Molecular Detection of Vector-Borne Pathogens and Their Association with Feline Immunodeficiency Virus and Feline Leukemia Virus in Cats from Northeastern Thailand
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Collection
2.3. Blood Genomic DNA Extraction
2.4. Polymerase Chain Reaction (PCR) and Agarose Gel Electrophoresis
2.5. Blood Smear and Microscopic Examination
2.6. Statistical Analysis
3. Results
3.1. Prevalence of FIV, FeLV, and VBPs in Domestic Cats
3.2. Pattern of Infection of FIV, FeLV, and VBPs in Domestic Cats
3.3. Correlation Analysis of FIV, FeLV, and VBPs in Domestic Cats
3.4. Prevalence of VBPs in FIV/FeLV-Positive and FIV/FeLV-Negative Groups
3.5. White Blood Cell Counts and Their Association with FIV/FeLV Infections in Domestic Cats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melo, T.B.; Silva, T.R.M.; Almeida, T.L.A.C.; Tutija, J.F.; Silva, A.O.D.; Lira, M.D.S.; Amorim, D.; Giannelli, A.; Ramos, C.A.D.N.; Alves, L.C.; et al. Molecular detection of vector-borne pathogens in cats tested for FIV and FeLV. Vet. Parasitol. Reg. Stud. Rep. 2023, 40, 100857. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Saz, S.; Martínez, M.; Nijhof, A.M.; Gerst, B.; Gentil, M.; Müller, E.; Fernández, A.; González, A.; Yusuf, M.S.M.; Greco, G.; et al. Molecular survey on vector-borne pathogens in clinically healthy stray cats in Zaragoza (Spain). Parasit. Vectors 2023, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Raimundo, J.M.; Peixoto, M.P.; da Silva, C.B.; Pires, M.S.; Santos, H.A.; Baldani, C.D. Molecular detection, characterization of Anaplasma spp. in domestic cats from Rio de Janeiro state. Acta. Trop. 2019, 191, 239–242. [Google Scholar] [CrossRef]
- Schäfer, I.; Kohn, B. Anaplasma phagocytophilum infection in cats: A literature review to raise clinical awareness. J. Feline Med. Surg. 2020, 22, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Stepanić, M.; Duvnjak, S.; Reil, I.; Hađina, S.; Kempf, V.A.J.; Špičić, S.; Mihaljević, Ž.; Beck, R. Epidemiology of Bartonella henselae infection in pet and stray cats in Croatia with risk factors analysis. Parasit. Vectors 2024, 17, 48. [Google Scholar] [CrossRef]
- Bullard, R.L.; Olsen, E.L.; Cheslock, M.A.; Embers, M.E. Evaluation of the available animal models for Bartonella infections. One Health 2023, 18, 100665. [Google Scholar] [CrossRef]
