Simple Summary
Heat stress is a common environmental factor that adversely affects the reproductive health of sheep. In this study, Hu sheep were used as a model to investigate the effects of heat stress on ovarian function. The results showed that heat stress impaired follicular development in the ovaries, reduced antioxidant capacity, and promoted the apoptosis of ovarian granulosa cells. Further circular RNA sequencing revealed that several key circRNAs were involved in stress responses and cell death pathways. This study contributes to the understanding of the molecular mechanisms by which heat stress damages ovarian function and provides a theoretical basis for improving heat tolerance in sheep breeding.
Abstract
Climate change poses an increasing threat to livestock reproduction, with heat stress (HS) known to significantly impair ovarian function. This study aimed to elucidate the impact of HS on ovarian function and circRNA expression profiles in Hu sheep. Twelve ewes were randomly assigned to a control (Con, n = 6) or HS group (n = 6) and exposed to different temperatures for 68 days. Compared with the Con group, HS significantly increased the respiratory rate (108.33 ± 3.72 vs. 63.58 ± 2.42 breaths/min), pulse rate (121.17 ± 3.98 vs. 78.08 ± 3.31 beats/min), and rectal temperature (40.17 ± 0.14 °C vs. 39.02 ± 0.21 °C; p < 0.05). Concurrently, serum antioxidant levels were markedly decreased, including total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-Px) (p < 0.05). Histological analysis revealed a significant reduction in the numbers of primordial, primary, secondary, and mature follicles, alongside an increase in antral follicles (p < 0.05). TUNEL staining demonstrated enhanced granulosa cell apoptosis (p < 0.05), accompanied by the upregulation of pro-apoptotic genes Bax and Caspase-3 and downregulation of the anti-apoptotic gene Bcl-2, as confirmed by qPCR (p < 0.05). CircRNA sequencing identified 152 differentially expressed circRNAs (120 upregulated, 32 downregulated), and enrichment analyses indicated their involvement in apoptosis, mitophagy, and the FoxO signaling pathway. Collectively, these findings demonstrate that HS impairs ovarian physiology and antioxidant defense, induces follicular damage and cell apoptosis, and alters circRNA expression profiles, providing new insights into the molecular mechanisms underlying HS-induced reproductive dysfunction in Hu sheep.
1. Introduction
With global warming, heat stress (HS) poses an increasing threat to the sheep industry worldwide [1]. Studies have shown that HS disrupts sheep physiology, alters heat shock protein gene expression, and impairs antioxidant capacity, growth, and reproduction [2,3,4,5]. In summer, sheep show increased respiratory rate, heart rate, rectal temperature, and oxidative stress levels compared to winter [6], along with significantly reduced lambing rates [7]. Heat stress reduces healthy ovarian follicles, leading to decreased fertility [8,9]. Sheep are one of the most important livestock species globally, with a population exceeding 1.2 billion worldwide [10]. More than 40% of the global sheep population is raised in Asia, and China is among the leading sheep-producing countries [11]. Hu sheep (Ovis aries), widely raised in southern China, are known for their strong reproductive performance and adaptability [12]. However, frequent high temperatures during the summer in southern regions severely threaten their fertility. Therefore, understanding the effects of heat stress on Hu sheep and their underlying mechanisms is crucial for improving their heat tolerance. Despite the significant economic and social value of Hu sheep in China, molecular studies on how heat stress affects their ovarian function remain limited.
With technological advancements, omics approaches have become key tools for exploring biological phenotypes, gene expression, and regulatory mechanisms [13]. Various omics approaches, including transcriptomics [14], metabolomics [15], and microbiomics [16,17], have been widely applied to investigate heat stress in sheep, revealing associated physiological changes and underlying molecular mechanisms. Transcriptome sequencing enables comprehensive analysis of gene expression and regulation under specific conditions [18]. Circular RNAs (circRNAs), a class of stable, non-coding RNAs with tissue-specific expression patterns, typically ranging from 200 to 1000 nt [19,20], have attracted considerable attention due to their diverse regulatory roles in biological processes [21,22,23]. Studies suggest that circRNAs play critical roles in growth [24], meat quality [25], and reproduction [26] in livestock. Thus, identifying HS-related circRNAs and pathways in Hu sheep ovaries via circRNA-seq offers novel insights into the molecular basis of HS responses.
In recent years, circRNA-seq has been widely applied in livestock research to identify HS-related circRNAs and signaling pathways in pigs [27] and cattle [28]. For example, circRNA-seq of mammary tissues from dairy cows under summer HS and winter non-stress conditions revealed that differentially expressed circRNAs (DE circRNAs) were mainly enriched in lipid metabolism-related pathways [29]. In pig pituitary tissues, 59 DE circRNAs were identified between heat-stressed and non-stressed groups, some of which may regulate pituitary-specific gene expression [30]. Moreover, circRNAs associated with thermotolerance were identified in the peripheral blood of heat-tolerant and heat-sensitive cows, including circRNA3685, which interacts with bta-miR-138 to target HIF1A and potentially modulate lactation function under HS [31]. However, studies on circRNAs related to HS in sheep remain limited. Based on previous studies in other species showing that heat stress significantly impairs reproductive performance in livestock and poultry, with circRNAs playing key regulatory roles in this process, we hypothesize that circRNAs may serve important regulatory functions in the ovarian response to heat stress in Hu sheep.
Therefore, this study aims to establish a heat stress model in Hu sheep to compare changes in physiology, antioxidant capacity, ovarian tissue damage, and gene expression between heat-stressed and control groups, with the goal of gaining a deeper understanding of the impact of heat stress on reproductive function in Hu sheep. Additionally, we aim to identify candidate circRNAs affected by heat stress and analyze their functional roles in ovarian tissue, in order to elucidate the molecular mechanisms underlying heat stress-induced ovarian damage in Hu sheep and provide new insights and molecular targets for improving heat tolerance in Hu sheep.
2. Materials and Methods
2.1. Ethics Statement
All experimental procedures involving animals were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) and were approved by the Animal Ethics Committee of Guangxi University (approval No. GXU-2023-0135).
