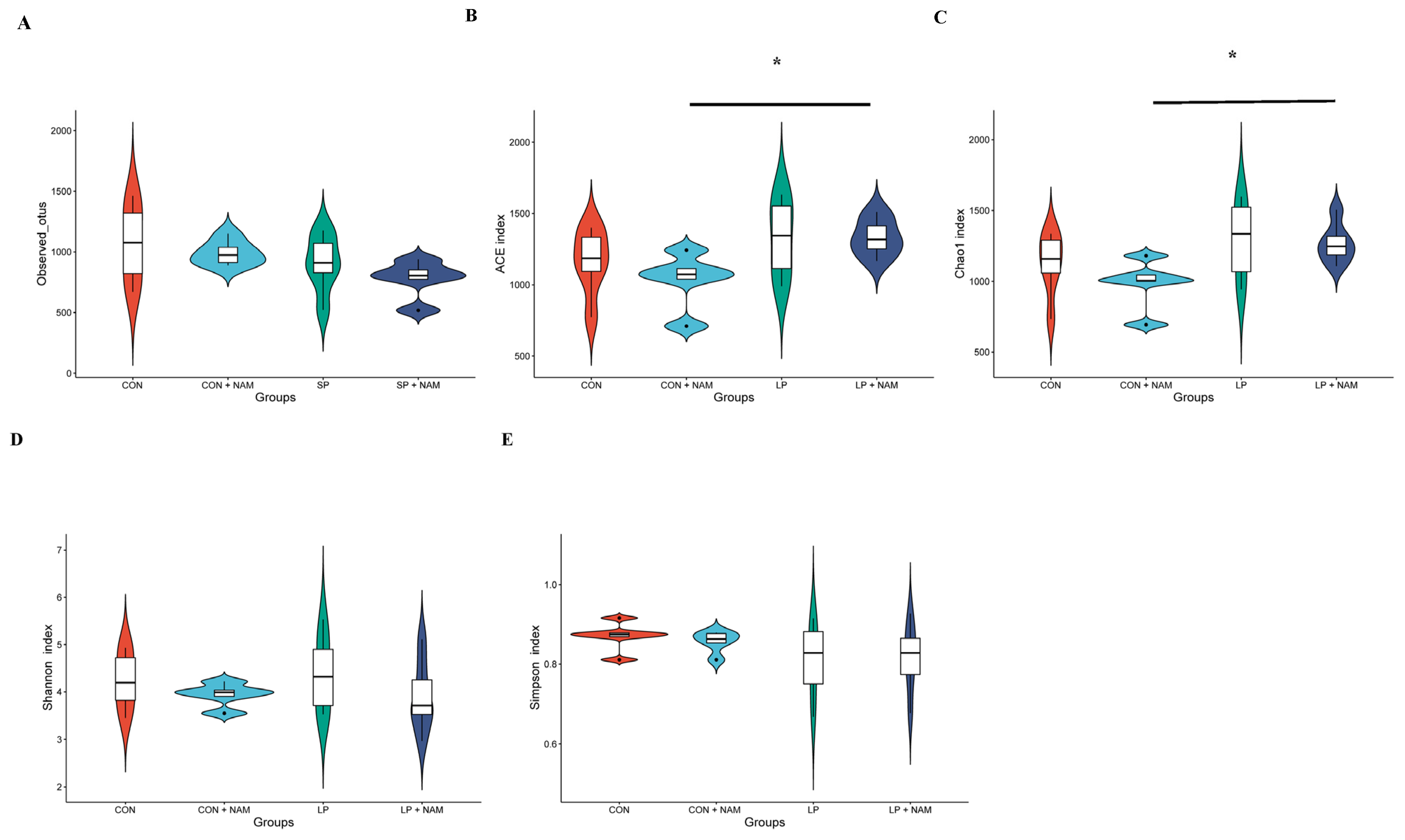
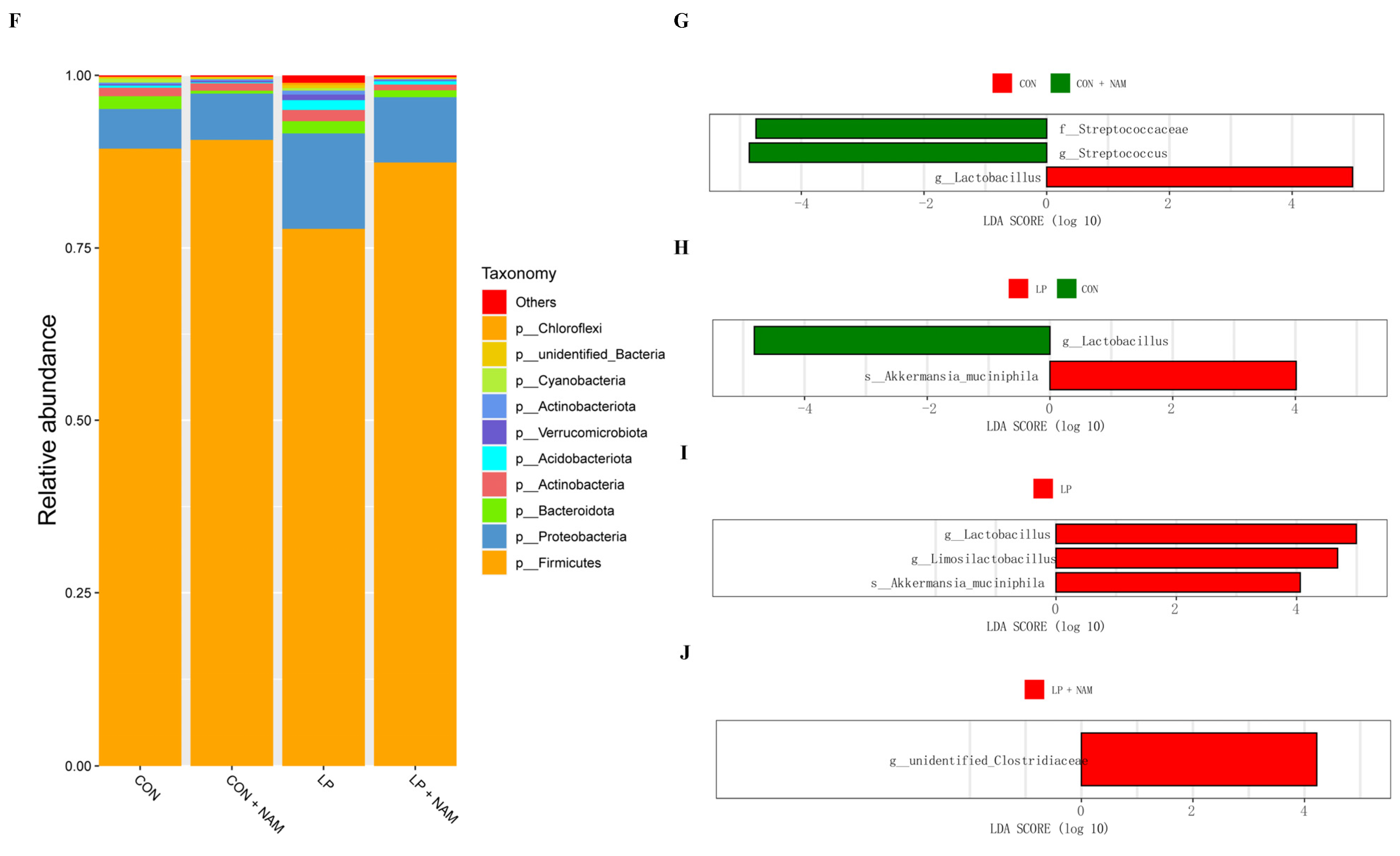
3.8. Changes in the Ileum Microbiota
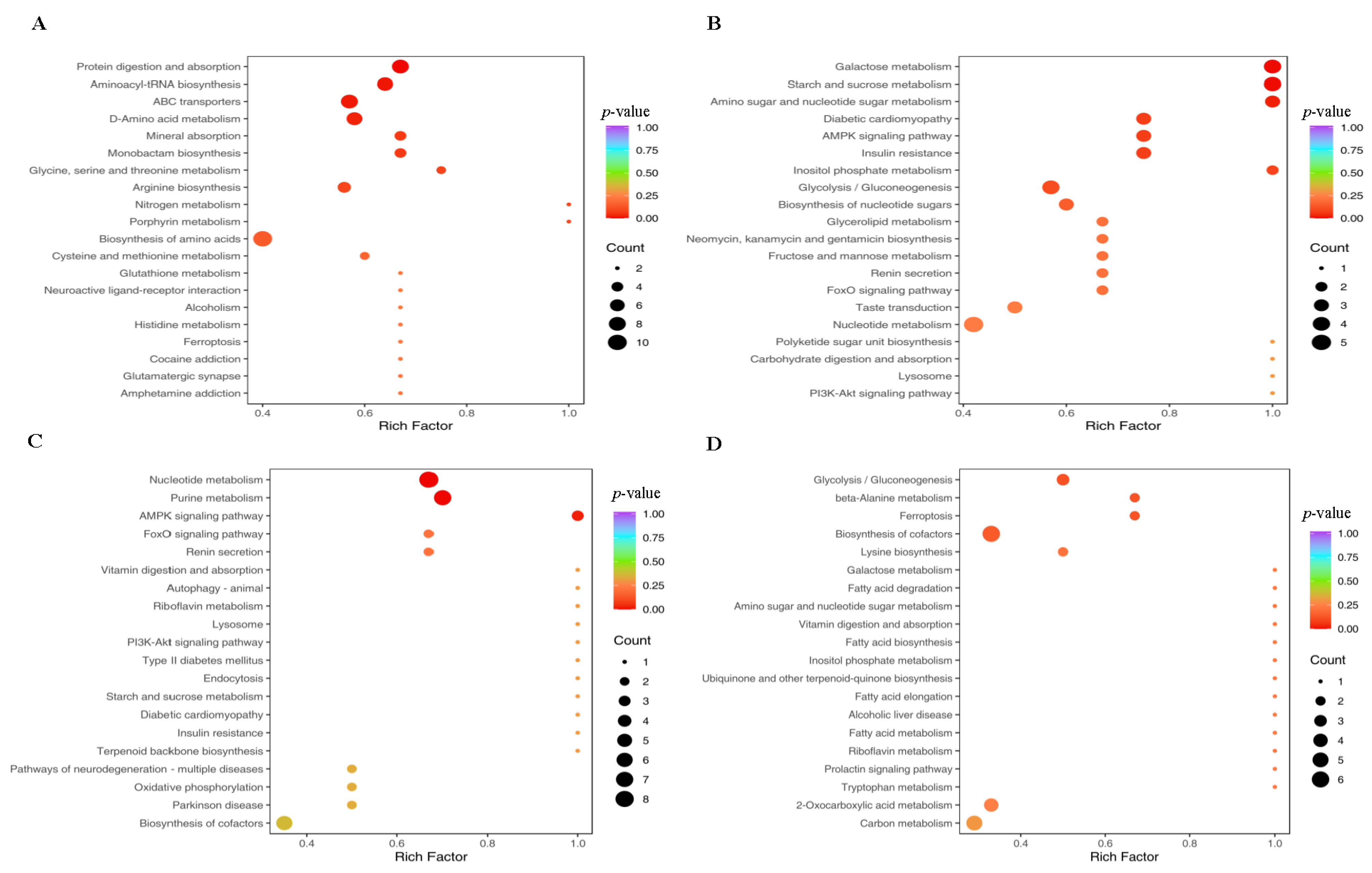
3.9. Metabolomic Analysis of Ileum Microbes
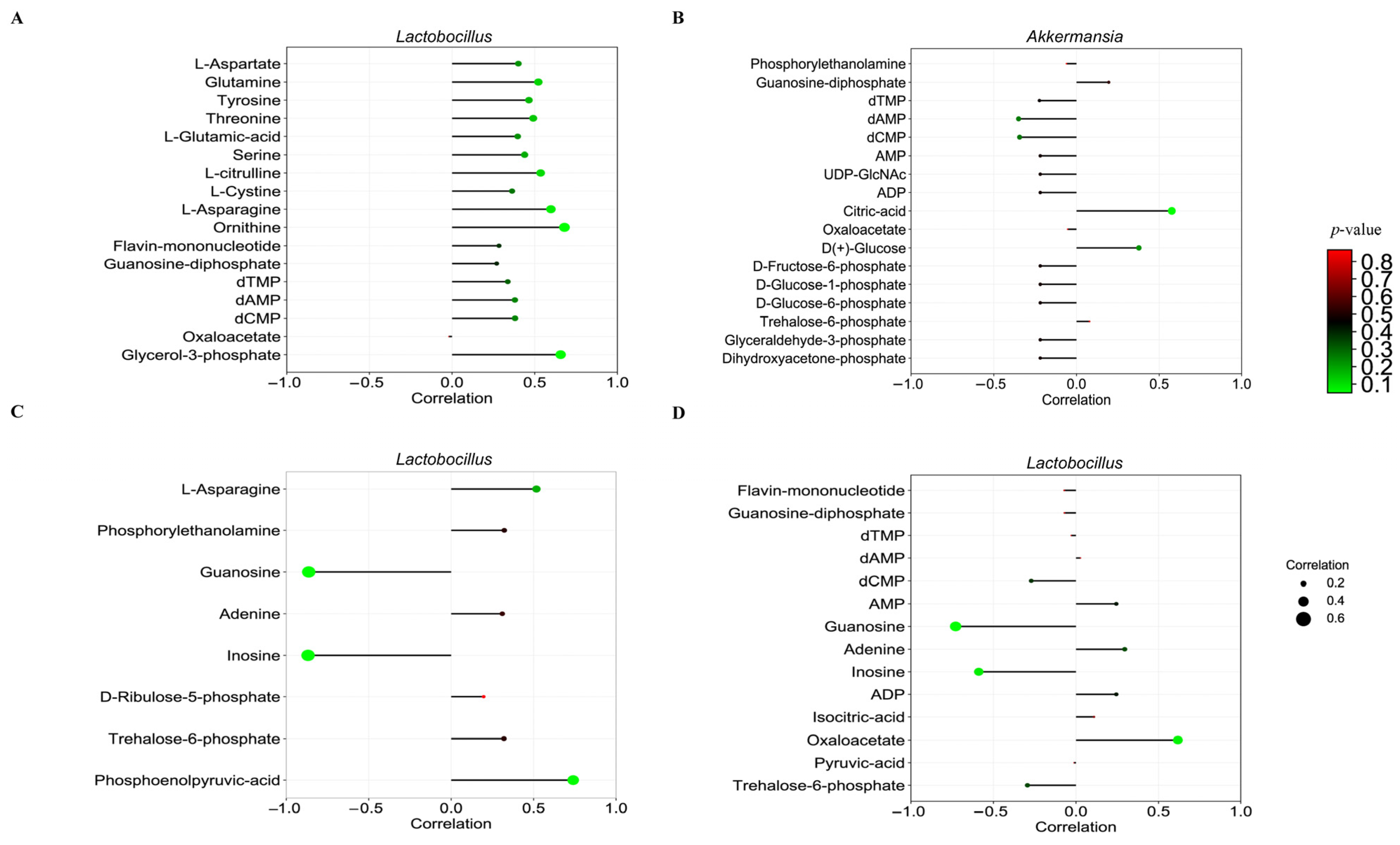
3.10. Association Between the Ileum Microbiome and Metabolome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NAMPT | nicotinamide phosphoribosyltransferase |
| SIRT1 | sirtuin 1 |
| LKB1 | liver Kinase B1 |
| AMPK-1 | AMP-activated protein kinase 1 |
| PPARα | peroxisome proliferator-activated receptor-α |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| ACC1 | acetyl-CoA carboxylase 1 |
| mTORC1 | mammalian target of rapamycin 1 |
| FOXO1 | forkhead box transcription factor O1 |
| CREB | cAMP response element-binding protein |
| GLUT1 | glucose transporter 1 |
| GLUT2 | glucose transporter 2 |
| ASCT1 | alanine-serine-cysteine transporter 1 |
| ASCT2 | alanine-serine-cysteine transporter 2 |
| SLC7A5 | solute carrier family 7 member 5 |
| GDH1 | glutamine dehydrogenase 1 |
| NMNAT1 | NMN adenylyl transferase 1 |
| NMRK1 | nicotinamide ribonucleoside kinase 1 |
| BUN | blood urea nitrogen |
| GLU | glucose |
| TG | triglyceride |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| GH | growth hormone |
| FINS | fasting insulin |
| GC | glycogen |
| GPT | glutamic-pyruvic transaminase |
| GOT | glutamic-oxaloacetic transaminase |
| PDH | pyruvate dehydrogenase |
| LDH | lactate dehydrogenase |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| TP | total protein |
| UN | urea nitrogen |
| CPS-1 | carbamyl phosphate synthetase-I |
| OTC | ornithine transcarbamylase |
| GS | glutamine synthetase |
| GLS | glutaminase |
| CS | citrate synthase |
| GDH | glutamate dehydrogenase |
| CPT1 | carnitine palmitoyltransferase 1 |
| HK | hexokinase |
| NAM | nicotinamide |
| LP | low-protein |
| CP | crude protein |
| NAD+ | nicotinamide adenine dinucleotide |
| CON | control |
| TCA | tricarboxylic acid |
References
- Kalscheur, K.F.; Baldwin, R.L.; Glenn, B.P.; Kohn, R.A. Milk production of dairy cows fed differing concentrations of rumen-degraded protein. J. Dairy. Sci. 2006, 89, 249–259. [Google Scholar] [CrossRef]
- Shriver, J.A.; Carter, S.D.; Sutton, A.L.; Richert, B.T.; Senne, B.W.; Pettey, L.A. Effects of adding fiber sources to reduced-crude protein, amino acid-supplemented diets on nitrogen excretion, growth performance, and carcass traits of finishing pigs. J. Anim. Sci. 2003, 81, 492–502. [Google Scholar] [CrossRef]
