The Effect of Yupingfeng Polysaccharides on Immune Performance and Intestinal Microbiota in Goslings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Treatments and Design
2.3. Measurement of Growth Performance
2.4. Immune Organ Indexes
2.5. Blood Biochemistry
2.6. Morphological Analysis of the Duodenum, Jejunum, and Ileum
2.7. 16S rRNA Sequencing and Analysis
2.8. Short-Chain Fatty Acid (SCFA) Quantification
2.9. Statistical Analysis
3. Results
3.1. Effect of YPF-P on Growth Performance of Goslings
3.2. Effects of YPF-P on Serum Antioxidant Indexes
3.3. Effect of YPF-P on Immunoglobulin Concentrations in Goslings
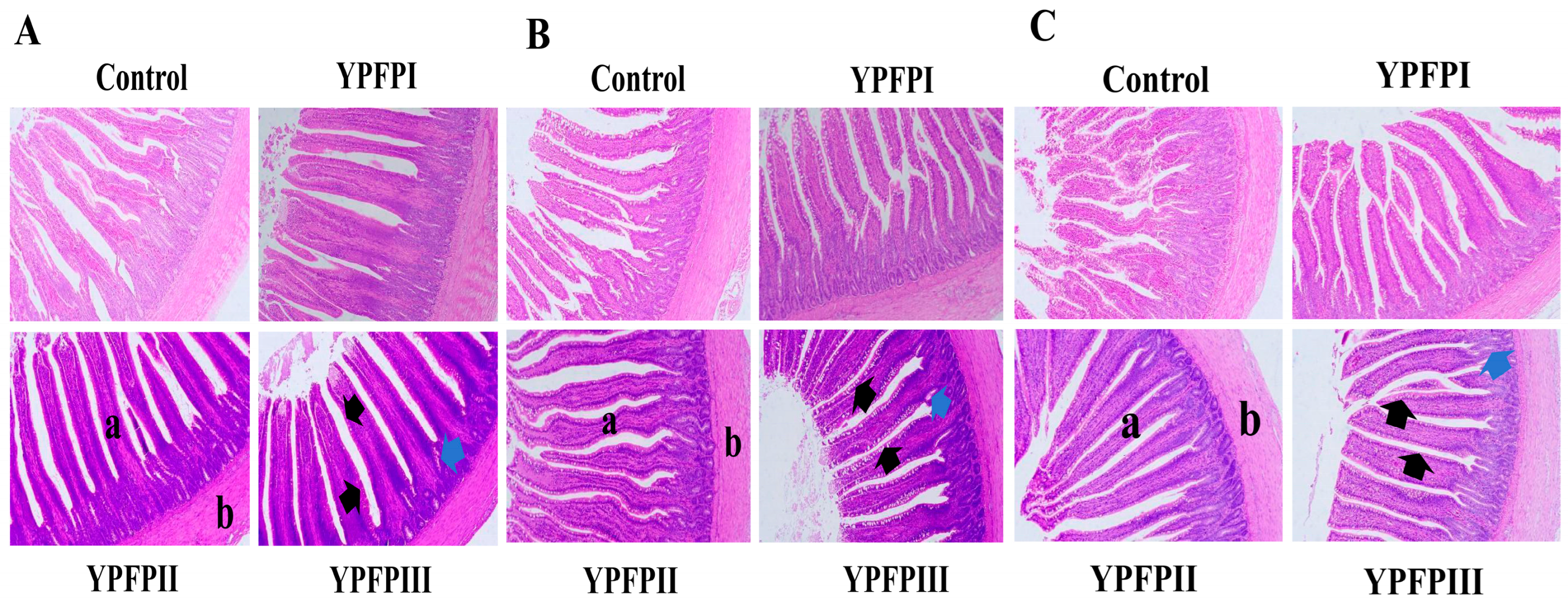
3.4. Influence of YPF-P on Duodenum, Jejunum, and Ileum Intestinal Morphology
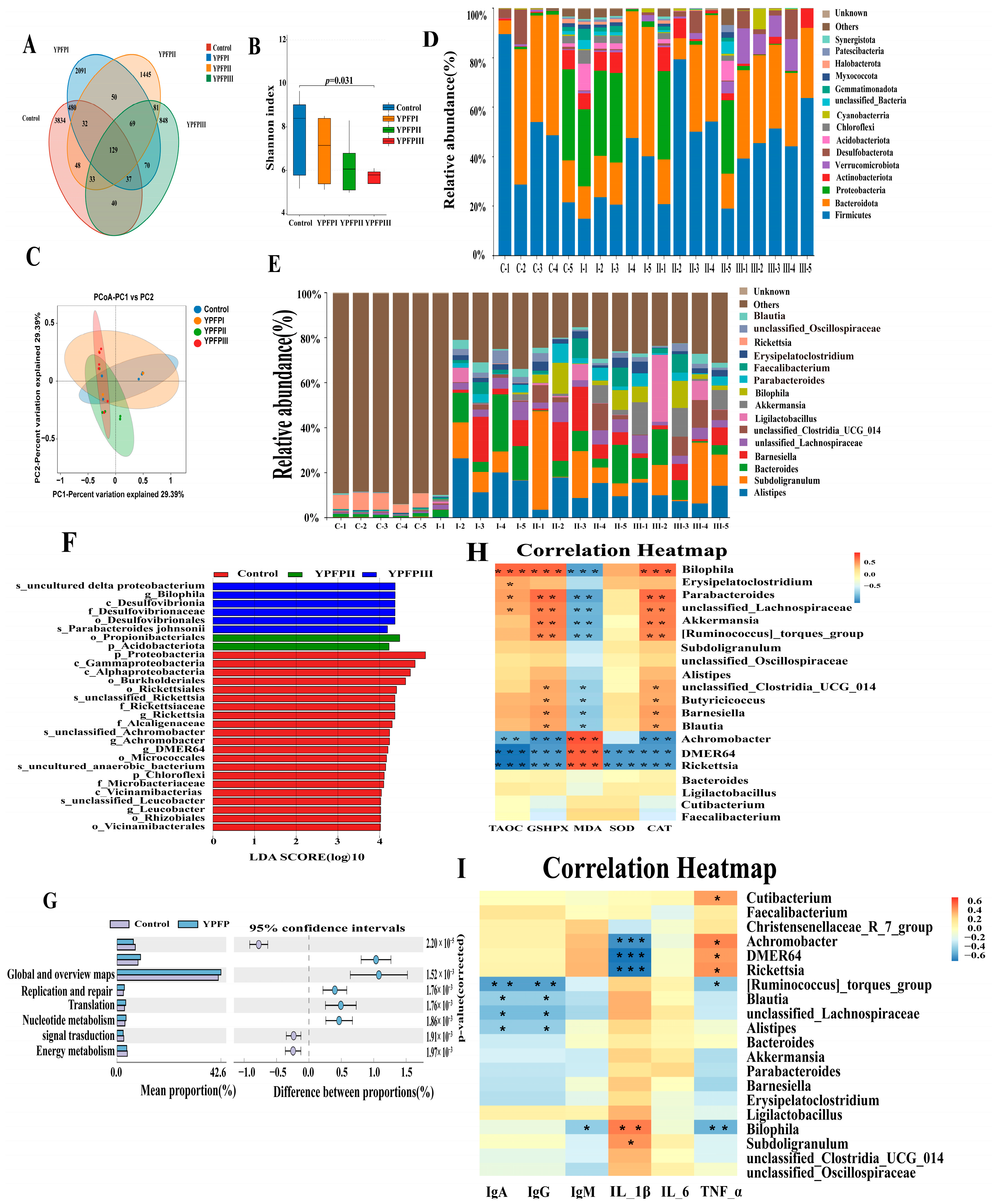
3.5. Influence of YPF-P on Cecal Microflora
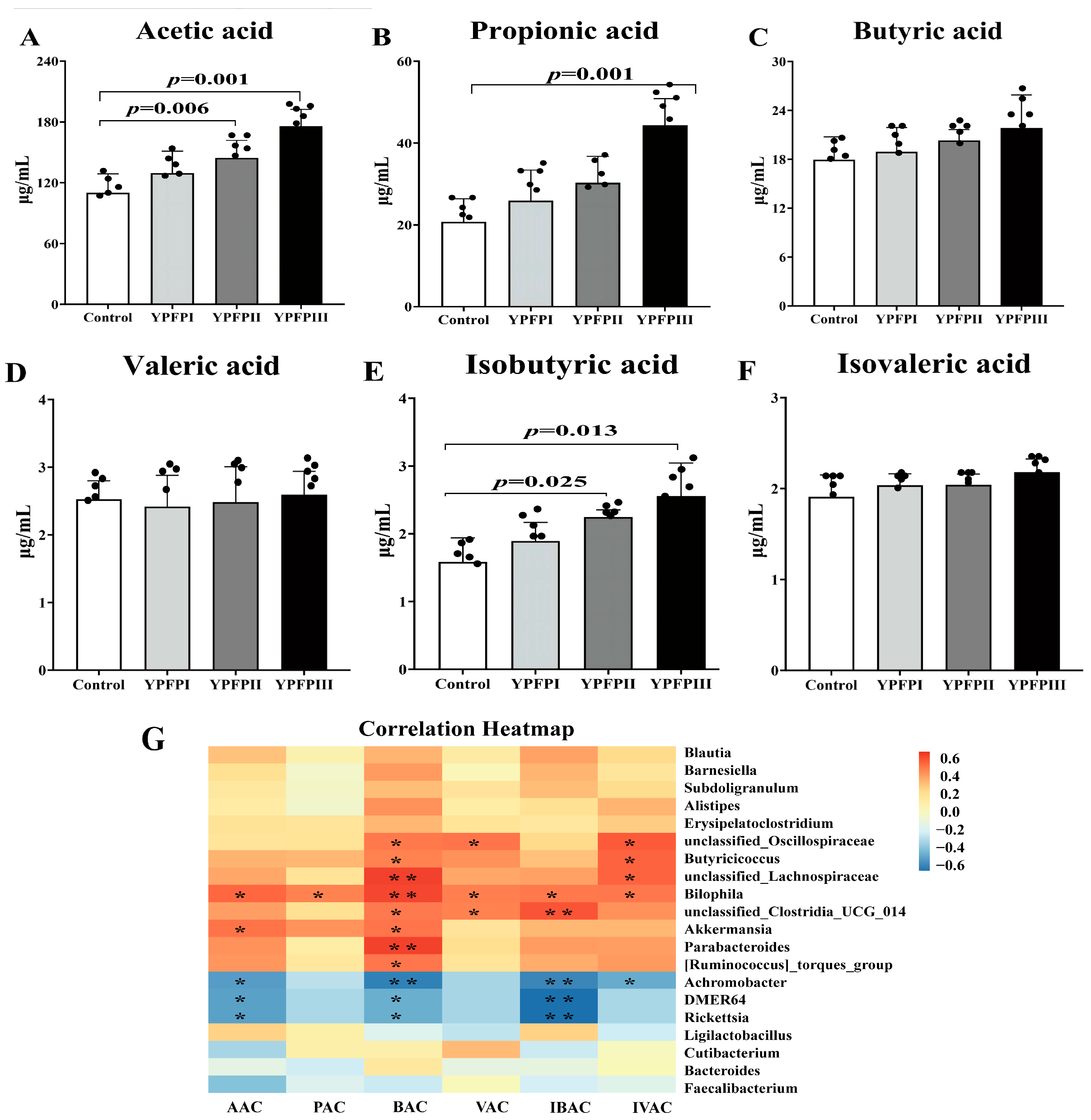
3.6. The Effect of Yupingfeng Polysaccharides on Short-Chain Fatty Acids in the Cecum of Goslings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, C.; Wang, X.; Zhang, D.; Wei, Y.; Cui, Y.; Shi, W.; Bao, Y. Synergistic use of florfenicol and Salvia miltiorrhiza polysaccharide can enhance immune responses in broilers. Ecotoxicol. Environ. Saf. 2021, 210, 111825. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Li, C.; Zhao, M.; Wang, H.; Tang, Y. Withdrawal of antibiotic growth promoters in China and its impact on the foodborne pathogen Campylobacter coli of swine origin. Front. Microbiol. 2022, 13, 1004725. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ni, X.; Zeng, D.; Zou, F.; Yang, M.; Peng, Z.; Zhou, Y.; Zeng, Y.; Zhu, H.; Wang, H.; et al. Bidirectional immunomodulating activity of fermented polysaccharides from Yupingfeng. Res. Vet. Sci. 2017, 110, 22–28. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Yi, M.; Huang, Y.; Yu, H.; Lai, M. Effect of Yupingfeng polysaccharide compound microecological preparation on growth and intestinal microflora of grass carp. Feed. Res. 2023, 46, 66–70. [Google Scholar]