2.2. Experimental Site and Environmental Conditions
This study was conducted from July to September 2023 at the Hu sheep breeding base of Guangxi Anxin Animal Husbandry Co., Ltd., located in Dahua County, Hechi City, Guangxi Zhuang Autonomous Region, China (24.04° N, 107.33° E; elevation ~340 m). The region has a subtropical monsoon climate, with an average summer daylight duration of 13–14 h, an average temperature of approximately 28–32 °C, a maximum temperature of 38–40 °C, and relative humidity ranging from 75% to 85%.
2.3. Animals and Experimental Diets
The experimental animals used in this study were Hu sheep, a non-seasonal estrus breed of sheep that can exhibit estrus and be bred throughout the year, with regular estrous cycles and clearly observable estrus behavior. Twelve clinically healthy, 2- to 3-year-old multiparous Hu ewes with similar body weights (41.69 ± 1.17 kg) that had recently completed estrus were selected from the Hu sheep breeding base of Guangxi Anxin Animal Husbandry Co., Ltd. and randomly assigned to the control group (Con, n = 6) or the heat stress group (HS, n = 6). The animals were housed in two separate artificial climate-controlled rooms. The control group was maintained at a constant temperature of 23 °C throughout the day, while the HS group was exposed to 38 °C from 08:00 to 18:00 and 28 °C from 18:00 to 08:00 the next day. This time-spanning experimental protocol is illustrated in Figure 1. Both groups were fed the same diet and had ad libitum access to water. The composition and nutritional values of the feed are provided in Table 1. The trial lasted for 68 days. Ambient temperature and humidity were recorded every 30 min using an electronic temperature and humidity data logger RC-4 (Jingchuang Electronics, Xuzhou, Jiangsu, China). The temperature–humidity index (THI) was calculated using the following formula: THI = (1.8 × Tdb + 32) − (0.55 − 0.0055 × RH) × (1.8 × Tdb − 26.8) [32], where Tdb is the dry-bulb temperature (°C) and RH is the relative humidity (%). Respiratory rate (RR), pulse rate (PR), and rectal temperature (RT) were measured every 7 days at 14:30. Estrus detection was conducted twice daily using teaser rams, with each observation lasting more than 30 min.
Figure 1.
Schematic diagram of the experimental protocol.

Table 1.
Dietary formula composition and nutritional level.
2.4. Sample Collection
At the end of the experiment, blood samples were collected from the jugular vein of the Hu ewes (n = 12) 12 h after the onset of estrus. Serum was separated by centrifugation at 3000× g for 15 min at 4 °C and stored at −80 °C for further analysis. In addition, according to the Chinese Agricultural Standard NY/T 3469-2019, three 2- to 3-year-old multiparous Hu ewes from each group were randomly selected and slaughtered 12 h after estrus. Ovarian tissues were harvested, halved, and processed: one portion was fixed in 4% paraformaldehyde for histological analysis (n = 6); the other was snap-frozen in liquid nitrogen for circRNA analysis (n = 6).
2.5. Serum Biochemical Level Analysis
The concentrations of several important antioxidant capacity indices were detected in the serum samples of sheep (n = 12). The antioxidant capacity indices included total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA). According to the manufacturer’s protocol, detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to detect the antioxidant capacity in the serum.
2.6. Histological Assay
Ovarian tissues (n = 6) fixed in 4% paraformaldehyde were processed by Servicebio (Wuhan, China). Samples were dehydrated using a graded ethanol series (75–100%), cleared in xylene (50–100%), embedded in paraffin, and sectioned at 5 μm thickness. Sections were deparaffinized and stained with hematoxylin and eosin (H&E). Follicle development was evaluated under an Olympus DP71 microscope. Images were analyzed using CaseViewer 2.4.0 (3DHISTECH, Budapest, Hungary).
2.7. TUNEL Assay
Ovarian sections (5 μm) were incubated with citrate buffer for antigen retrieval (8 min), followed by TUNEL reaction mix at 37 °C in the dark for 1 h. DAPI (Servicebio, Wuhan, China) was used to counterstain nuclei for 10 min. Slides were observed under a Zeiss fluorescence microscope, with at least 9 fields captured per replicate. TUNEL-positive cells were quantified, and apoptosis rates were calculated using ImageJ 1.53t.
2.8. RNA Extraction, Small RNA Library Construction, and Sequencing
The ovarian tissues of 3 randomly selected ewes from each group were sent to Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China) for circRNA-seq. Total RNA was treated with the Epicenter Ribo-Zero rRNA Removal Kit to remove rRNA, and then digested with RNase R. RNA was fragmented (~300 bp) and reverse-transcribed using random hexamers to generate cDNA. After second-strand synthesis, 400–500 bp fragments were selected using AMPure XP beads, PCR-amplified, and purified again. Library quality was assessed with an Agilent 2100 Bioanalyzer System (G2939BA, Agilent Technologies, Santa Clara, CA, USA). Libraries were sequenced using paired-end (PE) mode on an Illumina HiSeq platform (Personalbio, Shanghai, China).
2.9. Statistical Analyses
Raw data in FASTQ format were quality-checked using FastQC v0.11.9. Clean reads were evaluated for Q20, Q30, and GC content. Reference genome and annotation files were downloaded from NCBI (GCF_016772045.2_ARS-UI_Ramb_v3.0). CircRNA detection was performed using Find_circ and CIRI2. Circos plots were generated using Circos 0.69-6. Expression levels were normalized to TPM (transcripts per million), and differential expression analysis was conducted using DESeq v1.10.1. Significantly differentially expressed circRNAs were defined as those with adjusted p-values (p adj) < 0.05. Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed on the differentially expressed host genes of circRNAs using clusterProfiler (v4.6.0). GO terms and KEGG pathways with adjusted p-values (p adj) < 0.05 were considered significantly enriched.
2.10. RT-qPCR Validation
Eight circRNAs (six upregulated, two downregulated) were selected for RT-qPCR validation. Total RNA was extracted using TRIzol (Vazyme, Nanjing, China) and quantified by spectrophotometry (Miulab Instruments, Hangzhou, China). Reverse transcription was performed using PrimeScript™ RT Master Mix (TaKaRa, Otsu, Japan). Primers were designed using Primer Premier 6.0 (see Supplementary Table S1). RT-qPCR was carried out on a Bio-Rad CFX96 system. Relative expression was calculated using the 2−ΔΔCt method, with GAPDH as the internal control.