- Puiman, P.; Stoll, B.; Mølbak, L.; de Bruijn, A.; Schierbeek, H.; Boye, M.; Boehm, G.; Renes, I.; van Goudoever, J.; Burrin, D. Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G300–G310. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.T.; Luchini, D.; Murphy, M.R.; Cardoso, F.C. Effects of rumen-protected methionine on lactation performance and physiological variables during a heat stress challenge in lactating Holstein cows. J. Dairy. Sci. 2020, 103, 2800–2813. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, X.; Tang, Z.; Li, Y.; Li, T.; Xu, Q.; Zhen, J.; Huang, F.; Yang, J.; Chen, C.; et al. Low-protein diets decrease porcine nitrogen excretion but with restrictive effects on amino acid utilization. J. Agric. Food Chem. 2018, 66, 8262–8271. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, H.; Wan, K.; Zhou, K.; Wang, Y.; Li, J.; Tang, Z.; Sun, W.; Wu, L.; An, R.; et al. Effects of supplementing low-protein diets with sodium dichloroacetate and glucose on growth performance, carcass traits, and meat quality of growing-finishing pigs. J. Anim. Sci. 2022, 100, skab359. [Google Scholar] [CrossRef]
- Shi, W.; Hegeman, M.A.; van Dartel, D.A.M.; Tang, J.; Suarez, M.; Swarts, H.; van der Hee, B.; Arola, L.; Keijer, J. Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet. Mol. Nutr. Food Res. 2017, 61, 1600878. [Google Scholar] [CrossRef]
- Komatsu, M.; Kanda, T.; Urai, H.; Kurokochi, A.; Kitahama, R.; Shigaki, S.; Ono, T.; Yukioka, H.; Hasegawa, K.; Tokuyama, H.; et al. Nnmt activation can contribute to the development of fatty liver disease by modulating the NAD+ metabolism. Sci. Rep. 2018, 8, 8637. [Google Scholar] [CrossRef]
- Chellappa, K.; McReynolds, M.R.; Lu, W.; Zeng, X.; Makarov, M.; Hayat, F.; Mukherjee, S.; Bhat, Y.R.; Lingala, S.R.; Shima, R.T.; et al. NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 2022, 34, 1947–1959. [Google Scholar] [CrossRef]
- Heo, M.G.; Choung, S.Y. Anti-obesity effects of spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and sirt1. Food Funct. 2018, 9, 4906–4915. [Google Scholar] [CrossRef]
- Phillips, M.A.; Arnold, K.R.; Vue, Z.; Beasley, H.K.; Garza-Lopez, E.; Marshall, A.G.; Morton, D.J.; McReynolds, M.R.; Barter, T.T.; Hinton, A., Jr. Combining metabolomics and experimental evolution reveals key mechanisms underlying longevity differences in laboratory evolved drosophila melanogaster populations. Int. J. Mol. Sci. 2022, 23, 1067. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Sun, X.; Cao, Y.; Ning, S.; Zhang, H.; Chen, L.; Li, R.; Tian, Q.; Wang, L.; et al. Identifying a polymorphic switch that influences mirnas regulation of a myasthenia gravis risk pathway. PLoS ONE 2014, 9, e104827. [Google Scholar] [CrossRef]
