Effect and Optimal Level of Dietary Dried Watermeal (Wolffia globosa) Supplementation on the Production Performance of Two-Spotted Crickets (Gryllus bimaculatus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing and Management of Experimental Crickets
2.2. Experimental Diet
2.3. Growth Performance, Feed Efficiency, Survival Rate, and Production Index
2.4. Sample Collection
2.5. Chemical Analysis
2.6. Statistical Analysis
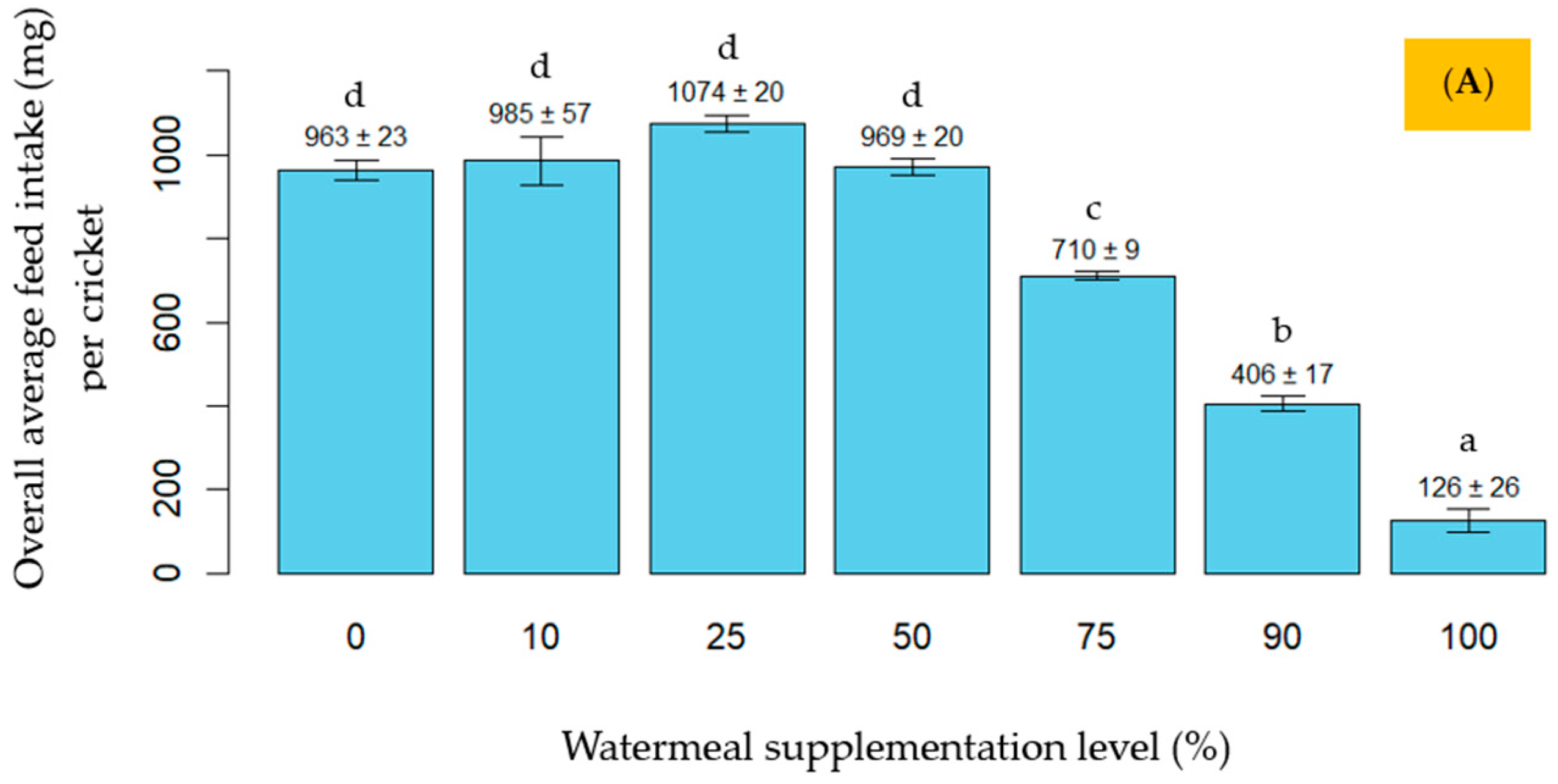
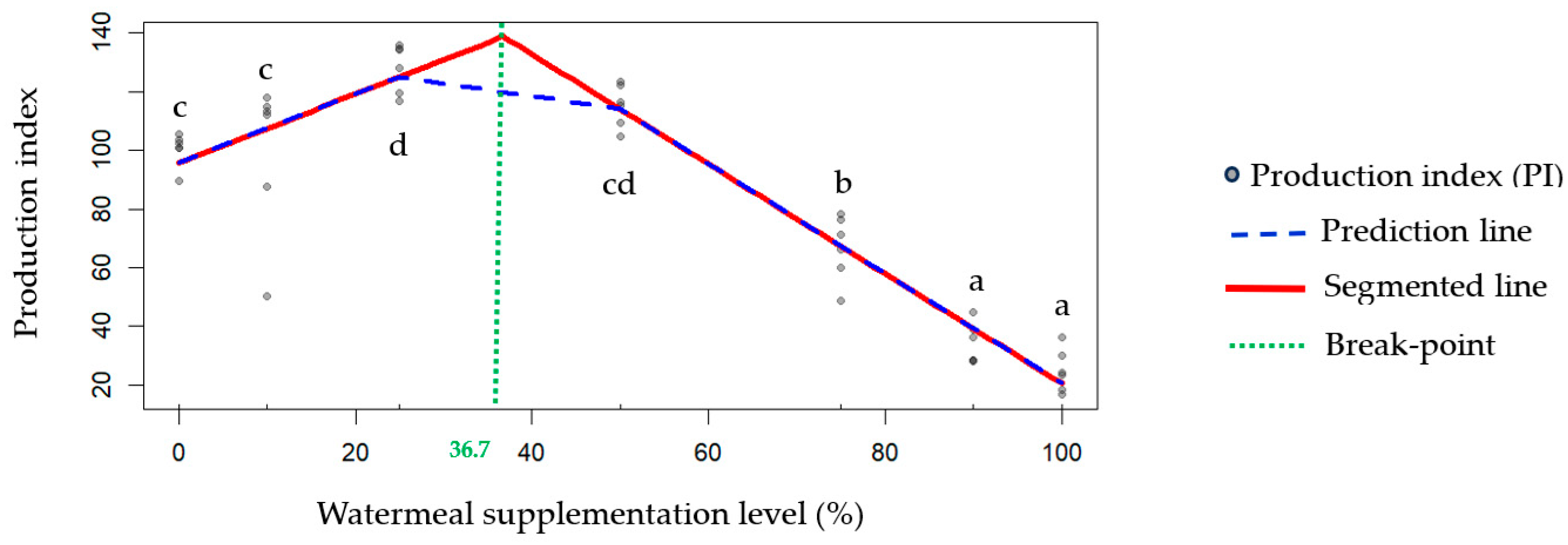
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safavi, A.; Thrastardottir, R.; Thorarinsdottir, R.I.; Unnthorsson, R. Insect Production: A circular economy strategy in Iceland. Sustainability 2024, 16, 9063. [Google Scholar] [CrossRef]
- Cattaneo, A.; Padula, C.; Meneguz, M.; Mileto, C.; Barbero, S.; Dabbou, S. Toward a circular economy in Italian agri-food: Upstream partners in insect biorefineries. Agric. Food Econ. 2024, 12, 43. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of diet on the growth performance, feed conversion, and nutrient content of the house cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef]
- Kuo, C.; Fisher, B.L. A literature review of the use of weeds and agricultural and food industry by-products to feed farmed crickets (Insecta; Orthoptera; Gryllidae). Front. Sustain. Food Syst. 2022, 5, 810421. [Google Scholar] [CrossRef]
- Govoni, C.; D’Odorico, P.; Pinotti, L.; Rulli, M.C. Preserving global land and water resources through the replacement of livestock feed crops with agricultural by-products. Nat. Food 2023, 4, 1047–1057. [Google Scholar] [CrossRef]
- Ruekaewma, N.; Piyatiratitivorakul, S.; Powtongsook, S. Culture system for Wolffia globosa L.(Lemnaceae) for hygiene human food. Songklanakarin J. Sci. Technol. 2015, 37, 575–580. [Google Scholar]
- Seephua, N.; Boonarsa, P.; Li, H.; Thammapat, P.; Siriamornpun, S. Nutritional composition and bioactive profiles of farmed and wild watermeal (Wolffia globosa). Foods 2025, 14, 1832. [Google Scholar] [CrossRef]
- Boonarsa, P.; Bunyatratchata, A.; Chumroenphat, T.; Thammapat, P.; Chaikwang, T.; Siripan, T.; Li, H.; Siriamornpun, S. Nutritional quality, functional properties, and biological characterization of watermeal (Wolffia globosa). Horticulturae 2024, 10, 1171. [Google Scholar] [CrossRef]
- Romano, L.E.; Aronne, G. The world smallest plants (Wolffia sp.) as potential species for bioregenerative life support systems in space. Plants 2021, 10, 1896. [Google Scholar] [CrossRef]
- Rassami, W.; Suwannarat, Y.; Sawasdikarn, J.; Jitsatta, N.; Prathumyot, W.; Vorakitpateep, N. Effect of spotless watermeal, durian peel and durian seeds instead of commercial formulation for house crickets, Acheta domesticus (Linnaeus). Rambhai Barni Rajabhat Agric. J. 2025, 3, 1–7. (In Thai) [Google Scholar]