- Tasker, S.; Hofmann-Lehmann, R.; Belák, S.; Frymus, T.; Addie, D.D.; Pennisi, M.G.; Boucraut-Baralon, C.; Egberink, H.; Hartmann, K.; Hosie, M.J.; et al. Haemoplasmosis in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2018, 20, 256–261. [Google Scholar] [CrossRef]
- Hoque, M.M.; Barua, S.; Kelly, P.J.; Chenoweth, K.; Kaltenboeck, B.; Wang, C. Identification of Rickettsia felis DNA in the blood of domestic cats and dogs in the USA. Parasit. Vectors 2020, 13, 581. [Google Scholar] [CrossRef]
- Penzhorn, B.L.; Oosthuizen, M.C. Babesia species of domestic cats: Molecular characterization has opened pandora’s box. Front. Vet. Sci. 2020, 7, 134. [Google Scholar] [CrossRef]
- Grillini, M.; Simonato, G.; Tessarin, C.; Dotto, G.; Traversa, D.; Cassini, R.; Marchiori, E.; Frangipane di Regalbono, A. Cytauxzoon sp. and Hepatozoon spp. in domestic cats: A Preliminary study in North-eastern Italy. Pathogens 2021, 10, 1214. [Google Scholar] [CrossRef]
- Remesar, S.; Arnal, J.L.; Gómez, A.; Prieto, A.; García-Dios, D.; Benito, A.; Panadero, R.; Morrondo, P.; Díaz, P. A case report of fatal feline babesiosis caused by Babesia canis in northwestern Spain. BMC Vet. Res. 2022, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Wikander, Y.M.; Reif, K.E. Cytauxzoon felis: An Overview. Pathogens 2023, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Cotey, S.R.; Dangoudoubiyam, S.; Weerarathne, P.; Tussey, K.; Wilkes, R.P.; Miller, C.A.; Mehringer, L.; Burcham, G.N. Cytauxzoonosis in Indiana, USA: A case series of cats infected with Cytauxzoon felis (2018–2022). J. Feline Med. Surg. 2024, 26, 1098612X231224139. [Google Scholar] [CrossRef]
- Pereira, C.; Maia, J.P.; Marcos, R.; Luzzago, C.; Puente-Payo, P.; Dall’Ara, P. Molecular detection of Hepatozoon felis in cats from Maio Island, Republic of Cape Verde and global distribution of feline hepatozoonosis. Parasit. Vectors 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Kamyingkird, K.; Chimnoi, W.; Inpankaew, T. Evaluation of hematological alteration of vector-borne pathogens in cats from Bangkok, Thailand. BMC Vet. Res. 2021, 17, 28. [Google Scholar] [CrossRef]
- Maggi, R.G.; Halls, V.; Krämer, F.; Lappin, M.; Pennisi, M.G.; Peregrine, A.S.; Roura, X.; Schunack, B.; Scorza, V.; Tasker, S.; et al. Vector-borne and other pathogens of potential relevance disseminated by relocated cats. Parasit. Vectors 2022, 15, 415. [Google Scholar] [CrossRef]