- Matsui, S.; Sasaki, T.; Kohno, D.; Yaku, K.; Inutsuka, A.; Yokota-Hashimoto, H.; Kikuchi, O.; Suga, T.; Kobayashi, M.; Yamanaka, A.; et al. Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signaling in mice. Nat. Commun. 2018, 9, 4604. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Porte, D., Jr.; Schwartz, M.W. Signals that regulate food intake and energy homeostasis. Science 1998, 280, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Katsyuba, E.; Auwerx, J. Modulating NAD(+) metabolism, from bench to bedside. EMBO J. 2017, 36, 2670–2683. [Google Scholar] [CrossRef] [PubMed]
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 2020, 31, 564–579. [Google Scholar] [CrossRef]
- Tan, B.; Xiao, H.; Li, F.; Zeng, L.; Yin, Y. The profiles of mitochondrial respiration and glycolysis using extracellular flux analysis in porcine enterocyte IPEC-J2. Anim. Nutr. 2015, 1, 239–243. [Google Scholar] [CrossRef]
- Wu, L.T.; Tan, L.M.; You, C.Y.; Lan, T.Y.; Li, W.X.; Xu, Y.T.; Ren, Z.X.; Ding, Q.; Zhou, C.Y.; Tang, Z.R.; et al. Effects of dietary niacinamide and CP concentrations on the nitrogen excretion, growth performance, and meat quality of pigs. Animal 2023, 17, 100869. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Xiao, H.; Zha, C.; Shao, F.; Wang, L.; Tan, B. Amino acids regulate energy utilization through mammalian target of rapamycin complex 1 and adenosine monophosphate activated protein kinase pathway in porcine enterocytes. Anim. Nutr. 2020, 6, 98–106. [Google Scholar] [CrossRef]
- Wu, L.; An, R.; Lan, T.; Tang, Z.; Xu, Y.; Peng, X.; Pang, J.; Sun, W.; Shi, B.; Tang, Q.; et al. Isocaloric diets with varying protein levels affected energy metabolism in young adult Sprague-Dawley rats via modifying the gut microbes: A lipid imbalance was brought on by a diet with a particularly high protein content. J. Nutr. Biochem. 2024, 124, 109534. [Google Scholar] [CrossRef]
- Wu, L.; An, R.; Xi, Y.; Tang, Z.; Li, T.; Xu, Y.; Pang, J.; Peng, X.; Sun, W.; Sun, Z. Immunomodulatory effect of isocaloric diets with different protein contents on young adult sprague dawley rats. Foods 2023, 12, 1597. [Google Scholar] [CrossRef]
- Matsen, F.A.; Kodner, R.B.; Armbrust, E.V. Pplacer: Linear time maximum-likelihood and bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinform. 2010, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; Warnow, T.; Pop, M.; White, B. A perspective on 16s rRNA operational taxonomic unit clustering using sequence similarity. NPJ Biofilms Microbiomes 2016, 2, 16004. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome. Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer. 2018, 18, 634–645. [Google Scholar] [CrossRef]