- Parajulee, M.N.; Defoliart, G.R.; Hogg, D.B. Model for use in mass-production of Acheta domesticus (Orthoptera: Gryllidae) as food. J. Econ. Entomol. 1993, 86, 1424–1428. [Google Scholar] [CrossRef][Green Version]
- Mitchaothai, J.; Grabowski, N.T.; Lertpatarakomol, R.; Trairatapiwan, T.; Chhay, T.; Keo, S.; Lukkananukool, A. Production performance and nutrient conversion efficiency of field cricket (Gryllus bimaculatus) in mass-rearing conditions. Animals 2022, 12, 2263. [Google Scholar] [CrossRef]
- Mitchaothai, J.; Grabowski, N.T.; Lertpatarakomol, R.; Trairatapiwan, T.; Lukkananukool, A. Bacterial contamination and antimicrobial resistance in two-spotted (Gryllus bimaculatus) and house (Acheta domesticus) cricket rearing and harvesting processes. Vet. Sci. 2024, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.M.S.; Schaeublin, H.; Wenk, C.; Wanner, M.; Liesegang, A. Effect of dietary citric acid on the performance and mineral metabolism of broiler. J. Anim. Physiol. Anim. Nutr. 2012, 96, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Kaewtapee, C.; Triwai, P.; Inson, C.; Masmeatathip, R.; Sriwongras, P. Effects of protein levels on production performance, nutritional values, and phase feeding of two-spotted cricket. J. Insect Sci. 2024, 24, 18. [Google Scholar] [CrossRef]
- AOAC [Association of Official Agricultural Chemists]. Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species-Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 19 May 2025).
- Sorjonen, J.M.; Valtonen, A.; Hirvisalo, E.; Karhapää, M.; Lehtovaara, V.J.; Lindgren, J.; Marnila, P.; Mooney, P.; Mäki, M.; Siljander-Rasi, H. The plant-based by-product diets for the mass-rearing of Acheta domesticus and Gryllus bimaculatus. PLoS ONE 2019, 14, e0218830. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Hugel, S.; Fisher, B.L. Effect of feed on the growth performance, nutrition content and cost of raising the field cricket (Gryllus madagascarensis) as a sustainable nutrient source in Madagascar. Foods 2024, 13, 3139. [Google Scholar] [CrossRef]
- Nakagaki, B.J.; Defoliart, G.R. Comparison of diets for mass-rearing Acheta domesticus (Orthoptera: Gryllidae) as a novelty food, and comparison of food conversion efficiency with values reported for livestock. J. Econ. Entomol. 1991, 84, 891–896. [Google Scholar] [CrossRef]
- Vaga, M.; Berggren, Å.; Jansson, A. Growth, survival and development of house crickets (Acheta domesticus) fed flowering plants. J. Insects Food Feed. 2021, 7, 151–161. [Google Scholar] [CrossRef]