- Rungsuriyawiboon, O.; Jarudecha, T.; Hannongbua, S.; Choowongkomon, K.; Boonkaewwan, C.; Rattanasrisomporn, J. Risk factors and clinical and laboratory findings associated with feline immunodeficiency virus and feline leukemia virus infections in Bangkok, Thailand. Vet. World 2022, 15, 1601–1609. [Google Scholar] [CrossRef]
- De Mello, L.S.; Ribeiro, P.R.; de Almeida, B.A.; Bandinelli, M.B.; Sonne, L.; Driemeier, D.; Pavarini, S.P. Diseases associated with feline leukemia virus and feline immunodeficiency virus infection: A retrospective study of 1470 necropsied cats (2010–2020). Comp. Immunol. Microbiol. Infect. Dis. 2023, 95, 101963. [Google Scholar] [CrossRef]
- Fusco, G.; Marati, L.; Pugliese, A.; Levante, M.; Ferrara, G.; de Carlo, E.; Amoroso, M.G.; Montagnaro, S. Prevalence of feline leukemia virus and feline immunodeficiency virus in cats from southern Italy: A 10-year cross-sectional study. Front. Vet. Sci. 2023, 10, 1260081. [Google Scholar] [CrossRef]
- Moyadee, W.; Chiteafea, N.; Tuanthap, S.; Choowongkomon, K.; Roytrakul, S.; Rungsuriyawiboon, O.; Boonkaewwan, C.; Tansakul, N.; Rattanasrisomporn, A.; Rattanasrisomporn, J. The first study on clinicopathological changes in cats with feline infectious peritonitis with and without retrovirus coinfection. Vet. World 2023, 16, 820–827. [Google Scholar] [CrossRef]
- Vojtek, B.; Čechvala, P.; Zemanová, S.; Korytár, Ľ.; Prokeš, M.; Drážovská, M.; Petroušková, P.; Kožiarská Tomčová, J.; Ondrejková, A. Incidence of Chlamydia spp., FIV, FeLV in free-roaming cats in Slovakia. Vet. Med. 2024, 15, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Nedumpun, T.; Piamsomboon, P.; Chanchaithong, P.; Taweethavonsawat, P.; Chungpivat, S.; Suradhat, S. Prevalence and distributions of feline immunodeficiency virus and feline leukemia virus infections in Bangkok and its vicinity, Thailand During 2013–2014. Thai J. Vet. Med. 2015, 45, 449–453. [Google Scholar] [CrossRef]
- Sprißler, F.; Jongwattanapisan, P.; Luengyosluechakul, S.; Pusoonthornthum, R.; Reese, S.; Bergmann, M.; Hartmann, K. Prevalence and risk factors of feline immunodeficiency virus and feline leukemia virus infection in healthy cats in Thailand. Front. Vet. Sci. 2022, 8, 764217. [Google Scholar] [CrossRef]
- Phyu, E.M.; Charoenkul, K.; Nasamran, C.; Chamsai, E.; Thaw, Y.N.; Phyu, H.W.; Soe, H.W.; Chaiyawong, S.; Amonsin, A. Whole genome characterization of feline coronaviruses in Thailand: Evidence of genetic recombination and mutation M1058L in pathotype switch. Front. Vet. Sci. 2025, 12, 1451967. [Google Scholar] [CrossRef]