- Belbekouche, M.; Gautier, C.; Vaillant, R. Changes in ornithine transcarbamylase activity and protein level during perinatal period in rat liver. Eff. Actinomycin D Biochem. Biophys. Res. Commun. 1985, 129, 780–788. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Hepatic glutaminase–A special role in urea synthesis? Nutrition 2002, 18, 455–457. [Google Scholar] [CrossRef]
- Liu, W.; Wei, Z.; Zhang, Y.; Liu, Y.; Bai, R.; Ma, C.; Yang, J.; Sun, D. Identification of three novel pathogenic mutations in sarcomere genes associated with familial hypertrophic cardiomyopathy based on multi-omics study. Clin. Chim. Acta 2021, 520, 43–52. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; Manucha, W.; Chuffa, L.G.A.; Zuccari, D.A.P.C. Melatonin and pathological cell interactions: Mitochondrial glucose processing in cancer cells. Int. J. Mol. Sci. 2021, 22, 12494. [Google Scholar] [CrossRef]
- Amato, P.; Besaury, L.; Joly, M.; Penaud, B.; Deguillaume, L.; Delort, A.M. Metatranscriptomic exploration of microbial functioning in clouds. Sci. Rep. 2019, 9, 4383. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, S.B.; Kim, Y.E.; Yoo, H.M.; Hong, J.; Choi, K.J.; Kim, K.Y.; Kang, D. Itraq-based quantitative proteomic comparison of 2D and 3D adipocyte cell models co-cultured with macrophages using online 2D-nanolc-ESI-MS/MS. Sci. Rep. 2019, 9, 16746. [Google Scholar] [CrossRef] [PubMed]
- Alberghina, L.; Gaglio, D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014, 5, e1561. [Google Scholar] [CrossRef] [PubMed]
- Anderton, C.R.; Mobberley, J.M.; Cole, J.K.; Nunez, J.R.; Starke, R.; Boaro, A.A.; Yesiltepe, Y.; Morton, B.R.; Cory, A.B.; Cardamone, H.C.; et al. Nitrogen source governs community carbon metabolism in a model hypersaline benthic phototrophic biofilm. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Freeman, H.J.; Kim, Y.S.; Sleisenger, M.H. Protein digestion and absorption in man. Normal mechanisms and protein-energy malnutrition. Am. J. Med. 1979, 67, 1030–1036. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The influence of nutritional factors on immunological outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef]
- Banerjee, S.K.; McGaffin, K.R.; Pastor-Soler, N.M.; Ahmad, F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc. Res. 2009, 84, 111–118. [Google Scholar] [CrossRef]