- Chapman, R. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Lorenz, M.W.; Anand, A.N. Changes in the biochemical composition of fat body stores during adult development of female crickets, Gryllus bimaculatus. In Archives of Insect Biochemistry and Physiology; The Entomological Society of America: Lanham, MD, USA, 2004; Volume 56, pp. 110–119. [Google Scholar]
- Orinda, M.A.; Mosi, R.O.; Ayieko, M.A.; Amimo, F.A. Growth performance of common house cricket (Acheta domesticus) and field cricket (Gryllus bimaculatus) crickets fed on agro-byproducts. J. Entomol. Zool. Stud. 2017, 5, 1664–1668. [Google Scholar]
- Jucker, C.; Belluco, S.; Oddon, S.B.; Ricci, A.; Bonizzi, L.; Lupi, D.; Savoldelli, S.; Biasato, I.; Caimi, C.; Mascaretti, A. Impact of some local organic by-products on Acheta domesticus growth and meal production. J. Insects Food Feed. 2022, 8, 631–640. [Google Scholar] [CrossRef]
- Orinda, M.A.; Oloo, J.; Magara, H.J.; Ayieko, M.; Ekesi, S.; Roo, N. Cricket Rearing Handbook; Services for Science and Education: Stockport, UK, 2021. [Google Scholar]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Muzzatti, M.J.; Harrison, S.J.; McColville, E.R.; Brittain, C.T.; Brzezinski, H.; Manivannan, S.; Stabile, C.C.; MacMillan, H.A.; Bertram, S.M. Applying nutritional ecology to optimize diets of crickets raised for food and feed. R. Soc. Open Sci. 2024, 11, 241710. [Google Scholar] [CrossRef] [PubMed]
- Khempaka, S.; Okrathok, S.; Schonewille, J.T.; Pukkung, C.; Sirisopapong, M.; Jantasaeng, O.; Pasri, P. Responses of house crickets (Orthoptera: Gryllidae) to various dietary gross energy levels: Effects on growth performance and nutrient deposition. J. Insect Sci. 2025, 25, 21. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.J.; Raubenheimer, D.; Simpson, S.J.; Godin, J.-G.J.; Bertram, S.M. Towards a synthesis of frameworks in nutritional ecology: Interacting effects of protein, carbohydrate and phosphorus on field cricket fitness. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140539. [Google Scholar] [CrossRef]
- Joern, A.; Behmer, S.T. Importance of dietary nitrogen and carbohydrates to survival, growth, and reproduction in adults of the grasshopper Ageneotettix deorum (Orthoptera: Acrididae). Oecologia 1997, 112, 201–208. [Google Scholar] [CrossRef]
- Taesuk, N.; Upan, J.; Siriamornpun, S.; Bunphan, D.; Sangkaew, M. Utilising brewer’s spent grain as a cost-effective feed formula for sustainable house cricket (Acheta domesticus) rearing. J. Insects Food Feed. 2024, 1, 987–999. [Google Scholar] [CrossRef]
- Kasdorf, S.Y.; Muzzatti, M.J.; Haider, F.; Bertram, S.M.; MacMillan, H.A. Brewery waste as a sustainable protein source for the banded cricket (Gryllodes sigillatus). J. Insects Food Feed. 2025, 1, 1417–1429. [Google Scholar] [CrossRef]