2.11. Statistical Analysis
All data were analyzed using SPSS 26.0 software. The Shapiro–Wilk test was used to assess data normality. Normally distributed data are expressed as the mean ± standard error of the mean (SEM), and differences between groups were analyzed using independent-sample t-tests. For non-normally distributed data, the Mann–Whitney U test was applied. A p-value of <0.05 was considered statistically significant. Dependent variables included serum antioxidant indices, gene expression levels, and follicle counts; the treatment group was used as the independent variable.
3. Results
3.1. The Effects of HS on the Physiological Characteristics of Hu Sheep
As shown in Table 2, the average temperature–humidity index (THI) in the barn of the HS group was 91.54 ± 0.93, which was higher than that of the Con group (69.36 ± 1.9; p < 0.05). Meanwhile, the respiratory rate (RR), pulse rate (PR), and rectal temperature (RT) of Hu sheep in the HS group were also higher than those in the control group (p < 0.05). In addition, compared with the Con group, the serum HSP70 level was significantly elevated in the HS group (p < 0.001), and the mRNA expression levels of HSP60, HSP70, and HSP110 in ovarian tissues were also significantly higher in the HS group (p < 0.05), while HSP90 showed no significant difference (Figure 2A,B). These results indicate that Hu sheep in the HS group experienced severe heat stress, whereas those in the Con group did not exhibit obvious signs of heat stress.

Table 2.
THI in sheep barns and physiological parameters of Hu sheep during the experimental period.
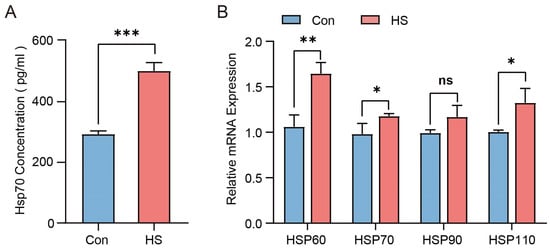
Figure 2.
The effect of heat stress on the HSP family in Hu sheep. (A) Concentration of HSP70 in the serum of Con- and HS-group sheep. (B) Relative expression patterns of HSP genes in ovarian tissues of Con and HS groups. ns, not significant; * p < 0.05; ** p < 0.01; *** p < 0.001.
3.2. Effects of HS on Antioxidant Capacity in Hu Sheep
Compared to the control group, the heat-stressed (HS) group of Hu sheep exhibited lower serum levels of total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) (p < 0.05, Figure 3A). Meanwhile, the mRNA expression levels of antioxidant-related genes (CAT, GPX1, and SOD2) in ovarian tissues were also lower than those in the control group (p < 0.05, Figure 3B).
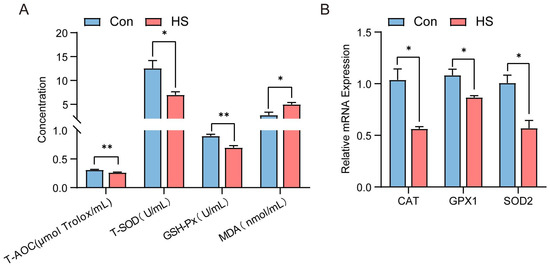
Figure 3.
Effect of heat stress on antioxidant enzyme levels in serum and mRNA expression of antioxidant genes in ovaries of Hu sheep. (A) Concentrations of T-AOC, T-SOD, GSH-Px, and MDA in the serum of Con- and HS-group sheep. (B) Relative expression levels of mRNAs of antioxidant-related genes (CAT, GPX1, and SOD2) in ovarian tissues of Hu sheep in the Con group and the HS group. ns, not significant; * p < 0.05; ** p < 0.01.
3.3. Effects of HS on Ovarian Tissue Morphology and Cell Apoptosis in Hu Sheep
As shown in Table 3, the average ovarian weight of Hu sheep in the heat-stressed (HS) group was higher than that in the control group (1.40 ± 0.16 vs. 1.01 ± 0.13, p < 0.05). Further statistical analysis of follicle numbers in Hu sheep ovaries using hematoxylin and eosin (H&E) staining revealed that the numbers of primordial, primary, secondary, and mature follicles in the HS group were lower than those in the control group (p < 0.05). Meanwhile, compared to the control group, the number of antral follicles in the HS group was significantly increased (p < 0.05, Figure 4A). TUNEL assay results showed that the level of granulosa cell apoptosis in the ovarian tissues of the HS group was higher than that of the control group (p < 0.05, Figure 4B,C). In addition, the mRNA expression levels of pro-apoptotic genes (Bax and Caspase-3) in the ovaries of the HS group were higher, while the expression level of the anti-apoptotic gene (Bcl-2) was lower, compared to the control group (p < 0.05, Figure 4D).

Table 3.
Number of follicles at different developmental stages in the ovaries of Hu sheep from the Con and HS groups.
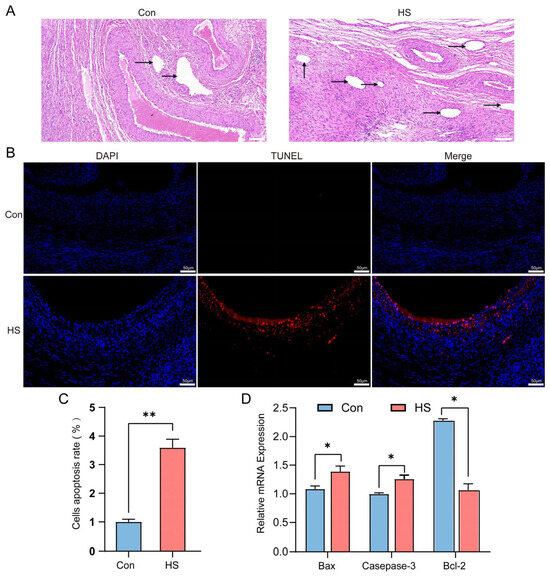
Figure 4.
Effect of heat stress on ovarian follicle development, apoptosis, and mRNA expression of apoptosis-related genes in the ovaries of Hu sheep. (A) HE staining of ovarian tissues from the Con and HS groups. Arrows indicate antral follicles. (B,C) TUNEL staining and quantification of apoptotic cells in ovarian tissues. Apoptosis rate = TUNEL-positive cells/DAPI-stained cells. (D) Relative mRNA expression levels of apoptosis-related genes (Bax, Caspase-3, and Bcl-2) in ovarian tissues from the Con and HS groups. * p < 0.05; ** p < 0.01.