- Scopelliti, A.J.; Font, J.; Vandenberg, R.J.; Boudker, O.; Ryan, R.M. Structural characterisation reveals insights into substrate recognition by the glutamine transporter ASCT2/SLC1A5. Nat. Commun. 2018, 9, 38. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, Y.; Li, T.; Sun, W.; Tang, Z.; Wang, Y.; Zhou, K.; Li, J.; Ding, Q.; Liang, K.; et al. NAD+ and its possible role in gut microbiota: Insights on the mechanisms by which gut microbes influence host metabolism. Anim. Nutr. 2022, 10, 360–371. [Google Scholar] [CrossRef]
- Liu, X.; Blouin, J.M.; Santacruz, A.; Lan, A.; Andriamihaja, M.; Wilkanowicz, S.; Benetti, P.H.; Tomé, D.; Sanz, Y.; Blachier, F.; et al. High-protein diet modifies colonic microbiota and luminal environment but not colonocyte metabolism in the rat model: The increased luminal bulk connection. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G459–G470. [Google Scholar] [CrossRef]
- Chen, X.; Song, P.; Fan, P.; He, T.; Jacobs, D.; Levesque, C.L.; Johnston, L.J.; Ji, L.; Ma, N.; Chen, Y.; et al. Moderate dietary protein restriction optimized gut microbiota and mucosal barrier in growing pig model. Front. Cell Infect. Microbiol. 2018, 8, 246. [Google Scholar] [CrossRef]
- Li, R.; Hou, G.; Jiang, X.; Song, Z.; Fan, Z.; Hou, D.X.; He, X. Different dietary protein sources in low protein diets regulate colonic microbiota and barrier function in a piglet model. Food Funct. 2019, 10, 6417–6428. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal integrity and akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Walter, J.; Heng, N.C.; Hammes, W.P.; Loach, D.M.; Tannock, G.W.; Hertel, C. Identification of lactobacillus reuteri genes specifically induced in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 2003, 69, 2044–2051. [Google Scholar] [CrossRef]
- Mishima, E.; Ichijo, M.; Kawabe, T.; Kikuchi, K.; Akiyama, Y.; Toyohara, T.; Suzuki, T.; Suzuki, C.; Asao, A.; Ishii, N.; et al. Germ-free conditions modulate host purine metabolism, exacerbating adenine-induced kidney damage. Toxins 2020, 12, 547. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wu, Q. Sulforaphane protects intestinal epithelial cells against lipopolysaccharide-induced injury by activating the AMPK/SIRT1/PGC-1α pathway. Bioengineered 2021, 12, 4349–4360. [Google Scholar] [CrossRef]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Raimondi, F.; Portella, G.; Orozco, M.; Fanelli, F. Nucleotide binding switches the information flow in Ras GTPases. PLoS Comput. Biol. 2011, 7, e1001098. [Google Scholar] [CrossRef]
- Sinha, A.K.; Winther, K.S.; Roghanian, M.; Gerdes, K. Fatty acid starvation activates RelA by depleting lysine precursor pyruvate. Mol. Microbiol. 2019, 112, 1339–1349. [Google Scholar] [CrossRef]