- Maruyama, S.; Sakai, T.; Morita, Y.; Tanaka, S.; Kabeya, H.; Boonmar, S.; Poapolathep, A.; Chalarmchaikit, T.; Chang, C.C.; Kasten, R.W.; et al. Prevalence of Bartonella species and 16s rRNA gene types of Bartonella henselae from domestic cats in Thailand. Am. J. Trop. Med. Hyg. 2001, 65, 783–787. [Google Scholar] [CrossRef]
- Srisanyong, W.; Takhampunya, R.; Boonmars, T.; Kerdsin, A.; Suksawat, F. Prevalence of Bartonella henselae, Bartonella clarridgeiae, and Bartonella vinsonii subsp. berkhoffii in pet cats from four provincial communities in Thailand. Thai J. Vet. Med. 2016, 46, 663–670. [Google Scholar] [CrossRef]
- Saengsawang, P.; Kaewmongkol, G.; Inpankaew, T. Molecular detection of Bartonella spp. and hematological evaluation in domestic cats and dogs from Bangkok, Thailand. Pathogens 2021, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Saengsawang, P.; Kaewmongkol, G.; Phoosangwalthong, P.; Chimnoi, W.; Inpankaew, T. Detection of zoonotic Bartonella species in ticks and fleas parasitizing free-ranging cats and dogs residing in temples of Bangkok, Thailand. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100612. [Google Scholar] [CrossRef]
- Hirunkanokpun, S.; Ahantarig, A.; Baimai, V.; Pramual, P.; Rakthong, P.; Trinachartvanit, W. Spotted fever group Rickettsia, Anaplasma and Coxiella-like endosymbiont in Haemaphysalis ticks from mammals in Thailand. Vet. Res. Commun. 2022, 46, 1209–1219. [Google Scholar] [CrossRef]
- Ceylan, O.; Ma, Z.; Ceylan, C.; Ider, M.; Evci, A.; Mavinehir, A.; Xuan, X.; Sevinc, F. Feline vector-borne haemopathogens in Türkiye: The first molecular detection of Mycoplasma wenyonii and ongoing Babesia ovis DNA presence in unspecific hosts. BMC Vet. Res. 2024, 20, 365. [Google Scholar] [CrossRef]
- Kumsiri, R.; Kanchanaphum, P. Comparison of time course detection of human male DNA from blood stains on various objects on surface in a natural environment and in a laboratory using Loop-Mediated Isothermal Amplification (LAMP). Scientifica 2021, 2021, 4811608. [Google Scholar] [CrossRef] [PubMed]