- Wang, D.; Zhang, B.B.; Qu, X.X.; Gao, F.; Yuan, M.Y. Microwave-assisted extraction of polysaccharides from Yupingfeng powder and their antioxidant activity. Pharmacogn. Mag. 2015, 11, 546–554. [Google Scholar]
- Yao, S.; Yang, X.; Wu, W.; Jiang, Q.; Deng, S.; Zheng, B.; Chen, L.; Chen, Y.; Xiang, X. Effect of Paecilomyces cicadae polysaccharide Pc0-1 on cyclophosphamide-induced immunosuppression and regulation of intestinal flora in mice. Food Biosci. 2023, 51, 102340. [Google Scholar] [CrossRef]
- Makino, T.; Ito, Y.; Sasaki, S.Y.; Fujimura, Y.; Kano, Y. Preventive and curative effects of Gyokuheifu-san, a formula of traditional Chinese medicine, on allergic rhinitis induced with Japanese cedar pollens in guinea pig. Biol. Pharm. Bull. 2004, 27, 554–558. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, F.; Hu, K. A study on the action mechanism of Yupingfeng san in treating non-small cell Lungcancer based on network pharmacology. Clin. J. Chin. Med. 2023, 15, 1–8. [Google Scholar]
- Hu, X.; Cai, W.; Deng, Y.; Zhang, X.; Chen, C.; Zhang, C. Effect of Yupingfeng San Polysaccharide on Serum Immunity Index and CSFV Antibod of Piglets. Swine Prod. 2017, 2, 121–123. [Google Scholar]
- Zhang, Z.; Li, H.; Chen, G.; Yang, H.; Li, J.; Li, Y.; Dong, H. The Study of The Effects of Yupingfeng Polysaccharide Liposomes on Immune System of Chickens. Prog. Vet. Med. 2019, 40, 54–59. [Google Scholar]
- Zheng, W.; Guan, Y.; Wu, B. Effects of Yupingfeng Polysaccharides as Feed Supplement on Immune Function and Intestinal Microbiome in Chickens. Microorganisms 2023, 11, 2774. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y.; Tian, Y.; Wang, Y.; Du, J.; Ye, X.; Lu, L.; Sun, C. Dietary supplementation with Dendrobium officinale leaves improves growth, antioxidant status, immune function, and gut health in broilers. Front. Microbiol. 2023, 14, 1255894. [Google Scholar] [CrossRef]
- Li, P.; Qiu, Q.; Qin, C.; Mo, H.; Liu, X.; Shi, J.; Gu, B. Research progress of Yupingfengsan and predictive analysis on quality markers. China J. Tradit. Chin. Med. Pharm. 2024, 42, 101–107. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, H. Research progress on pharmacological effects of Astraglus polysaccharide. Chin. J. Biochem. Pharm. 2012, 33, 692–694. [Google Scholar]