3.4. Identification and Characterization of circRNAs in Ovarian Tissues of Hu Sheep Under HS
CircRNA-Seq analysis was performed on ovarian tissues from three randomly selected Hu sheep in each of the Con and HS groups (Table 4). cDNA libraries from six samples were sequenced using the Illumina HiSeq platform. Raw sequencing data were processed for quality control, filtering, and alignment. A total of 422,209,300 reads were obtained, of which 396,667,822 were successfully mapped to the reference genome. The GC content ranged from 47% to 52%.

Table 4.
Basic statistics of circRNA sequencing data.
Based on specific selection criteria, circRNAs were annotated, and their length distribution was analyzed. A relatively high proportion (21.22%) of circRNAs ranged from 300 to 400 nt in length (Figure 5A). The majority of circRNAs identified in Hu sheep ovarian samples (average = 87.55%) were exonic, primarily annotated as “annot_exons” (Figure 5B). Transcript abundance was quantified using transcripts per million (TPM), and TPM analysis indicated consistent circRNA expression levels and data density across samples (Figure 5C). High correlation was observed among ovarian tissue samples from both the control and heat-stressed groups, with correlation coefficients ranging from 0.82 to 1 (Figure 5D).

Figure 5.
Quality assessment of circRNA-seq data. (A) Length distribution of circRNAs across samples. (B) Distribution of circRNA types across samples. (C) TPM density distribution of circRNAs in each sample. (D) Correlation analysis among samples.
3.5. Differential Expression Patterns of circRNAs in Hu Sheep Ovaries Under HS
Differential expression analysis of circRNAs was conducted using edgeR 3.36.0, with thresholds set at |log2FoldChange| > 1 and p < 0.05. A total of 152 differentially expressed (DE) circRNAs were identified between the Con and HS groups, including 120 upregulated and 32 downregulated circRNAs (Figure 6A). Hierarchical clustering analysis revealed distinct expression patterns of DE circRNAs, clearly separating the Con and HS groups (Figure 6B).

Figure 6.
Screening and identification of differentially expressed circRNAs in ovarian tissue of Hu sheep. (A) Volcano plot of differentially expressed circRNAs. The x-axis represents log2FoldChange, and the y-axis represents −log10(p-value). The two vertical dashed lines indicate a two-fold change threshold, and the horizontal dashed line represents a p-value threshold of 0.05. Red dots indicate upregulated circRNAs, blue dots represent downregulated ones, and gray dots correspond to non-significantly changed circRNAs. (B) Hierarchical clustering of differentially expressed circRNAs. The x-axis represents circRNAs, with each column corresponding to a sample. Red indicates high expression, and green indicates low expression.
3.6. Functional Enrichment Analysis of Source Genes of Differentially Expressed circRNAs in Hu Sheep Ovaries Under HS
To explore the potential biological functions of DE circRNAs between the Con and HS groups, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed on the source genes of 152 DE circRNAs. GO classification divided these genes into three categories: cellular component (CC), biological process (BP), and molecular function (MF) (Figure 7A). Most source genes were significantly enriched (p < 0.01) in terms such as intracellular membrane-bounded organelle (GO:0043231), membrane-bounded organelle (GO:0043227), organelle (GO:0043226), 2-oxoglutarate-dependent dioxygenase activity (GO:0016706), protein binding (GO:0005515), enzyme activator activity (GO:0008047), and centriole elongation (GO:0061511). KEGG pathway analysis (Figure 7B) revealed significant enrichment in Apoptosis (PATH:oas04210), Adherens junction (PATH:oas04520), Mitophagy—animal (PATH:oas04137), Tight junction (PATH:oas04530), FoxO signaling pathway (PATH:oas04068), Th17 cell differentiation (PATH:oas04659), and RNA degradation (PATH:oas03018).

Figure 7.
GO and KEGG enrichment analyses of source genes of differentially expressed circRNAs. (A) GO annotation of source genes of DE circRNAs. The x-axis represents GO level 10 terms, and the y-axis indicates the enrichment level as −log10(p-value) for each term. (B) KEGG enrichment analysis of source genes of DE circRNAs. The x-axis shows the pathway names, and the y-axis represents the enrichment level as −log10(p-value) for each pathway.
3.7. RT-qPCR Validation of circRNAs
To validate the circRNA-seq results, eight differentially expressed circRNAs were randomly selected for analysis. RT-qPCR results confirmed that the expression patterns of these circRNAs were consistent with the sequencing data (Figure 8A). Subsequently, Sanger sequencing was performed to confirm the back-spliced junctions and verify the expected RNA sequences of the selected DEcircRNAs (Figure 8B–I). These findings further confirmed the reliability of the circRNA-seq results.
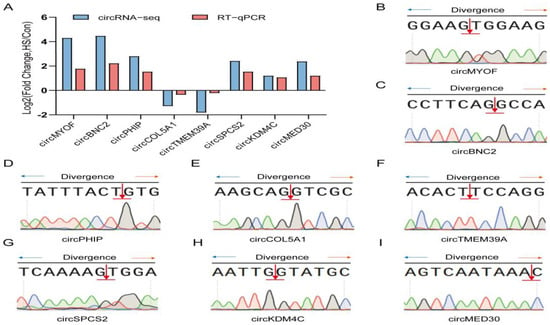
Figure 8.
Validation of DE circRNAs in Hu sheep ovarian tissues. (A) Validation of circRNA-seq results. Eight DE circRNAs were randomly selected, and their expression levels were validated by qRT-PCR. (B–I) Sanger sequencing confirmed the back-spliced junction sequences of the specified circRNAs. The blue and orange arrows indicate divergent primers for amplifying the back-splice junction, and the red arrow marks the circularization site. Colored peaks below show the Sanger sequencing chromatogram (A: green, T: red, G: black, C: blue).
