Incorporating Morphological Evaluations into Breeding Soundness Examinations for Female Dogs
Simple Summary
Abstract
1. Introduction
2. Breeding Soundness Examination
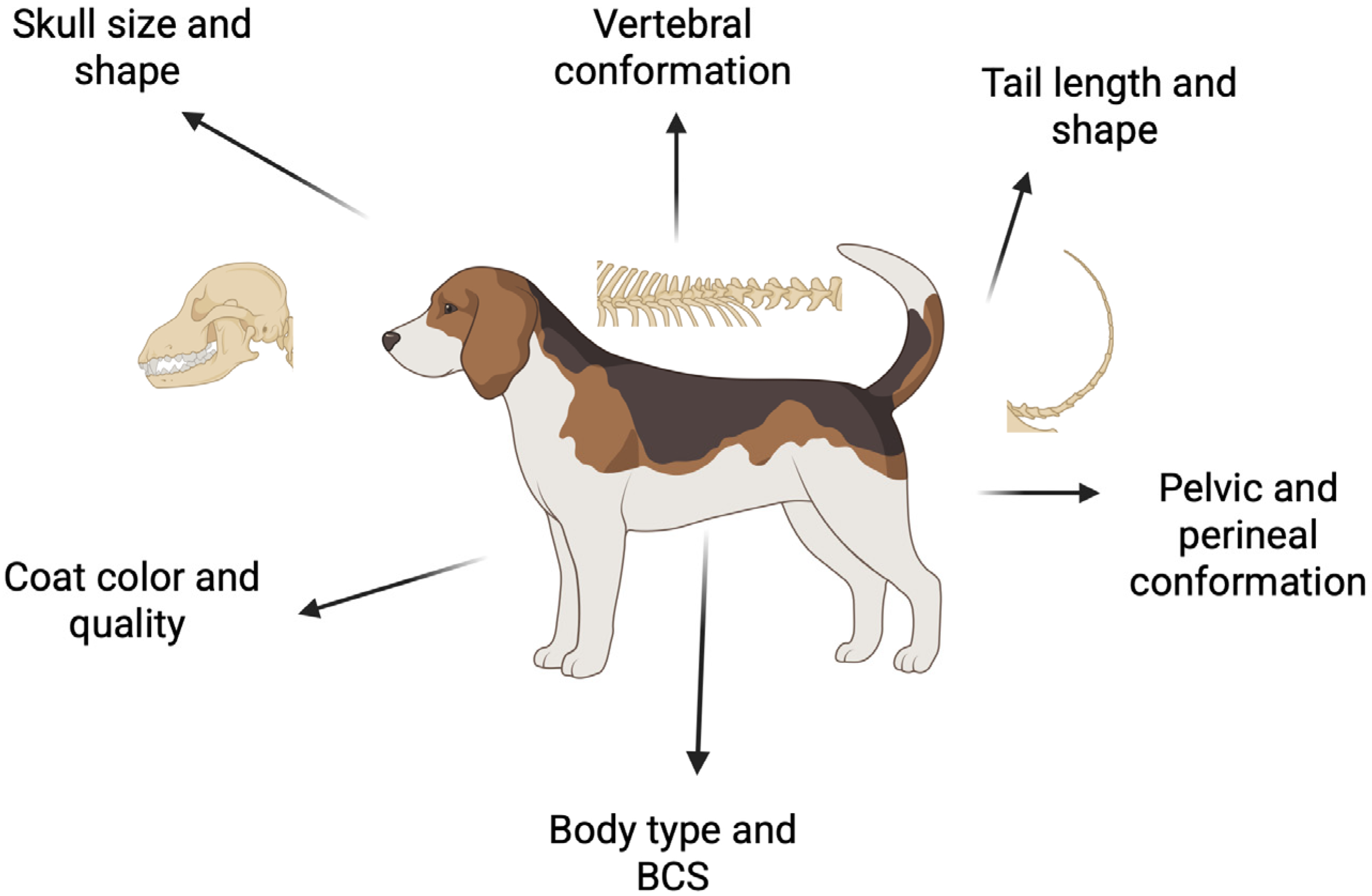
3. Morphologic Characteristics
3.1. Body Type
3.2. Body Condition Score
3.3. Coat Color and Quality