- Bai, J.; Ling, Y.; Li, W.-J.; Wang, L.; Xue, X.-B.; Gao, Y.-Y.; Li, F.-F.; Li, X.-J. Analysis of intestinal microbial diversity of four species of grasshoppers and determination of cellulose digestibility. Insects 2022, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.; Dean, D.; Southern, L.; Bidner, T. Effect of protein and energy sources and bulk density of diets on growth performance of chicks. Poult. Sci. 2005, 84, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Nation, J.L. Insect Diets: Science and Technology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Iwuji, T.C.; Okoli, I.C.; Ogbuewu, I.P.; Etuk, I.F.; Opara, M.N.; Ugwu, C.C. Particle size effect on bulk density of selected commercial broiler starter feed produced in Nigeria. Adv. Agric. Sci. Eng. Res. 2013, 3, 1045–1049. [Google Scholar]
- Dobermann, D.; Michaelson, L.; Field, L. The effect of an initial high-quality feeding regime on the survival of Gryllus bimaculatus (black cricket) on bio-waste. J. Insects Food Feed. 2019, 5, 117–124. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Ngóngá, C.A.; Gor, C.O.; Okuto, E.O.A.; Ouko, K.O. Economic efficiency of cricket production reared under improvised cage system for improved food production. Afr. J. Agric. Res. 2021, 17, 1453–1462. [Google Scholar]
- Bhamre, K.S. Nutritive Evaluation of Cashew Apple Waste in Broilers. Ph.D. Thesis, College of Veterinary and Animal Sciences, Pookode Wayanad, India, 2016. [Google Scholar]
- Kryeziu, A.; Mestani, N.; Berisha, S.; Kamberi, M. The European performance indicators of broiler chickens as influenced by stocking density and sex. J. Anim. Prod. Adv. 2018, 8, 45–52. [Google Scholar]
- Sorjonen, J.; Karhapää, M.; Holm, S.; Valtonen, A.; Roininen, H. Performance of the house cricket (Acheta domesticus) on by-product diets in small-scale production. J. Insects Food Feed. 2022, 8, 289–294. [Google Scholar] [CrossRef]
- Magara, H.J.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.; Hugel, S. Edible crickets (Orthoptera) around the world: Distribution, nutritional value, and other benefits—A review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional values and functional properties of house cricket (Acheta domesticus) and field cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Loypimai, P.; Moontree, T.; Pranil, T.; Moongngarm, A. A comparative study of nutritional components of Gryllus bimaculatus and Acheta domesticus cricket powder prepared using different drying methods. J. Food Meas. Charact. 2024, 18, 3974–3983. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]


| Item | Experimental Treatment | ||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 * | |
| Ingredients | |||||||
| Commercial diet (%) | 100 | 90 | 75 | 50 | 25 | 10 | 0 |
| Watermeal (%) | 0 | 10 | 25 | 50 | 75 | 90 | 100 |
| Analyzed nutrient composition | |||||||