4. Discussion
With the continued intensification of global climate warming, heat stress (HS) is expected to cause long-term damage to livestock production worldwide [32]. As an important component of animal husbandry, sheep farming is particularly susceptible, with HS exerting adverse effects on sheep health [33]. Based on previous studies in other species showing that HS impairs reproductive function and that circRNAs play a regulatory role in this process, we hypothesized that circRNAs are involved in regulating the ovarian response of Hu sheep to HS. Therefore, in this study, we established a Hu sheep HS model and conducted circRNA sequencing of ovarian tissues to investigate how HS affects the physiological status of Hu sheep. Our results confirmed the hypothesis, demonstrating that HS induces ovarian dysfunction accompanied by altered circRNA expression, suggesting that circRNAs may play a potential regulatory role in the ovarian response to HS. These findings provide new insights into the molecular mechanisms underlying HS-induced impairments in the health and fertility of Hu sheep.
We first assessed the physiological response of Hu sheep to HS, which revealed significant stress indicators, confirming the successful establishment of the HS model. Hu sheep, as homeothermic animals, regulate their body temperature in response to environmental changes. When ambient temperatures exceed their thermoneutral zone, they experience heat stress (HS) [34]. The temperature–humidity index (THI) is a widely used indicator of HS, with values above 72 indicating stress [35]. Physiological indicators such as the respiratory rate (RR), pulse rate (PR), and rectal temperature (RT) also rise significantly under HS in a THI-dependent manner [36]. In this study, the average THI in the HS group was 91.54 ± 0.93. The RR, PR, and RT were significantly higher in the HS group than in the control group. Heat shock proteins (HSPs) help maintain cellular homeostasis under HS, and their expression reflects the animal’s stress response. Among them, HSP70 is a key marker of thermotolerance [37,38]. Previous studies have shown that HSP70 expression increases significantly in sheep during summer [39]. Consistent with this, serum HSP70 levels were significantly elevated in Hu sheep exposed to HS. Moreover, ovarian expression of HSP60, HSP70, and HSP110 was markedly upregulated, while HSP90 showed no significant change. These results confirm that the HS group experienced severe heat stress, which adversely affected their physiological state.
This study found that heat stress disrupts ovarian tissue morphology and increases granulosa cell apoptosis. In addition to promoting cell apoptosis, heat stress also significantly impairs redox balance and weakens the antioxidant defense capacity of the ovary. Previous studies reported reduced ovarian weight and increased granulosa cell apoptosis in sheep, mice, and cattle under HS [40,41,42,43]. Consistently, HS in Hu sheep significantly decreased ovarian weight, increased follicular granulosa cell apoptosis, upregulated pro-apoptotic genes (Bax and Caspase-3), and downregulated anti-apoptotic Bcl-2. Follicle numbers at all stages declined, while atretic follicles increased. In addition, previous studies have shown that heat stress (HS) negatively affects oxidative stress balance, leading to weakened antioxidant defenses in sheep and rabbits [44,45]. Consistent with these findings, our study demonstrated that HS reduced the levels of key antioxidant enzymes in Hu sheep, including catalase (CAT), glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC) (p < 0.05). These changes may result from excessive production of reactive oxygen species (ROS) in tissues induced by heat stress, which overwhelms the antioxidant defense system. Studies have shown that elevated ROS levels can directly damage cellular structures, increasing the demand for antioxidant enzymes [46]. However, HS may interfere with the expression and activity of antioxidant-related genes such as CAT and GSH-Px, which are essential for ROS clearance [47,48]. In our study, the downregulation of antioxidant genes (such as SOD2, GPx, and CAT) suggests that HS may suppress the transcriptional activation of these genes, reducing their enzymatic activity and consequently impairing overall antioxidant capacity. Given their regulatory potential, we next investigated circRNA expression profiles in the ovaries of Hu sheep under HS conditions. CircRNAs are a class of stable endogenous non-coding RNAs generated by the back-splicing of precursor mRNAs [49]. Increasing evidence suggests that circRNAs participate in various biological processes under physiological and pathological conditions in mammals through mechanisms such as acting as microRNA sponges, interacting with proteins, or encoding small peptides, thereby playing important roles in gene regulatory networks [50,51]. The ovary is a key reproductive organ in female mammals, and gene expression within the ovary directly influences reproductive capacity. Studies have shown that heat stress (HS) impairs ovarian function in livestock by disrupting critical signaling pathways, ultimately leading to reduced fertility [52]. However, the role of circRNAs in the ovarian response to HS remains largely unexplored. In this study, we performed circRNA-seq analysis on ovarian tissues from Hu sheep under HS and non-HS conditions to investigate the potential molecular mechanisms underlying the ovarian response to heat stress.
We identified and validated a total of 152 differentially expressed circRNAs. Functional enrichment analysis of the host genes of these circRNAs suggested that they may be involved in heat stress-induced apoptosis and stress responses through multiple signaling pathways. In our study, a total of 152 differentially expressed (DE) circRNAs were identified in ovarian tissues of Hu sheep between the control (Con) and heat stress (HS) groups, with 120 upregulated and 32 downregulated under HS conditions. Eight DE circRNAs were randomly selected for RT-qPCR validation, and their expression patterns were consistent with the circRNA-seq results, confirming the reliability of the sequencing data. GO and KEGG enrichment analyses of the host genes of DE circRNAs revealed significant enrichment in pathways related to apoptosis, mitophagy, and the FoxO signaling pathway, suggesting that circRNAs may regulate ovarian cell fate through these pathways. HS-induced germ cell apoptosis is a major factor contributing to reduced reproductive performance in livestock [53]. Abnormally high summer temperatures have been shown to trigger apoptosis in mammary and ovarian cells of dairy cows, impairing lactation and fertility [54,55]. Both mitophagy and the FoxO signaling pathway have been implicated as key regulators of heat stress-induced apoptosis [56,57], consistent with our enrichment findings. Additionally, GO analysis showed that the host genes of DE circRNAs were significantly enriched in functions such as 2-oxoglutarate-dependent dioxygenase activity, protein binding, dioxygenase activity, intracellular membrane-bounded organelle, and centriole elongation, which are closely associated with cellular homeostasis and responses to environmental stress.