- Wang, X.; Su, W.; Jiang, Y.; Jia, F.; Huang, W.; Zhang, J.; Yin, Y.; Wang, H. Regulation of nucleotide metabolism with nutrient-sensing nanodrugs for cancer therapy. Adv. Sci. 2022, 9, 2200482. [Google Scholar] [CrossRef]
- Swain, J.L.; Hines, J.J.; Sabina, R.L.; Holmes, E.W. Accelerated repletion of ATP and GTP pools in postischemic canine myocardium using a precursor of purine de novo synthesis. Circ. Res. 1982, 51, 102–105. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Ramos da Silva, S.; Lee, J.J.; Lu, C.; Eoh, H.; Jung, J.U.; Gao, S.J. A critical role of glutamine and asparagine γ-nitrogen in nucleotide biosynthesis in cancer cells hijacked by an oncogenic virus. Mbio 2017, 8, e01179-17. [Google Scholar] [CrossRef]
- Chen, Y.; Coorey, N.J.; Zhang, M.; Zeng, S.; Madigan, M.C.; Zhang, X.; Gillies, M.C.; Zhu, L.; Zhang, T. Metabolism dysregulation in retinal diseases and related therapies. Antioxidants 2022, 11, 942. [Google Scholar] [CrossRef]
- Mullen, N.J.; Singh, P.K. Nucleotide metabolism: A pan-cancer metabolic dependency. Nat. Rev. Cancer 2023, 23, 275–294. [Google Scholar] [CrossRef]
- Yamada, N.; Saito-Iwamoto, C.; Nakamura, M.; Soeda, M.; Chiba, Y.; Kano, H.; Asami, Y. Lactobacillus gasseri pa-3 uses the purines imp, inosine and hypoxanthine and reduces their absorption in rats. Microorganisms 2017, 5, 10. [Google Scholar] [CrossRef]
- Yamada, N.; Saito, C.; Kano, H.; Fukuuchi, T.; Yamaoka, N.; Kaneko, K.; Asami, Y. Lactobacillus gasseripa-3 directly incorporates purine mononucleotides and utilizes them for growth. Nucleosides Nucleotides Nucleic Acids 2022, 41, 221–230. [Google Scholar] [CrossRef]
| Gene | Primers | Sequences (5′ to 3′) | Annealing Temperature (°C) | Accession No. |
|---|---|---|---|---|
| NAMPT | F | CCGACTCGTACAAGGTTACTC | 58 | NM_001031793.2 |
| R | TGGATCTTCTCTTTGGTCACTAC | 58 | ||
| SIRT1 | F | GCTATTGGGTACCGAGATAACCTT | 60 | NM_001145750.2 |
| R | TCGAGGATCTGTGCCAATCA | 59 | ||
| LKB1 | F | TGGGGTCACGCTCTACAAC | 59 | NM_001407255.1 |
| R | CTGCCGGATCTGCTGTATGG | 60 | ||
| AMPK-1 | F | GGCAAAGTGAAGGTTGGCA | 59 | NM_001167633.1 |
| R | AGATGGTGTACTGATGACCTGG | 59 | ||
| PPARα | F | GAAGGTTGCAAGGGCTTCTT | 59 | NM_001044526.1 |
| R | TGGCTTTTTCAGACCTTGGC | 59 | ||
| PGC-1α | F | CCCACAGAGACCCGAAACAG | 60 | NM_213963.2 |
| R | ACCCTTGGGGTCATTTGGTG | 60 | ||
| ACC1 | F | GGATGAACCGTCTCCCTTGG | 58 | NM_001114269.1 |
| R | CCAGACATGCTGGACCTCAT | 57 | ||
| mTORC1 | F | GTGAAACCGGAGGCCCTAAA | 60 | XM_003127584.6 |
| R | CAGAAAGGACACCAGCCGAT | 60 | ||
| FOXO1 | F | CTGAGTGAGTGAGCAGGCTA | 59 | NM_214014.3 |
| R | GGAAAAGTGTCTTCGCTGCC | 60 | ||
| CREB | F | GGAGCTTGTACCACCGGTAA | 59 | NM_001361427.1 |