- Thongseesuksai, T.; Boonmars, T.; Laummaunwai, P. Comparison of three methods to extract Plasmodium falciparum DNA from whole blood and dried blood spots. Am. J. Trop. Med. Hyg. 2024, 110, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Ibrahim, O.; Bara Allawe, A.; Ali Kadhim, H. Isolation and molecular detection of Feline infectious peritonitis virus. Arch. Razi. Inst. 2022, 77, 1709–1714. [Google Scholar] [PubMed]
- Arjona, A.; Barquero, N.; Doménech, A.; Tejerizo, G.; Collado, V.M.; Toural, C.; Martín, D.; Gomez-Lucia, E. Evaluation of a novel nested PCR for the routine diagnosis of feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV). J. Feline Med. Surg. 2007, 9, 14–22. [Google Scholar] [CrossRef]
- Lima, M.L.F.; Soares, P.T.; Ramos, C.A.N.; Araújo, F.R.; Ramos, R.A.N.; Souza, I.I.F.; Faustino, M.A.G.; Alves, L.C.A. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz. J. Microbiol. 2010, 41, 381–385. [Google Scholar] [CrossRef]
- Paulauskas, A.; Radzijevskaja, J.; Rosef, O. Molecular detection and characterization of Anaplasma phagocytophilum strains. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 187–195. [Google Scholar] [CrossRef]
- Leulmi, H.; Aouadi, A.; Bitam, I.; Bessas, A.; Benakhla, A.; Raoult, D.; Parola, P. Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasit. Vectors 2016, 9, 27. [Google Scholar] [CrossRef]
- Prusinski, M.A.; Kokas, J.E.; Hukey, K.T.; Kogut, S.J.; Lee, J.; Backenson, P.B. Prevalence of Borrelia burgdorferi (spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (rickettsiales: Anaplasmataceae), and Babesia microti (piroplasmida: Babesiidae) in Ixodes scapularis (acari: Ixodidae) collected from recreational lands in the hudson valley region, New York State. J. Med. Entomol. 2014, 51, 226–236. [Google Scholar]
- Maggi, R.G.; Compton, S.M.; Trull, C.L.; Mascarelli, P.E.; Robert Mozayeni, B.; Breitschwerdt, E.B. Infection with hemotropic Mycoplasma species in patients with or without extensive arthropod or animal contact. J. Clin. Microbiol. 2013, 51, 3237–3241. [Google Scholar] [CrossRef]
- Duarte, S.C.; Linhares, G.F.C.; Romanowsky, T.N.; Neto, O.J.S.; Borges, L.M.F. Assessment of primers designed for the subspecies-specific discrimination among Babesia canis canis, Babesia canis vogeli and Babesia canis rossi by PCR assay. Vet. Parasitol. 2008, 152, 16–20. [Google Scholar] [CrossRef]
- Persing, D.H.; Mathiesen, D.; Marshall, W.F.; Telford, S.R.; Spielman, A.; Thomford, J.W. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 2097–2103. [Google Scholar] [CrossRef]