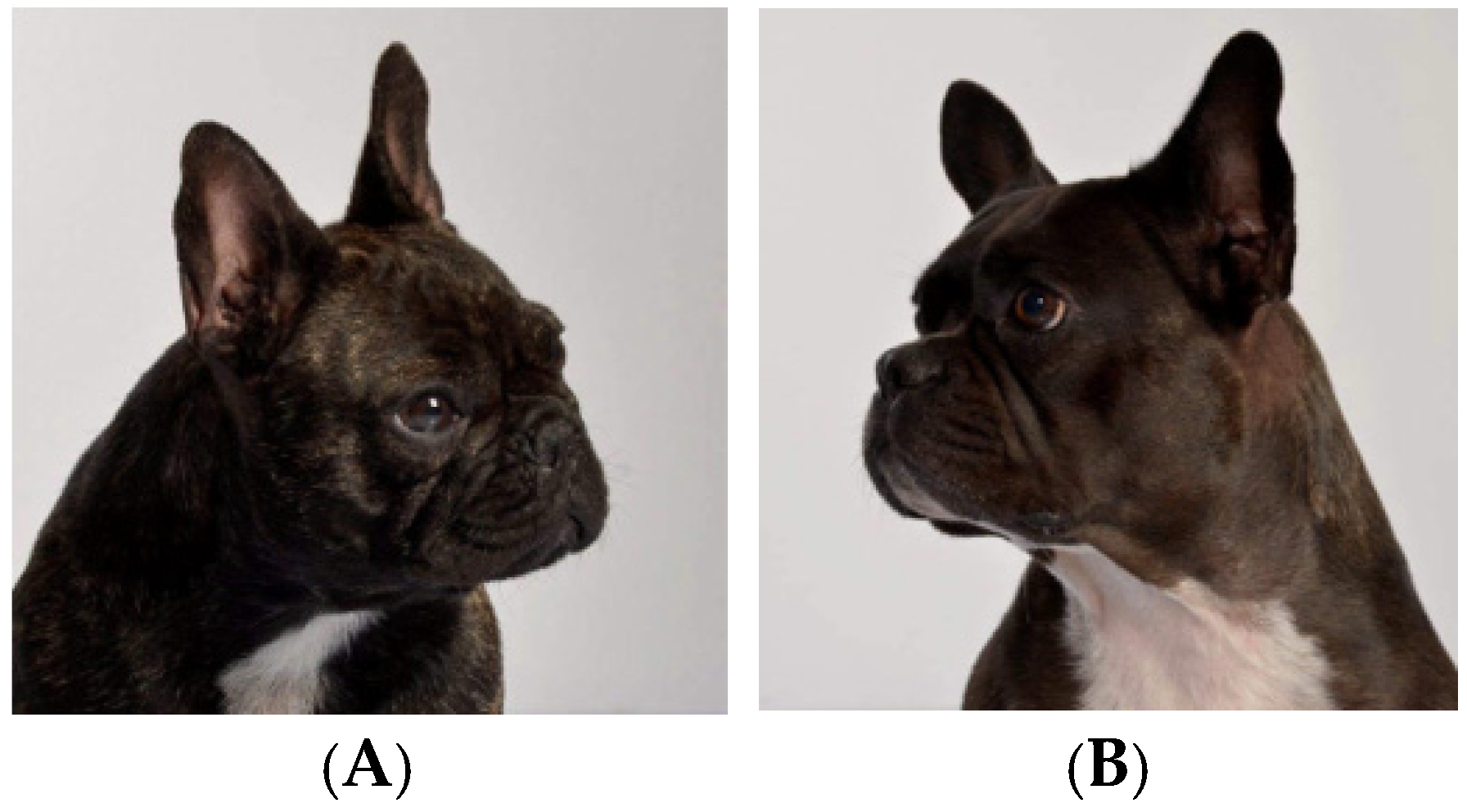
3.4. Skull Size and Shape
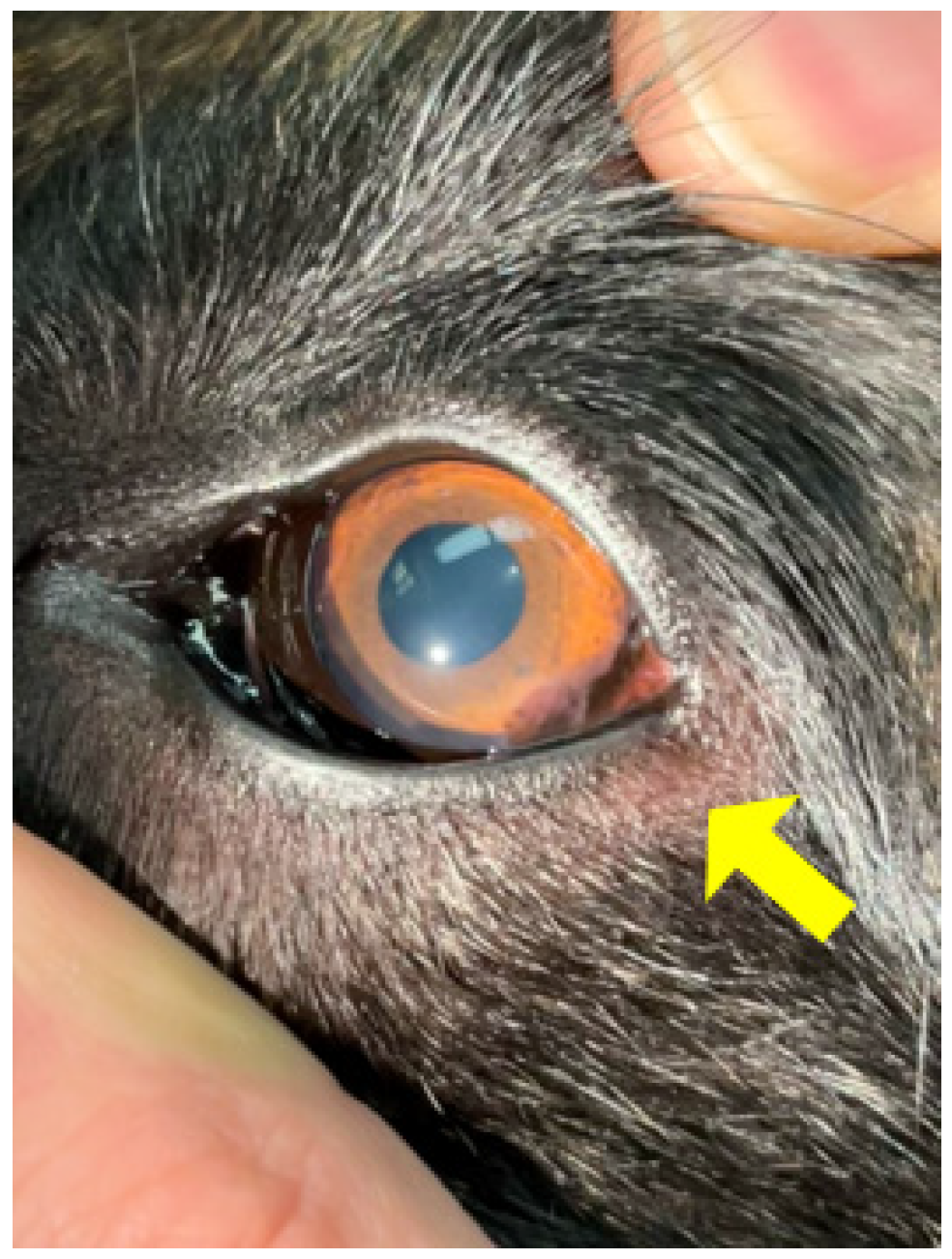
3.5. Ocular Conditions
3.6. Vertebral Conformation