- Tang, M.; Yang, M.; Wang, S.; Du, J.; Liu, W.; Hu, W.; Sun, H.; Sun, L. Progress of Atractylodis macrocephalae polysaccharide. Acta Chin. Med. Pharmacol. 2025, 53, 109–116. [Google Scholar]
- Yuan, C.; Tan, W. The effect of Saposhnikovia divaricata polysaccharides on cytokine secretion by macrophages. J. Guiyang Coll. Tradit. Chin. Med. 2011, 33, 31–33. [Google Scholar]
- Zhang, X.; Zhang, D. Overview of the Chemical Constituents and Pharmacological Activities of Atractylodes macrocephala. Inf. Tradit. Chin. Med. 2018, 35, 101–106. [Google Scholar]
- Zhang, Z.; Zhang, J.; Bai, H. Optimization of the Extraction Process of Saposhnikovia divaricata Polysaccharides. Chin. J. Bioprocess Eng. 2008, 3, 34–38. [Google Scholar]
- Tang, Y.; Zhang, J.; Li, Q.; Jiang, J.; Huang, R. Effects of Atractylodes macrocephala Polysaccharides on Growth Performance and Serum Biochemical Parameters in Weaned Piglets. Chin. J. Anim. Sci. 2009, 45, 45–48. [Google Scholar]
- Gao, Y.; Zhang, Z.; Sun, L. Effects of Saposhnikovia divaricata Polysaccharides on Immune Factors in Rats with Allergic Rhinitis. China Pharm. 2017, 20, 1188–1191. [Google Scholar]
- Jie, F. Effects of Astragalus Polysaccharides on the Growth Performance of Weaned Piglets. China Swine Ind. 2022, 17, 67–69. [Google Scholar]
- Gao, H.; Gao, Q.; Yang, J.; Jiang, S.; Wang, R. Effects of Atractylodes macrocephala Polysaccharides on Growth Performance and Serum Immune Parameters in Heat-Stressed Broilers. China Feed. 2025, 2, 29–32. [Google Scholar]
- Chu, H.; Zong, Y.; Yang, H.; Chen, S.; Ma, Z.; Li, H. Effects of Yu-Ping-Feng polysaccharides on animal growth performance and immune function: A review. Front. Vet. Sci. 2023, 10, 1260208. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Y.; Li, Y.; Chen, W.; Ma, S.; Peng, W. Immunomodulatory effects of yupingfeng complex polysaccharide on immunosuppressed mice. Chin. Agric. Sci. Bull. 2014, 30, 93–96. [Google Scholar]