Among the DE circRNAs, circBNC2 and circPHIP emerged as promising candidates, possibly contributing to ovarian cell stress responses and granulosa cell function under HS. Moreover, our data showed that both circBNC2 and circPHIP were significantly upregulated in the HS group. CircBNC2 has been reported to inhibit ovarian cancer cell proliferation and migration by targeting the miR-223-3p/FBXW7 axis [58]. BNC2 is considered a key tumor suppressor gene in the ovary and plays a crucial role in regulating oxidative stress responses within ovarian cells [59]. PHIP is a potential candidate gene associated with egg production in chickens; its knockout significantly reduces progesterone (PROG) levels and inhibits granulosa cell proliferation in the ovary [60]. These findings suggest that circBNC2 and circPHIP may serve as important regulators in the ovarian response to heat stress.
5. Conclusions
In summary, our results demonstrate that heat stress (HS) negatively affects the physiological health and antioxidant capacity of Hu sheep, impairing follicular development and promoting the apoptosis of ovarian granulosa cells. Under HS conditions, a total of 152 differentially expressed circRNAs were identified, including 120 upregulated and 32 downregulated circRNAs. Functional enrichment analysis revealed that these circRNAs are involved in apoptosis, mitophagy, and the FoxO signaling pathway. These findings provide new insights into how circRNAs regulate ovarian function under heat stress and suggest that circRNAs such as circBNC2 and circPHIP may serve as potential biomarkers for heat stress resilience. However, further studies with larger sample sizes and broader age ranges are needed to validate these results and explore the potential mechanisms by which circRNAs modulate heat stress responses in livestock, which may contribute to the development of new strategies for enhancing livestock adaptability to climate change.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani15142063/s1, Table S1: Gene-specific primer pairs used for RT-qPCR.
Author Contributions
Conceptualization, W.L. and S.W.; methodology, formal analysis, and investigation, J.Z. (Juhong Zou), H.H., F.W., J.L. and J.Z. (Jianwei Zou); validation, Y.L., Z.M., L.W. and J.Z. (Jianwei Zou); writing—original draft preparation, J.Z. (Jianwei Zou); writing—review and editing, J.Z. (Jianwei Zou), L.W., Z.M., Y.L., J.L., J.Z. (Juhong Zou), F.W., S.W., H.H., W.L., Y.H. and Q.J.; resources, project administration, and funding acquisition, Y.H. and Q.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Key Research and Development Program of Guangxi (no. Guike AB23026081), the National Modern Agricultural Industry Technology System Guangxi Innovation Team (nycytxgxcxtd-2021-09), and the Innovation Project of Guangxi Graduate Education (YCBZ2024017).
Institutional Review Board Statement
The project was approved by the Animal Experimentation Ethics Committee of Guangxi University, with the approval number GXU-2023-0135.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely thank the College of Animal Science and Technology, Guangxi University, and the Guangxi Key Laboratory of Animal Breeding and Disease Control for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Wettere, W.; Culley, S.; Swinbourne, A.; Leu, S.; Lee, S.; Weaver, A.; Kelly, J.; Walker, S.; Kleemann, D.; Thomas, D.; et al. Heat stress from current and predicted increases in temperature impairs lambing rates and birth weights in the Australian sheep flock. Nat. Food 2024, 5, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Arero, G.; Ozmen, O. Effects of heat stress on reproduction and gene expression in sheep. Anim. Reprod. 2025, 22, e20240067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dunshea, F.; Warner, R.; DiGiacomo, K.; Osei-Amponsah, R.; Chauhan, S. Impacts of heat stress on meat quality and strategies for amelioration: A review. Int. J. Biometeorol. 2020, 64, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- van Wettere, W.H.E.J.; Kind, K.L.; Gatford, K.L.; Swinbourne, A.M.; Leu, S.T.; Hayman, P.T.; Kelly, J.M.; Weaver, A.C.; Kleemann, D.O.; Walker, S.K. Review of the impact of heat stress on reproductive performance of sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wei, Y.; Zou, J.; Yang, B.; Wang, Q.; Lu, J.; Lu, J.; Zheng, Z.; Huang, Y.; et al. Effect of heat stress on growth performance, carcase characteristics, meat quality and rumen-muscle axis of Hu sheep. Ital. J. Anim. Sci. 2024, 23, 87–100. [Google Scholar] [CrossRef]
- Karthik, D.; Suresh, J.; Reddy, Y.; Sharma, G.; Ramana, J.; Gangaraju, G.; Reddy, P.; Reddy, Y.; Yasaswini, D.; Adegbeye, M.; et al. Adaptive profiles of Nellore sheep with reference to farming system and season: Physiological, hemato-biochemical, hormonal, oxidative-enzymatic and reproductive standpoint. Heliyon 2021, 7, e07117. [Google Scholar] [CrossRef]
- De, K.; Kumar, D.; Balaganur, K.; Naqvi, S. Effect of environmental factors on estrus synchronization and artificial insemination success in farmers flock in sheep under semi-arid tropical region. Reprod. Domest. Anim. 2020, 55, 777–784. [Google Scholar] [CrossRef]
- Mossa, F.; Evans, A. Review: The ovarian follicular reserve—Implications for fertility in ruminants. Animal 2023, 17, 100744. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Shu, J.; Meng, C.; Zhang, J.; Zhang, J.; Qian, Y.; Wang, H.; Ding, Q.; Cao, S. Acute heat stress regulates estradiol synthesis in ovine ovarian granulosa cells through the SREBPs/MVK-LHR pathway. Anim. Reprod. Sci. 2025, 272, 107649. [Google Scholar] [CrossRef]
- Dalgleish, M. Where the Global Sheep Flock Can Be Found and Where It’s Going. In Agricultural Outlook; FAO: Rome, Italy, 2024. [Google Scholar]