| Dry matter (%) | 87.72 | 87.99 | 88.74 | 89.61 | 90.89 | 91.84 | 93.53 |
| Crude protein (%) | 21.72 | 21.74 | 21.78 | 22.02 | 22.51 | 22.91 | 23.24 |
| Crude fat (%) | 3.33 | 3.28 | 3.03 | 2.95 | 2.39 | 2.17 | 0.97 |
| Crude fiber (%) | 3.32 | 3.60 | 4.53 | 6.12 | 7.40 | 8.37 | 11.70 |
| Ash (%) | 6.99 | 7.32 | 8.23 | 9.85 | 11.29 | 12.46 | 13.17 |
| Nitrogen-free extract (NFE) (%) | 52.35 | 52.08 | 51.18 | 48.67 | 47.29 | 45.93 | 44.44 |
| Calcium (%) | 1.01 | 0.99 | 0.98 | 0.89 | 0.76 | 0.65 | 0.64 |
| Phosphorus, (%) | 0.94 | 0.92 | 0.86 | 0.74 | 0.70 | 0.61 | 0.57 |
| Crude protein: NFE | 1:2.41 | 1:2.39 | 1:2.35 | 1:2.21 | 1:2.10 | 1:2.00 | 1:1.91 |
| Gross energy (kcal/kg) | 3928.85 | 3934.65 | 3951.20 | 3950.85 | 3972.85 | 3989:95 | 3926.60 |
| Bulk density (g/cm3) | 0.553 | 0.534 | 0.432 | 0.329 | 0.266 | 0.236 | 0.231 |
| Item | Control (T1); n = 6 | Watermeal Supplementation | SEM | |
|---|---|---|---|---|
| ≤50% (T2–T4); n = 18 | >50% (T5–T7); n = 6 | |||
| Dry matter (%) | 92.48 | 92.71 | 91.00 | 0.32 |
| Crude protein (%) | 42.33 a | 44.41 a | 51.40 b | 1.14 |
| Crude fat (%) | 28.83 b | 24.51 b | 12.92 a | 1.86 |
| Crude fiber (%) | 6.21 | 7.57 | 8.43 | 0.35 |
| Ash (%) | 3.74 a | 4.10 a | 5.25 b | 0.21 |
| Calcium (%) | 0.19 a | 0.17 a | 0.49 b | 0.06 |
| Phosphorus (%) | 0.75 | 0.76 | 0.88 | 0.02 |
| Gross energy (kcal/kg) | 6066.50 b | 5860.34 b | 5167.55 a | 116.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchaothai, J.; Grabowski, N.T.; Lertpatarakomol, R.; Trairatapiwan, T.; Lukkananukool, A. Effect and Optimal Level of Dietary Dried Watermeal (Wolffia globosa) Supplementation on the Production Performance of Two-Spotted Crickets (Gryllus bimaculatus). Animals 2025, 15, 2052. https://doi.org/10.3390/ani15142052
Mitchaothai J, Grabowski NT, Lertpatarakomol R, Trairatapiwan T, Lukkananukool A. Effect and Optimal Level of Dietary Dried Watermeal (Wolffia globosa) Supplementation on the Production Performance of Two-Spotted Crickets (Gryllus bimaculatus). Animals. 2025; 15(14):2052. https://doi.org/10.3390/ani15142052
Chicago/Turabian StyleMitchaothai, Jamlong, Nils T. Grabowski, Rachakris Lertpatarakomol, Tassanee Trairatapiwan, and Achara Lukkananukool. 2025. "Effect and Optimal Level of Dietary Dried Watermeal (Wolffia globosa) Supplementation on the Production Performance of Two-Spotted Crickets (Gryllus bimaculatus)" Animals 15, no. 14: 2052. https://doi.org/10.3390/ani15142052
APA StyleMitchaothai, J., Grabowski, N. T., Lertpatarakomol, R., Trairatapiwan, T., & Lukkananukool, A. (2025). Effect and Optimal Level of Dietary Dried Watermeal (Wolffia globosa) Supplementation on the Production Performance of Two-Spotted Crickets (Gryllus bimaculatus). Animals, 15(14), 2052. https://doi.org/10.3390/ani15142052