- Xue, L.G.; Guo, T.K.; Wang, J.; Shan, Y.Q.; Guo, L.; Zhang, D.X.; Wei, Z.; Wang, D. Effects of in-ovo injection of Yu ping feng polysaccharides on growth performance, intestinal development, and immunity in broiler chickens. Poult. Sci. 2025, 104, 104574. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Guo, L.; Xu, L.; Zhang, J. Effects of Astragalus Polysaccharides on Growth Performance, Organ Indices, and Antioxidant Capacity of 1–14 Day-Old Broiler Chickens. Anim. Nutr. 2024, 36, 7967–7974. [Google Scholar]
- Long, L.N.; Zhang, H.H.; Wang, F.; Yin, Y.X.; Yang, L.Y.; Chen, J.S. Research Note: Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult. Sci. 2021, 100, 101028. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, N.; Xu, D.; Tian, Y.; Shen, X.; Jiang, D.; Huang, Y.; Li, W.; Li, B. Polysaccharide of Atractylodes macrocephala Koidz Alleviates Cyclophosphamide-Induced Thymus Ferroptosis in Gosling. Animals 2022, 12, 3394. [Google Scholar] [CrossRef]
- Ma, M.; Wang, K.; Qiu, L.; Dou, T.; Guo, J.; Wang, X.; Hu, Y.; Li, J.; Li, Y. Study on the growth and development of some digestive organs and immune organs in broilers with different growth rate. China Feed 2023, 11, 20–24. [Google Scholar]
- Liu, W.; Cheng, H.; Zhang, H.; Liu, G.; Yin, X.; Zhang, C.; Jiang, R.; Wang, Z.; Ding, X. Effect of Lactobacillus paracasei LK01 on Growth Performance, Antioxidant Capacity, Immunity, Intestinal Health, and Serum Biochemical Indices in Broilers. Animals 2024, 14, 3474. [Google Scholar] [CrossRef]
- Wu, S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018, 97, 3489–3493. [Google Scholar] [CrossRef]
- Chunmei, K.; Zhujun, Z.; Xiuhui, Z. Effects of Gan lian Yu ping feng powder on the antibody titers to infectious laryngotracheitis vaccine and some nonspecific immune indexes in chickens. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 70–77. [Google Scholar]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. Growth Performance and Characterization of Meat Quality of Broiler Chickens Supplemented with Betaine and Antioxidants under Cyclic Heat Stress. Antioxidants 2019, 8, 366. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Liu, Q.; Zhang, S.; Peng, M.; Zhou, J.; Chen, L.; Fang, F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019, 85, 102420. [Google Scholar] [CrossRef]