- Brown, H.M.; Latimer, K.S.; Erikson, L.E.; Cashwell, M.E.; Britt, J.O.; Peterson, D.S. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J. Vet. Diagn. Investig. 2008, 20, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Cabello, J.; Altet, L.; Napolitano, C.; Sastre, N.; Hidalgo, E.; Dávila, J.A.; Millán, J. Survey of infectious agents in the endangered Darwin’s fox (Lycalopex fulvipes): High prevalence and diversity of hemotrophic mycoplasmas. Vet. Microbiol. 2013, 167, 448–454. [Google Scholar] [CrossRef]
- Harvey, J.W. The feline blood film. J. Feline Med. Surg. 2017, 19, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Karalyan, Z.; Zakaryan, H.; Arzumanyan, H.; Sargsyan, K.; Voskanyan, H.; Hakobyan, L.; Abroyan, L.; Avetisyan, A.; Karalova, E. Pathology of porcine peripheral white blood cells during infection with African swine fever virus. BMC Vet. Res. 2012, 8, 18. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Little, S.; Levy, J.; Hartmann, K.; Hofmann-Lehmann, R.; Hosie, M.; Olah, G.; Denis, K.S. 2020 AAFP Feline retrovirus testing and management guidelines. J. Feline Med. Surg. 2020, 22, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.E.; Coggins, S.J.; van Dorsselaer, M.; Norris, J.M.; Squires, R.A.; Thompson, M.; Malik, R. Feline immunodeficiency virus (FIV) infection in domestic pet cats in Australia and New Zealand: Guidelines for diagnosis, prevention and management. Aust. Vet. J. 2022, 100, 345–359. [Google Scholar] [CrossRef]
- Nehring, M.; Dickmann, E.M.; Billington, K.; Vande Woude, S. Study of feline immunodeficiency virus prevalence and expert opinions on standards of care. J. Feline Med. Surg. 2024, 26, 1098612X241245046. [Google Scholar] [CrossRef]
- De Miranda, L.H.M.; Meli, M.; Conceição-Silva, F.; Novacco, M.; Menezes, R.C.; Pereira, S.A.; Sugiarto, S.; Reis, É.G.; Gremião, I.D.F.; Hofmann-Lehmann, R. Co-infection with feline retrovirus is related to changes in immunological parameters of cats with sporotrichosis. PLoS ONE 2018, 13, e0207644. [Google Scholar] [CrossRef]
- Murphy, B.G.; Castillo, D.; Cook, S.; Eckstrand, C.; Evans, S.; Sparger, E.; Grant, C.K. The late asymptomatic and terminal immunodeficiency phases in experimentally FIV-infected cats—A Long-term Study. Viruses 2023, 15, 1775. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Breitschwerdt, E.B.; Solano-Gallego, L. Bartonella infections in cats and dogs including zoonotic aspects. Parasit. Vectors 2018, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Torrejón, E.; Sanches, G.S.; Moerbeck, L.; Santos, L.; André, M.R.; Domingos, A.; Antunes, S. Molecular survey of Bartonella species in stray cats and dogs, humans, and questing ticks from Portugal. Pathogens 2022, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Shaker Chouhan, C.; Habib, T.; Pratik Siddique, M.; Nazir, K.H.M.N.H.; Anisur Rahman, A.K.M.; Amimul Ehsan, M. Epidemiology of feline bartonellosis and molecular characteristics of Bartonella henselae in Bangladesh. Saudi J. Biol. Sci. 2024, 31, 103881. [Google Scholar] [CrossRef]
- Mojahed, N.; Mohammadkhani, M.A.; Mohamadkhani, A. Climate crises and developing vector-borne diseases: A narrative review. Iran J. Public Health 2022, 51, 2664–2673. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R.; Tasker, S.; Roura, X. Role of vector-borne pathogens in the development of fever in cats: 1. Flea-associated diseases. J. Feline Med. Surg. 2020, 22, 31–39. [Google Scholar] [CrossRef]
- Muz, M.N.; Erat, S.; Mumcuoglu, K.Y. Protozoan and microbial pathogens of house cats in the province of tekirdag in western turkey. Pathogens 2021, 10, 1114. [Google Scholar] [CrossRef]
- Chan, P.K.; Hawley, J.R.; Lappin, M.R. Evaluation of the role of Babesia species and Cytauxzoon felis in feline anemia cases in Colorado, USA. JFMS Open Rep. 2021, 7, 0551169211024967. [Google Scholar] [CrossRef]
- Antognoni, M.T.; Rocconi, F.; Ravagnan, S.; Vascellari, M.; Capelli, G.; Miglio, A.; Di Tommaso, M. Cytauxzoon sp. Infection and coinfections in three domestic cats in central Italy. Vet. Sci. 2022, 9, 50. [Google Scholar] [CrossRef]
- Carvalho, S.F.; Pádua, G.T.; Paula, W.V.F.; Tavares, M.A.; Neves, L.C.; Pereira, B.G.; Santos, R.A.; Dos Santos, G.C.; Cardoso, E.R.N.; Qualhato, A.F.; et al. Feline vector-borne diseases and their possible association with hematological abnormalities in cats from midwestern Brazil. Microorganisms 2024, 12, 2171. [Google Scholar] [CrossRef]
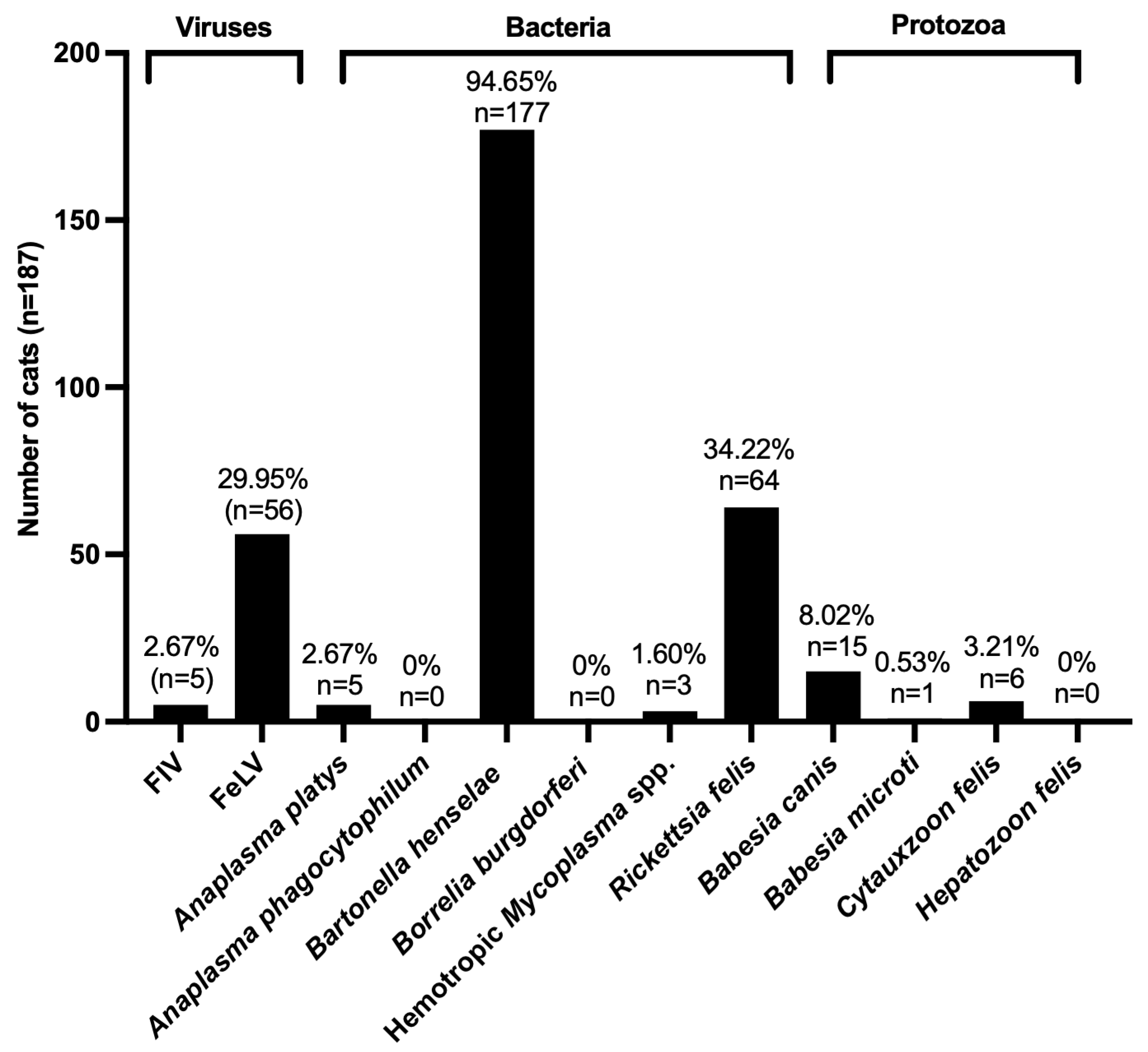
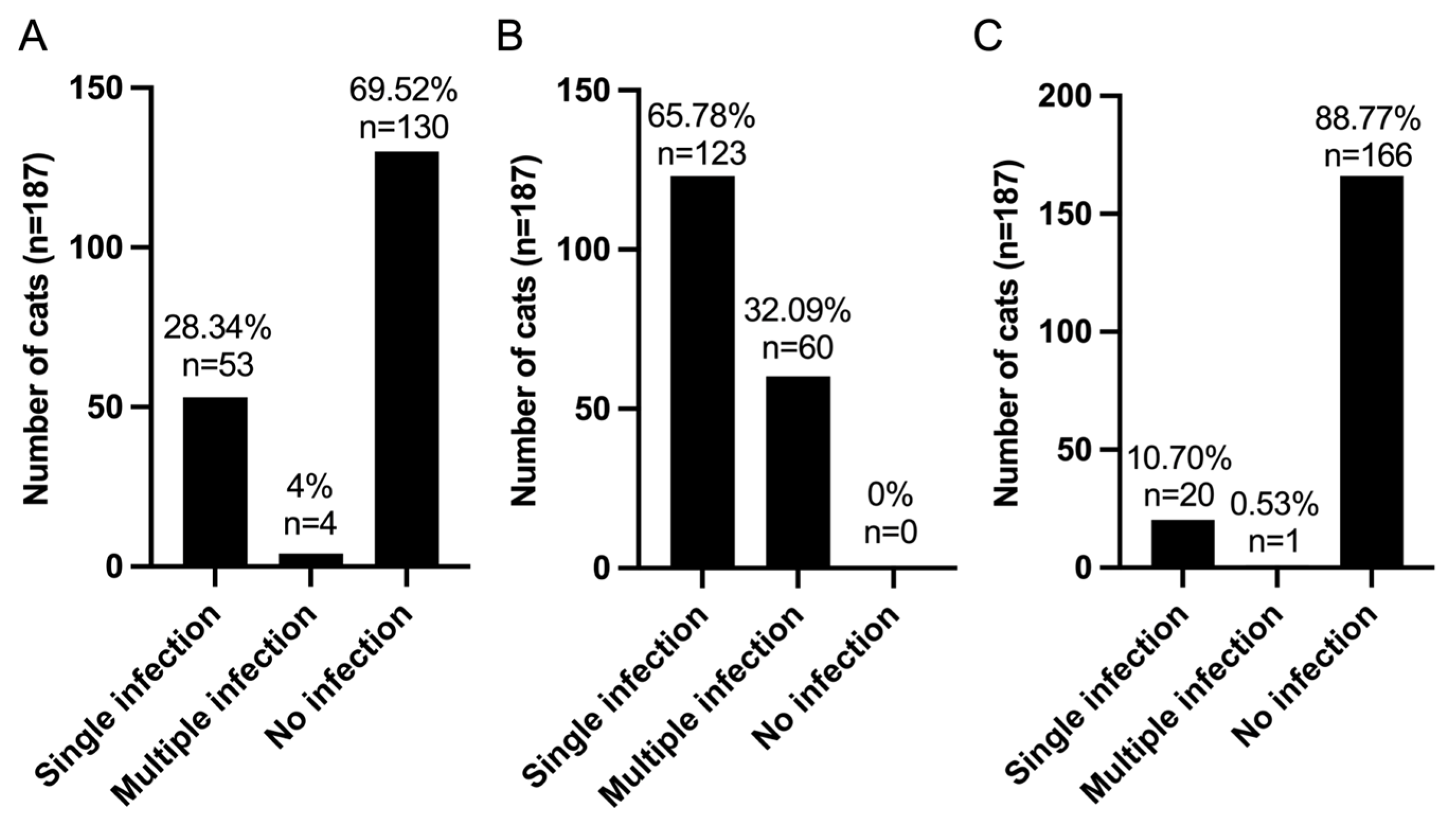
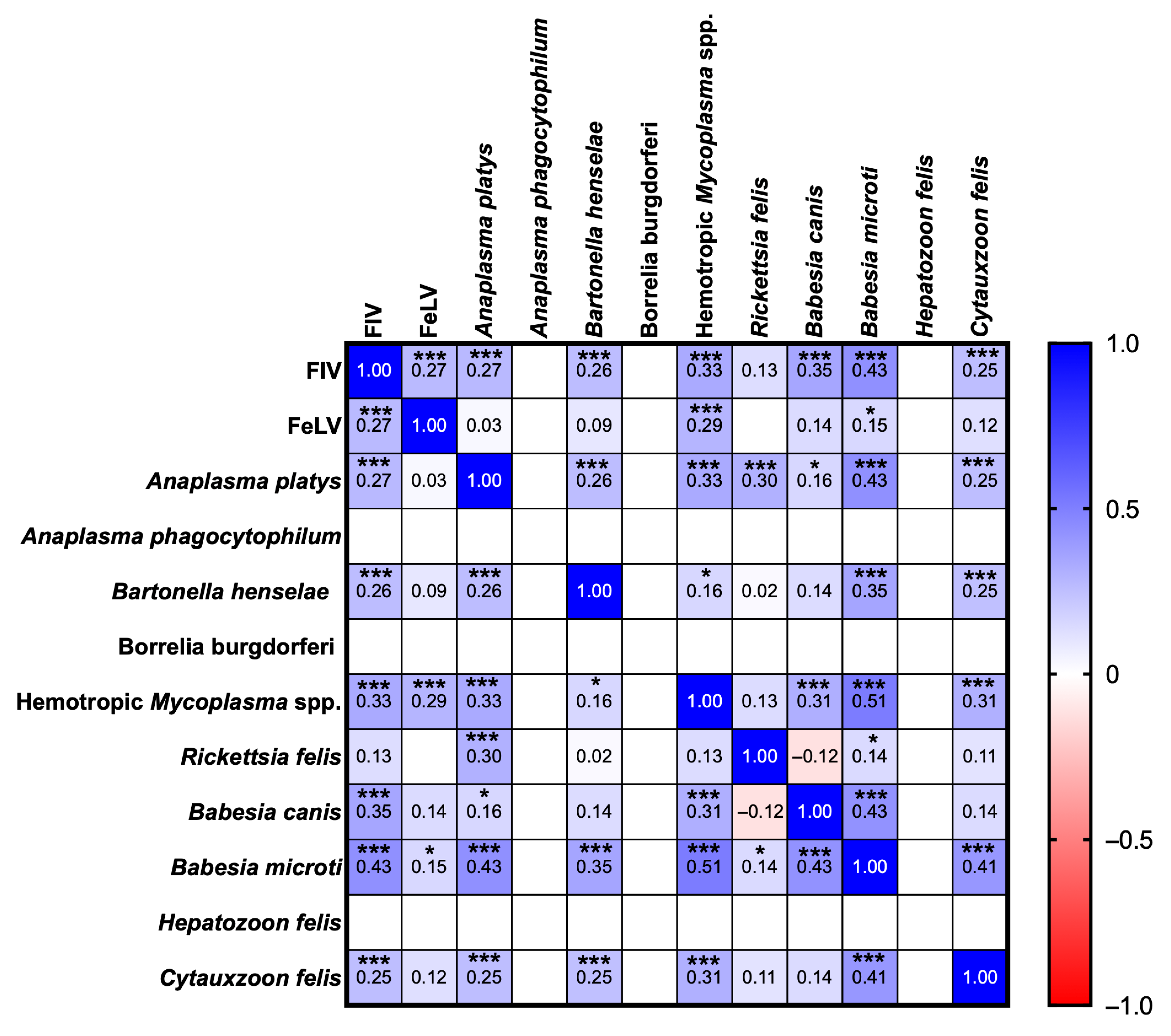
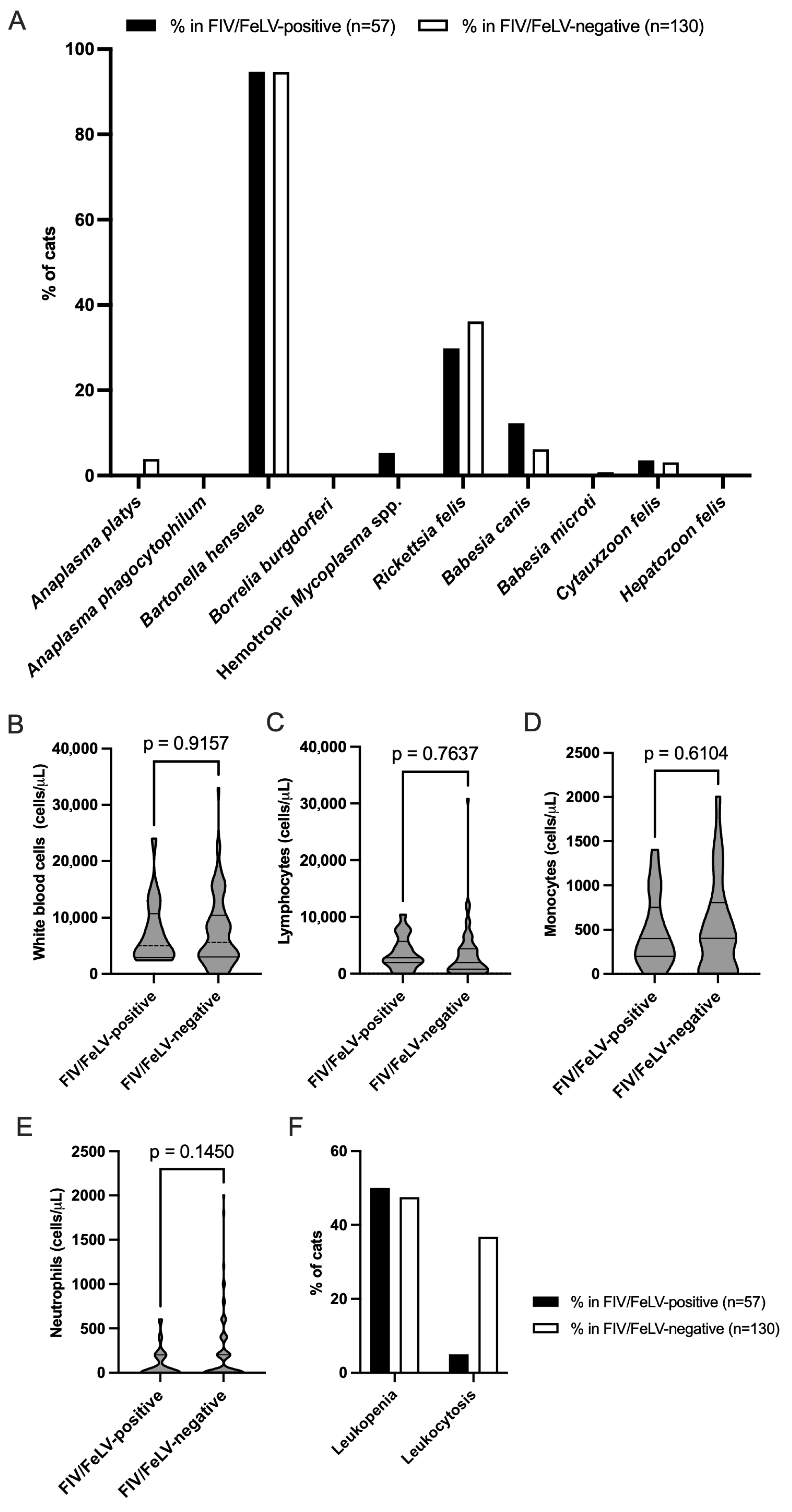
| Pathogen Category | Organisms and Primers | Annealing Temperature (°C) | PCR Product Size (Base Pair) | References |
|---|---|---|---|---|