- IWTO. World Sheep Numbers and Wool Production; IWTO: Brussels, Belgium, 2022. [Google Scholar]
- Lingying, K.; Yaojing, Y.; Jianye, L.; Bohui, Y.; Bowen, C.; Jianbin, L.; Zengkui, L. Transcriptomics and metabolomics reveal improved performance of Hu sheep on hybridization with Southdown sheep. Food Res. Int. 2023, 173, 113240. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Dai, Y.; Zhao, Z.; Zhu, J.; Guo, H.; Yang, R. Single-cell sequencing to multi-omics: Technologies and applications. Biomark. Res. 2024, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Haire, A.; Bai, J.; Zhao, X.; Song, Y.; Zhao, G.; Dilixiati, A.; Li, J.; Sun, W.Q.; Wan, P.; Fu, X.; et al. Identifying the heat resistant genes by multi-tissue transcriptome sequencing analysis in Turpan Black sheep. Theriogenology 2021, 179, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-D.; Wang, L.-W.; Fu, S.-Y.; E, R.-G.; Ren, X.-Q.; Sun, H.; Liu, F.; Wang, B.; An, J.-H.; Zhao, M.-R.; et al. Heat Tolerance Differences Between Hu Sheep and Hu Crossbred Sheep in Microbial Community Structure and Metabolism. Metabolites 2025, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; E, G.; Zhang, M.; Zhang, Y.; Bai, T.; Pu, X.; Liu, J.; Guo, X.; Sarker, S.; Cheng, L. No rumen fermentation profiles and associated microbial diversities difference were found between Hu sheep and Karakul sheep fed a cottonseed hull diet. Microbiome 2025, 13, 22. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, L.; Li, F.; Zhang, X.; Tian, H.; Ma, Z.; Zhang, D.; Zhang, Y.; Zhao, Y.; Huang, K.; et al. Whole-genome resequencing of Hu sheep identifies candidate genes associated with agronomic traits. J. Genet. Genom. 2024, 51, 866–876. [Google Scholar] [CrossRef]
- Wang, J.; Hua, G.; Cai, G.; Ma, Y.; Yang, X.; Zhang, L.; Li, R.; Liu, J.; Ma, Q.; Wu, K.; et al. Genome-wide DNA methylation and transcriptome analyses reveal the key gene for wool type variation in sheep. J. Anim. Sci. Biotechnol. 2023, 14, 88. [Google Scholar] [CrossRef]
- Xu, F.; Xiao, Q.; Du, W.W.; Wang, S.; Yang, B.B. CircRNA: Functions, Applications and Prospects. Biomolecules 2025, 14, 1503. [Google Scholar] [CrossRef]
- Dong, J.; Zeng, Z.; Huang, Y.; Chen, C.; Cheng, Z.; Zhu, Q. Challenges and opportunities for circRNA identification and delivery. Crit. Rev. Biochem. Mol. Biol. 2023, 58, 19–35. [Google Scholar] [CrossRef]
- Chen, L.-L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Misir, S.; Wu, N.; Yang, B.B. Specific expression and functions of circular RNAs. Cell Death Differ. 2022, 29, 481–491. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Y.; Chen, C.; Wang, Z. Pervasive translation of circular RNAs driven by short IRES-like elements. Nat. Commun. 2022, 13, 3751. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Tang, Q.; Hu, S.; Chen, Z.; Zhou, X.; Zeng, B.; Wang, Y.; He, M.; Li, Y.; Gui, L.; et al. A pig BodyMap transcriptome reveals diverse tissue physiologies and evolutionary dynamics of transcription. Nat. Commun. 2021, 12, 3715. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, M.; Sooranna, S.; Shi, D.; Liu, Q.; Li, H. The emerging roles of circRNAs in traits associated with livestock breeding. Wiley Interdiscip. Rev. RNA 2023, 14, e1775. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, W.; Wang, J.; Xu, X.; Zhang, T.; Wang, L.; Feng, X. Unveiling Circular RNA-Mediated Regulatory Mechanisms in Necroptosis in Premature Ovarian Failure. Front. Biosci. (Landmark Edit.) 2023, 28, 314. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Hu, B.; Xie, Y.; Wang, D.; Zhang, J.; Chen, T.; Luo, J.; Wang, S.; Jiang, Q.; et al. Emerging Roles of Heat-Induced circRNAs Related to Lactogenesis in Lactating Sows. Front. Genet. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Hu, L.; Fang, H.; Chen, G.; Ma, X.; Yu, Y.; Wang, Y.; Xu, Q. Analysis of CircRNA Expression in Peripheral Blood of Holstein Cows in Response to Heat Stress. Int. J. Mol. Sci. 2023, 24, 10150. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.; Zhuang, X.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Identification of circRNA-Associated-ceRNA Networks Involved in Milk Fat Metabolism under Heat Stress. Int. J. Mol. Sci. 2020, 21, 4162. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, B.; Xiong, J.; Chen, T.; Xi, Q.; Luo, J.; Jiang, Q.; Sun, J.; Zhang, Y. Genomewide analysis of circular RNA in pituitaries of normal and heat-stressed sows. BMC Genom. 2019, 20, 1013. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, L.; Wang, S.; Chen, G.; Brito, L.; Li, B.; Xu, Q.; Wang, Y. Heat Tolerance-Associated circRNA3685 Regulates Apoptosis and Autophagy in Bovine Mammary Epithelial Cells via Sponging bta-miR-138. J. Agric. Food Chem. 2025, 73, 1656–1671. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Caroprese, M.; Albenzio, M. Climate resilience in small ruminant and immune system: An old alliance in the new sustainability context. Small Rumin. Res. 2022, 210, 106662. [Google Scholar] [CrossRef]
- Laporta, J.; Khatib, H.; Zachut, M. Review: Phenotypic and molecular evidence of inter- and trans-generational effects of heat stress in livestock mammals and humans. Animal 2024, 18, 101121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, M.; Hou, P.; Wang, W.; Shen, X.; Zhang, L.; Han, S.; Pan, C. Analysis of microbial community structure and volatile compounds in pit mud used for manufacturing Taorong-type Baijiu based on high-throughput sequencing. Sci. Rep. 2022, 12, 7347. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.; Du, Y.; Wang, C.; Zhang, L.; Yin, L.; Zuo, F.; Huang, W. Effect of heat stress on blood biochemistry and energy metabolite of the Dazu black goats. Front. Veter. Sci. 2024, 11, 1338643. [Google Scholar] [CrossRef]