- Roche, J.F. The effect of nutritional management of the dairy cow on reproductive efficiency. Anim. Reprod. Sci. 2006, 96, 282–296. [Google Scholar] [CrossRef]
- Abecia, L.; Fondevila, M.; Balcells, J.; Edwards, J.E.; Newbold, C.J.; McEwan, N.R. Molecular profiling of bacterial species in the rabbit caecum. FEMS Microbiol. Lett. 2005, 244, 111–115. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Guo, X. Effects of Astragalus polysaccharides on anti-oxidation activity of chicks. Zhongshouyi Yiyao Zazhi 2017, 36, 50–53. [Google Scholar]
- Zhao, G.; Niu, Y.; Wang, H.; Qin, S.; Zhang, R.; Wu, Y.; Xiao, X.; Xu, Y.; Yang, C. Effects of three different plant-derived polysaccharides on growth performance, immunity, antioxidant function, and cecal microbiota of broilers. J. Sci. Food Agric. 2023, 104, 1020–1029. [Google Scholar] [CrossRef]
- Cheng, H.S.; Qin, C.Q.; Bing, C.Y.; Rong, Y.Y.; Ting, X.T.; Ke, Y.; Fang, L.; Xi, T.Z.; Bing, W.X. Morinda officinalis polysaccharides improve meat quality by reducing oxidative damage in chickens suffering from tibial dyschondroplasia. Food Chem. 2020, 344, 128688. [Google Scholar]
- Xu, Y.; Shen, Q.; Gao, L.; Wang, Y.; Zhang, C.; Yang, W.; Zhang, G. The biological functions of phytogenic polysaccharides as feed additives. Pratacult. Sci. 2016, 33, 503–511. [Google Scholar]
- Qiao, Y.; Liu, C.; Guo, Y.; Zhang, W.; Guo, W.; Oleksandr, K.; Wang, Z. Polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poult. Sci. 2022, 101, 101905. [Google Scholar] [CrossRef]
- Cai, G.; Mao, N.; Gu, P.; Zhu, T.; He, J.; Peng, S.; Yang, Y.; Liu, Z.; Hu, Y.; Wang, D. Effects of Alhagi Honey Polysaccharides as Feed Supplement on Intestine Function and Microbiome, Immune Function, and Growth Performance in Chicken. Int. J. Mol. Sci. 2022, 23, 14332. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Alqhtani, A.H.; Swelum, A.A.; Salem, H.M.; Elbestawy, A.R.; Noreldin, A.E.; Babalghith, A.O.; Khafaga, A.F.; Hassan, M.I.; et al. The relationship among avian influenza, gut microbiota and chicken immunity: An updated overview. Poult. Sci. 2022, 101, 102021. [Google Scholar] [CrossRef]