| Viruses | FIV FW: CAATGGCCATTAAATGAA RV: AGAGAGGCCTGGAATCAAAT FeLV FW: GAAAGTACACAAAAACAGGAG RV: CTTAAGTCCTGCACTGG | 54 | 1137 | [33] |
| 49 | 305 | [33] | ||
| Bacteria | Anaplasma platys FW: GATTTTTGTCGTAGCTTGCTATG RV: TAGCACTCATCGTTTACAGC Anaplasma phagocytophilum FW: ATGAATTACAGAGAATTGCTTGTAGG RV: TTAATTGAAAGCAAATCTTGCTCCTATG Bartonella henselae FW: TTCCGYCTTATGGGTTTTGG RV: CATTTCTGTTGGAAATCCTAG Borrelia burgdorferi FW: AATAGGTCTAATATTAGCCTTAATAGC RV: TCAAGTCTGGTTCCGTCTGCTC Hemotropic Mycoplasma spp. FW: GCCCATATTCCTACGGGAAGCAGCAGT RV: CTCCACCACTTGTTCAGGTCCCCGTC | 55 | 678 | [34] |
54 | 846 | [35] | ||
52 | 246 | [36] | ||
60 | 417 | [37] | ||
| 68 | 620 | [38] | ||
| Rickettsia felis FW: CCGATTCAGCAGGTTCTTCAA RV: ATGTTCGGGCTTCCGGTATG | 57 | 120 | [36] | |
| Protozoa | Babesia canis FW: GTGAACCTTATCACTTAAAGG RV: CTACACAGAGCACACAGCC | 56 | 746 | [39] |
| Babesia microti FW: ATAGGTCAGAAACTTGAATGATACA RV: CTTAGTATAAGCTTTTATACAGC Cytauxzoon felis FW: CCAGCTCCAATAGCGTATATT RV: AGGATGAACTCGATGAATGCA Hepatozoon felis FW: CTTACCGTGGCAGTGACGGT RV: TGTTATTTCTTGTCACTACCTCTCTTATGC | 55 | 238 | [40] | |
61 | 431 | [41] | ||
| 58 | 146 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So-In, C.; Watayotha, L.; Sonsupee, T.; Khankhum, S.; Sunthamala, N. Molecular Detection of Vector-Borne Pathogens and Their Association with Feline Immunodeficiency Virus and Feline Leukemia Virus in Cats from Northeastern Thailand. Animals 2025, 15, 2065. https://doi.org/10.3390/ani15142065
So-In C, Watayotha L, Sonsupee T, Khankhum S, Sunthamala N. Molecular Detection of Vector-Borne Pathogens and Their Association with Feline Immunodeficiency Virus and Feline Leukemia Virus in Cats from Northeastern Thailand. Animals. 2025; 15(14):2065. https://doi.org/10.3390/ani15142065
Chicago/Turabian StyleSo-In, Charinya, Laksanachan Watayotha, Thikhamporn Sonsupee, Surasak Khankhum, and Nuchsupha Sunthamala. 2025. "Molecular Detection of Vector-Borne Pathogens and Their Association with Feline Immunodeficiency Virus and Feline Leukemia Virus in Cats from Northeastern Thailand" Animals 15, no. 14: 2065. https://doi.org/10.3390/ani15142065
APA StyleSo-In, C., Watayotha, L., Sonsupee, T., Khankhum, S., & Sunthamala, N. (2025). Molecular Detection of Vector-Borne Pathogens and Their Association with Feline Immunodeficiency Virus and Feline Leukemia Virus in Cats from Northeastern Thailand. Animals, 15(14), 2065. https://doi.org/10.3390/ani15142065