| R | CGGTGGGAGCAGATGATGTT | 60 | ||
| GLUT1 | F | CGCTTCCTGCTCATCAACC | 59 | XM_021096908.1 |
| R | GACCTTCTTCTCCCGCATC | 58 | ||
| GLUT2 | F | TCTTTGGTGGGATGCTTGGA | 59 | NM_001097417.1 |
| R | AAGCCTGAAATTAGCCCACAG | 59 | ||
| ASCT1 | F | GTGACCCACAACACGAGCAA | 61 | XM_021087450.1 |
| R | TGCAAATGGCGTGACGAG | 59 | ||
| ASCT2 | F | CAAGATTGTGGAGATGGAGGAT | 58 | XM_003355984.4 |
| R | TTGCGAGTGAAGAGGAAGTAGAT | 59 | ||
| SLC7A5 | F | TCAACCCCTACAGAAACCTGC | 60 | XM_047790216.1 |
| R | GACAGGGTGGTGAAGTAGGC | 60 | ||
| GDH1 | F | AGGGCTTTATTGGTCCTGGC | 60 | NM_001244501.2 |
| R | TCCACGACCAGTAGCAGAGA | 60 | ||
| NMNAT1 | F | GGCCAGTAGCGTGAGTTACA | 60 | XM_021095304.1 |
| R | AAAAGGAAACCTCCGACCCC | 60 | ||
| NADSYN1 | F | GGAATCTCCGGTCACTCAGG | 60 | XM_021082640.1 |
| R | ACTCCTCTGTTTGCCGACTC | 60 | ||
| NMRK1 | F | GAGGGTTAGTGAGAGGCGTG | 60 | XM_003121961.4 |
| R | ACCATTTGTCACACCACCGA | 60 | ||
| β-actin | F | TTCTAGGCGGACTTGCAGC | 60 | XM_021086047.1 |
| R | GCTTCTCAGCAGACAGGAGG | 60 |
| Items | Normal-Protein Diet | Low-Protein Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 30 mg/kg NAM | 360 mg/kg NAM | 30 mg/kg NAM | 360 mg/kg NAM | CP | NAM | CP × NAM | ||
| BUN (mmol/L) | 7.2 a | 6.3 ab | 5.9 b | 6.2 ab | 0.26 | 0.012 | 0.266 | 0.023 |
| GLU (mmol/L) | 3.6 | 5.9 | 4.5 | 6.9 | 0.69 | 0.161 | 0.003 | 0.962 |
| TG (mmol/L) | 0.52 | 0.50 | 0.54 | 0.67 | 0.06 | 0.128 | 0.406 | 0.225 |
| HDL-C (mmol/L) | 1.2 b | 1.7 a | 1.5 ab | 1.2 b | 0.10 | 0.432 | 0.276 | 0.002 |
| LDL-C (mmol/L) | 2.4 | 2.4 | 2.3 | 2.8 | 0.15 | 0.458 | 0.175 | 0.175 |
| GH (ng/mL) | 2.6 a | 2.1 ab | 1.3 b | 1.6 b | 0.23 | 0.001 | 0.525 | 0.010 |
| FINS (mIU/L) | 1.7 | 2.3 | 1.6 | 1.4 | 0.32 | 0.123 | 0.575 | 0.193 |
| GC (pg/mL) | 12 | 18 | 12 | 17 | 3.8 | 0.840 | 0.204 | 0.953 |
| GPT (U/L) | 24 | 22 | 28 | 30 | 3.6 | 0.148 | 0.998 | 0.627 |
| GOT (U/L) | 9.6 | 7.6 | 5.7 | 14 | 3.7 | 0.748 | 0.409 | 0.179 |
| PDH (ng/L) | 1.8 | 3.7 | 1.3 | 2.7 | 0.34 | 0.042 | <0.001 | 0.487 |
| LDH (U/L) | 415 | 414 | 467 | 363 | 53 | 0.990 | 0.327 | 0.340 |
| HORA-IR | 0.26 | 0.55 | 0.32 | 0.42 | 0.06 | 0.576 | 0.005 | 0.122 |
| Items | Normal-Protein Diet | Low-Protein Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 30 mg/kg NAM | 360 mg/kg NAM | 30 mg/kg NAM | 360 mg/kg NAM | CP | NAM | CP × NAM | ||
| TP (mg/g) | 94 | 110 | 94 | 105 | 5.5 | 0.957 | 0.107 | 0.512 |
| GPT (U/g) | 14 | 12 | 10 | 13 | 2.1 | 0.493 | 0.875 | 0.260 |
| GOT (U/g) | 64 | 59 | 64 | 58 | 4.9 | 0.916 | 0.262 | 0.911 |
| CPS-1 (nmol/min/mg prot) | 18 | 13 | 17 | 15 | 1.3 | 0.900 | 0.013 | 0.254 |