- Farahat, M.; Ibrahim, D.; Kishawy, A.T.Y.; Abdallah, H.M.; Hernandez-Santana, A.; Attia, G. Effect of cereal type and plant extract addition on the growth performance, intestinal morphology, caecal microflora, and gut barriers gene expression of broiler chickens. Animal 2021, 15, 100056. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; El-Rayes, T.K.; Farahat, M.; Attia, G.; Alagawany, M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021, 100, 101266. [Google Scholar] [CrossRef]
- Depommier, C.; Van Hul, M.; Everard, A.; Delzenne, N.M.; De Vos, W.M.; Cani, P.D. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, Z. Role of Akkermansia muciniphila in Intestinal Diseases and Prospect of Microflora Therapy. Chin. J. Gastroenterol. 2020, 25, 43–46. [Google Scholar]
- Sun, H.; Ni, X.; Song, X.; Wen, B.; Zhou, Y.; Zou, F.; Yang, M.; Peng, Z.; Zhu, H.; Zeng, Y.; et al. Fermented Yupingfeng Polysaccharides Enhance Immunity by Improving the Foregut Microflora and Intestinal Barrier in Weaning Rex Rabbits. Appl. Microbiol. Biotechnol. 2016, 100, 8105–8120. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Hou, Y.; Wang, H. Regulatory Effects of Yellow Tea Polysaccharides on Gut Microbiota in High-Fat Diet-Induced Obese Mice. J. Change Inst. Technol. 2022, 36, 32–37+65. [Google Scholar]
- Qian, Y.; Zhang, K.; Zhang, H. The gut microbiota links disease to human genome evolution. Trends Genet. 2023, 39, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zhang, Y.; Li, Q.; Jin, J.; Jiang, Y.; Wang, Q.; Zhang, J. Effect of Lycium barbarum Leaves Polysaccharides on Biological Metabolism, Antioxidant Capacity, Immune Function and Intestinal Microbiota in Mice. Sci. Technol. Food Ind. 2023, 44, 413–419. [Google Scholar]

| Material | Content (%) | Nutrient Levels (2) | Content |
|---|---|---|---|
| Corn | 56.85 | ME (MJ/kg) | 11.50 |
| Soybean meal | 22.00 | CP (g/kg) | 17.84 |
| Wheat middling and red dog | 10.00 | CF (g/kg) | 4.61 |