- Fontoura, A.; Javaid, A.; de la Maza-Escolà, V.S.; Salandy, N.; Fubini, S.; Grilli, E.; McFadden, J. Heat stress develops with increased total-tract gut permeability, and dietary organic acid and pure botanical supplementation partly restores lactation performance in Holstein dairy cows. J. Dairy Sci. 2022, 105, 7842–7860. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Xu, W.; Meng, Z.; Deng, J.; Sun, X.; Liu, T.; Tang, Y.; Zhang, Z.; Liu, Y.; Zhu, W. Metabonomic identification of serum biomarkers related to heat stress tolerance of sheep. Anim. Sci. J. 2022, 93, e13792. [Google Scholar] [CrossRef]
- Abu Rawash, R.A.; Sharaby, M.A.; Hassan, G.E.-D.A.; Elkomy, A.E.; Hafez, E.E.; Abu Hafsa, S.H.; Salem, M.M.I. Expression profiling of HSP 70 and interleukins 2, 6 and 12 genes of Barki sheep during summer and winter seasons in two different locations. Int. J. Biometeorol. 2022, 66, 2047–2053. [Google Scholar] [CrossRef]
- Sammad, A.; Luo, H.; Hu, L.; Zhu, H.; Wang, Y. Transcriptome Reveals Granulosa Cells Coping through Redox, Inflammatory and Metabolic Mechanisms under Acute Heat Stress. Cells 2022, 11, 1443. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.; et al. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Lima, E.; Carvalho, L.; Orlandi, R.; Simões, L.; Bottino, M.; Santos, A.; de Oliveira Scarpa, F.; Sales, J. Effect of maternal heat stress at different stages of pregnancy on the reproductive performance and antral follicle count of the progeny of Holstein cows. Anim. Reprod. Sci. 2025, 272, 107665. [Google Scholar] [CrossRef]
- Vanselow, J.; Vernunft, A.; Koczan, D.; Spitschak, M.; Kuhla, B. Exposure of Lactating Dairy Cows to Acute Pre-Ovulatory Heat Stress Affects Granulosa Cell-Specific Gene Expression Profiles in Dominant Follicles. PLoS ONE 2016, 11, e0160600. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bai, X.; Xie, X.; Chen, G.; Jia, X.; Lei, M.; Li, C.; Lai, S. Negative effects of heat stress on ovarian tissue in female rabbit. Front. Veter. Sci. 2022, 9, 1009182. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xu, Y.; Mao, C.; Wang, Z.; Guo, S.; Jin, X.; Yan, S.; Shi, B. Effects of heat stress on antioxidant status and immune function and expression of related genes in lambs. Int. J. Biometeorol. 2020, 64, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Ye, X.-Q.; Zhu, Y.-R.; Yang, Y.-Y.; Qiu, S.-J.; Liu, W.-C. Biogenic Selenium Nanoparticles Synthesized with Alginate Oligosaccharides Alleviate Heat Stress-Induced Oxidative Damage to Organs in Broilers through Activating Nrf2-Mediated Anti-Oxidation and Anti-Ferroptosis Pathways. Antioxidants 2023, 12, 1973. [Google Scholar] [CrossRef]
- Jing, J.; Zeng, H.; Shao, Q.; Tang, J.; Wang, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; et al. Selenomethionine alleviates environmental heat stress induced hepatic lipid accumulation and glycogen infiltration of broilers via maintaining mitochondrial and endoplasmic reticulum homeostasis. Redox Biol. 2023, 67, 102912. [Google Scholar] [CrossRef]
- Yang, L.; Wilusz, J.; Chen, L. Biogenesis and Regulatory Roles of Circular RNAs. Annu. Rev. Cell Dev. Biol. 2022, 38, 263–289. [Google Scholar] [CrossRef]
- Yi, Q.; Feng, J.; Lan, W.; Shi, H.; Sun, W.; Sun, W. CircRNA and lncRNA-encoded peptide in diseases, an update review. Mol. Cancer 2024, 23, 214. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Roach, C.; Bidne, K.; Romoser, M.; Ross, J.; Baumgard, L.; Keating, A. Impact of heat stress on prolactin-mediated ovarian JAK-STAT signaling in postpubertal gilts. J. Anim. Sci. 2022, 100, skac118. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Chen, W.; Chai, W.; Wang, C. Advancements in Genetic Biomarkers and Exogenous Antioxidant Supplementation for Safeguarding Mammalian Cells against Heat-Induced Oxidative Stress and Apoptosis. Antioxidants 2024, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-F.; Xu, J.; Wang, X.-L.; Li, S.-J.; Han, Z.-Y. Nicotinamide mononucleotide alleviates heat stress-induced oxidative stress and apoptosis in BMECs through reducing mitochondrial damage and endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2022, 235, 113441. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.Z.; Umer, S.; Khan, I.M.; Xu, H.; Zhu, H.; Wang, Y. Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals 2020, 10, 110. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Q.; Wei, C.; Sun, Y.; Li, Y.; Wei, Y.; Jiang, Q.; Huang, Y. EGCG Alleviates Skeletal Muscle Oxidative Damage in Heat-Stressed Pigs via Keap1/PGAM5 Complex-Mediated Mitophagy. J Agric. Food Chem. 2024, 73, 425–437. [Google Scholar] [CrossRef]
- Ning, Z.; Deng, X.; Li, L.; Feng, J.; Du, X.; Amevor, F.K.; Tian, Y.; Li, L.; Rao, Y.; Yi, Z.; et al. miR-128-3p regulates chicken granulosa cell function via 14-3-3β/FoxO and PPAR-γ/LPL signaling pathways. Int. J. Biol. Macromol. 2023, 241, 124654. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, L.; Zou, X. Circular RNA circ-BNC2 (hsa_circ_0008732) inhibits the progression of ovarian cancer through microRNA-223-3p/FBXW7 axis. J. Ovarian Res. 2022, 15, 95. [Google Scholar] [CrossRef]
- Cesaratto, L.; Grisard, E.; Coan, M.; Zandonà, L.; De Mattia, E.; Poletto, E.; Cecchin, E.; Puglisi, F.; Canzonieri, V.; Mucignat, M.; et al. BNC2 is a putative tumor suppressor gene in high-grade serous ovarian carcinoma and impacts cell survival after oxidative stress. Cell Death Dis. 2016, 7, e2526. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, Y.; Dong, J.; Zhi, Y.; Wei, G.; Kang, X.; Liu, X. Exploring the functional variations of key candidate genes affecting egg production by hypothalamic-pituitary-ovarian axis in chickens. Poult. Sci. 2025, 104, 105027. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).