| OTC (nmol/min/mg prot) | 26 | 27 | 27 | 27 | 0.44 | 0.149 | 0.271 | 0.177 |
| GS (μmol/h/mg prot) | 207 | 189 | 170 | 154 | 16 | 0.034 | 0.947 | 0.300 |
| GLS (U/mg prot) | 17 | 11 | 10 | 10 | 2.2 | 0.093 | 0.236 | 0.228 |
| Items | Standard-Protein Diet | Low-Protein Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 30 mg/kg NAM | 360 mg/kg NAM | 30 mg/kg NAM | 360 mg/kg NAM | CP | NAM | CP × NAM | ||
| Liver tissue | ||||||||
| CS (U/mg prot) | 11 | 11 | 9.4 | 12 | 1.39 | 0.934 | 0.420 | 0.270 |
| GDH1 (U/mg prot) | 9.8 | 9.8 | 6.4 | 8.5 | 0.90 | 0.017 | 0.265 | 0.234 |
| CPT1 (U/mg prot) | 41 | 38 | 44 | 37 | 2.04 | 0.597 | 0.035 | 0.416 |
| PDH (U/mg prot) | 6.7 | 14 | 5.6 | 14 | 1.51 | 0.630 | <0.001 | 0.800 |
| LDH (U/g prot) | 2012 | 1936 | 2239 | 1653 | 250 | 0.912 | 0.322 | 0.204 |
| HK (nmol/min/mg prot) | 11 | 16 | 16 | 19 | 1.74 | 0.046 | 0.030 | 0.604 |
| Ileum tissue | ||||||||
| CS (U/mg prot) | 5.0 b | 11 a | 1.3 c | 2.4 bc | 0.82 | <0.001 | <0.001 | 0.008 |
| GDH1 (U/mg prot) | 10 | 14 | 5.6 | 7.5 | 1.0 | <0.001 | 0.018 | 0.486 |
| CPT1 (U/mg prot) | 42 | 44 | 53 | 53 | 3.55 | 0.010 | 0.803 | 0.846 |
| PDH (U/mg prot) | 10 | 17 | 6.1 | 13 | 1.65 | 0.018 | 0.001 | 0.971 |
| LDH (U/g prot) | 2925 | 2382 | 3641 | 2577 | 448 | 0.325 | 0.092 | 0.570 |
| HK (nmol/min/mg prot) | 17 | 16 | 19 | 28 | 6.52 | 0.311 | 0.602 | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Wei, H.; Wang, Z.; Tang, Z.; Xu, Y.; Peng, X.; Sun, Z.; Wu, L. Impacts of 360 mg/kg Niacinamide Supplementation in Low-Protein Diets on Energy and Nitrogen Metabolism and Intestinal Microbiota in Growing–Finishing Pigs. Animals 2025, 15, 2088. https://doi.org/10.3390/ani15142088
Long X, Wei H, Wang Z, Tang Z, Xu Y, Peng X, Sun Z, Wu L. Impacts of 360 mg/kg Niacinamide Supplementation in Low-Protein Diets on Energy and Nitrogen Metabolism and Intestinal Microbiota in Growing–Finishing Pigs. Animals. 2025; 15(14):2088. https://doi.org/10.3390/ani15142088
Chicago/Turabian StyleLong, Xiaoyi, Haiyang Wei, Zhenyang Wang, Zhiru Tang, Yetong Xu, Xie Peng, Zhihong Sun, and Liuting Wu. 2025. "Impacts of 360 mg/kg Niacinamide Supplementation in Low-Protein Diets on Energy and Nitrogen Metabolism and Intestinal Microbiota in Growing–Finishing Pigs" Animals 15, no. 14: 2088. https://doi.org/10.3390/ani15142088
APA StyleLong, X., Wei, H., Wang, Z., Tang, Z., Xu, Y., Peng, X., Sun, Z., & Wu, L. (2025). Impacts of 360 mg/kg Niacinamide Supplementation in Low-Protein Diets on Energy and Nitrogen Metabolism and Intestinal Microbiota in Growing–Finishing Pigs. Animals, 15(14), 2088. https://doi.org/10.3390/ani15142088