| Rice mill by-product | 4.00 | Ca (g/kg) | 1.00 |
| Limestone | 1.50 | AP (g/kg) | 0.40 |
| Expanded soybean | 3.00 | Lys (%) | 0.89 |
| NaCl | 0.30 | Met (%) | 0.42 |
| CaHPO4 | 1.20 | Met + Cys (%) | 0.75 |
| DL-Met | 0.15 | ||
| Premix (1) | 1.00 | ||
| Total | 100.00 |
| Items | Days of Age | Group | |||
|---|---|---|---|---|---|
| Control | YPFPI | YPFPII | YPFPIII | ||
| IW/g | 1 | 107.45 ± 4.78 | 107.20 ± 4.80 | 107.50 ± 3.84 | 107.15 ± 5.04 |
| FW/g | 7 | 231.77 ± 9.23 | 231.77 ± 9.23 | 224.93 ± 4.42 | 229.98 ± 10.36 |
| 14 | 577.85 ± 25.41 | 580.68 ± 24.02 | 577.91 ± 22.72 | 574.82 ± 18.27 | |
| 21 | 1015.10 ± 10.57 a | 1009.36 ± 12.28 a | 987.35 ± 6.67 b | 994.16 ± 13.10 ab | |
| ADFI/g | 1~7 | 26.92 ± 1.63 | 25.99 ± 1.42 | 25.63 ± 1.57 | 26.24 ± 2.14 |
| 1~14 | 57.81 ± 2.98 | 55.96 ± 2.62 | 54.85 ± 1.65 | 55.43 ± 2.11 | |
| 1~21 | 77.67 ± 4.26 | 76.53 ± 1.18 | 75.24 ± 1.69 | 76.55 ± 3.04 | |
| ADG/g | 1~7 | 17.76 ± 1.85 | 17.89 ± 1.24 | 16.61 ± 1.04 | 17.55 ± 1.76 |
| 1~14 | 33.60 ± 2.08 | 33.81 ± 1.54 | 33.60 ± 1.58 | 33.41 ± 1.50 | |
| 1~21 | 43.31 ± 0.81 | 42.86 ± 0.58 | 41.98 ± 0.43 | 42.22 ± 0.87 | |
| F/G | 1~7 | 1.52 ± 0.09 | 1.46 ± 0.09 | 1.55 ± 0.08 | 1.50 ± 0.10 |
| 1~14 | 1.72 ± 0.03 a | 1.66 ± 0.05 b | 1.63 ± 0.04 b | 1.66 ± 0.05 b | |
| 1~21 | 1.79 ± 0.07 | 1.79 ± 0.04 | 1.79 ± 0.05 | 1.81 ± 0.04 | |
| Items | Group | |||
|---|---|---|---|---|
| Control | YPFPI | YPFPII | YPFPIII | |
| T-AOC/(mM) | 0.19 ± 0.01 | 0.21 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.02 |
| GSH-PX/(μmol/L) | 345.11 ± 70.31 c | 364.26 ± 24.32 bc | 454.75 ± 21.37 b | 565.26 ± 74.16 a |
| MDA/(nmol/mL) | 2.32 ± 0.08 a | 2.10 ± 0.23 ac | 1.81 ± 0.26 bc | 1.70 ± 0.12 b |
| SOD/(U/mL) | 18.24 ± 8.57 b | 23.51 ± 2.45 ab | 34.03 ± 4.05 a | 27.23 ± 6.49 a |
| CAT/(U/mL) | 1.29 ± 0.67 | 1.43 ± 0.61 | 1.54 ± 0.36 | 1.76 ± 0.32 |
| Items | Group | |||
|---|---|---|---|---|
| Control | YPFPI | YPFPII | YPFPIII | |
| Thymus | 2.21 ± 0.46 c | 2.29 ± 0.31 bc | 2.89 ± 0.45 ab | 2.92 ± 0.27 a |
| Bursa | 0.78 ± 0.04 b | 0.76 ± 0.05 b | 0.92 ± 0.07 a | 0.99 ± 0.11 a |
| Spleen | 1.61 ± 0.32 | 1.69 ± 0.23 | 1.76 ± 0.35 | 1.94 ± 0.34 |
| Items | Group | ||||
|---|---|---|---|---|---|
| Control | YPFPI | YPFPII | YPFPIII | ||
| Villus Height (μm) | Duodenum | 665.54 ± 49.80 b | 698.02 ± 118.56 b | 889.00 ± 130.22 a | 968.88 ± 218.31 a |
| Jejunum | 865.59 ± 154.02 | 1027.83 ± 149.96 | 964.56 ± 165.57 | 941.47 ± 186.76 | |
| Ileum | 658.46 ± 142.70 c | 755.02 ± 113.68 bc | 817.32 ± 146.53 ab | 985.70 ± 194.25 a | |
| Crypt Depth (μm) | Duodenum | 293.01 ± 35.05 | 218.55 ± 42.88 | 229.28 ± 21.26 | 266.78 ± 73.01 |
| Jejunum | 197.24 ± 44.66 | 172.06 ± 41.15 | 221.48 ± 73.53 | 169.45 ± 40.02 | |
| Ileum | 203.16 ± 64.15 | 184.20 ± 56.36 | 175.06 ± 45.41 | 204.60 ± 58.00 | |
| V/C ratio | Duodenum | 2.30 ± 0.33 b | 3.40 ± 1.16 ab | 3.90 ± 0.63 a | 3.96 ± 1.55 a |
| Jejunum | 4.69 ± 1.63 | 6.32 ± 1.94 | 5.11 ± 2.72 | 5.89 ± 1.99 | |
| Ileum | 3.49 ± 1.20 c | 4.44 ± 1.36 bc | 5.15 ± 2.32 ab | 5.93 ± 1.42 a | |
| Group | Control | YPFPI | YPFPII | YPFPIII |
|---|---|---|---|---|
| Akkermansia (%) | 0.36 | 0.82 | 0.72 | 7.58 |
| Alistipes (%) | 5.64 | 8.86 | 4.42 | 11.56 |
| Rickettsia (%) | 4.34 | 3.74 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Huang, M.; Wang, T.; Gong, L.; Ma, Z.; Ye, F.; Li, H. The Effect of Yupingfeng Polysaccharides on Immune Performance and Intestinal Microbiota in Goslings. Animals 2025, 15, 2077. https://doi.org/10.3390/ani15142077
He Q, Huang M, Wang T, Gong L, Ma Z, Ye F, Li H. The Effect of Yupingfeng Polysaccharides on Immune Performance and Intestinal Microbiota in Goslings. Animals. 2025; 15(14):2077. https://doi.org/10.3390/ani15142077
Chicago/Turabian StyleHe, Qinxin, Miaoxin Huang, Tianyu Wang, Li Gong, Zheng Ma, Fei Ye, and Hua Li. 2025. "The Effect of Yupingfeng Polysaccharides on Immune Performance and Intestinal Microbiota in Goslings" Animals 15, no. 14: 2077. https://doi.org/10.3390/ani15142077
APA StyleHe, Q., Huang, M., Wang, T., Gong, L., Ma, Z., Ye, F., & Li, H. (2025). The Effect of Yupingfeng Polysaccharides on Immune Performance and Intestinal Microbiota in Goslings. Animals, 15(14), 2077. https://doi.org/10.3390/ani15142077