3.7. Tail Length and Shape
3.8. Pelvic Conformation and Pelvimetry
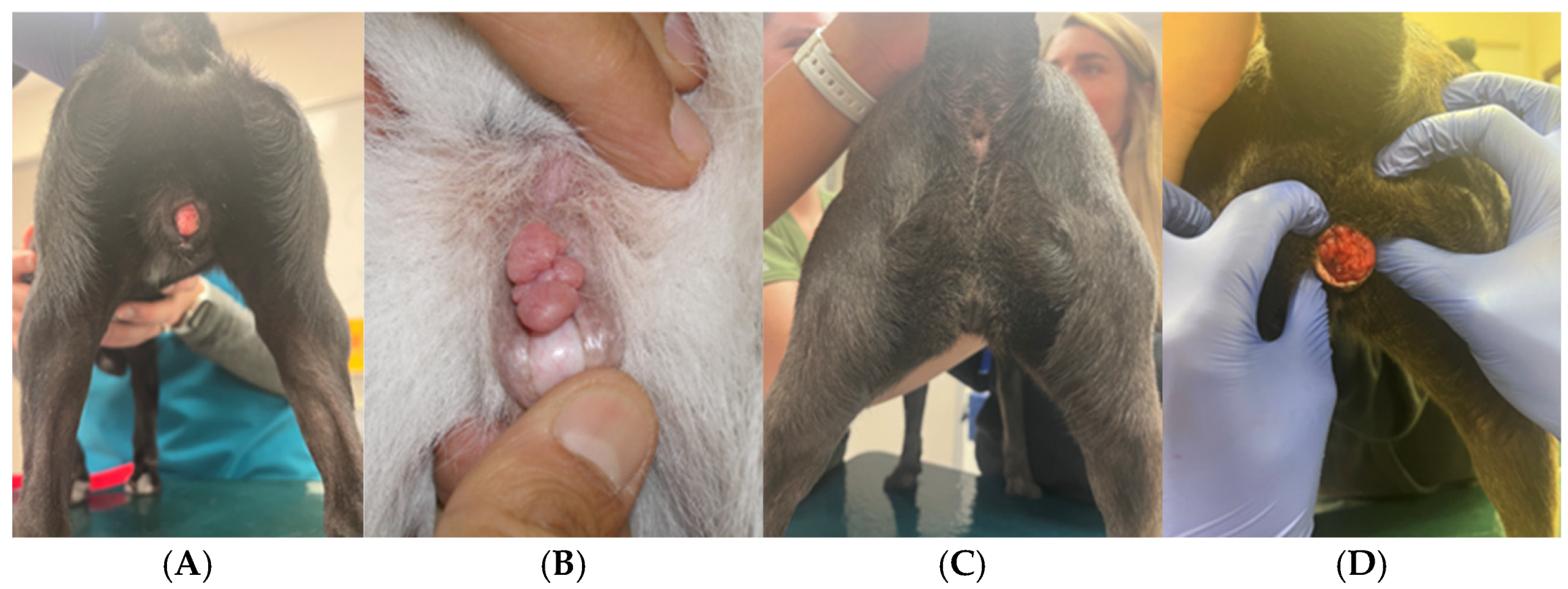
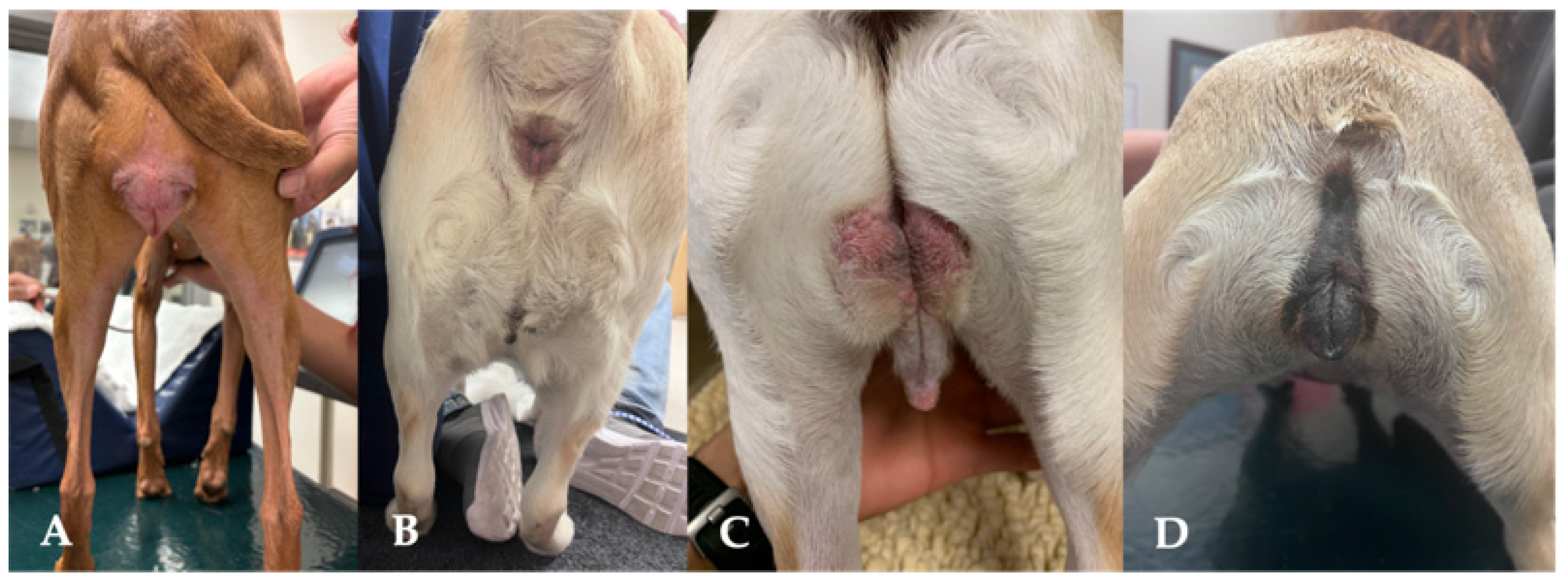
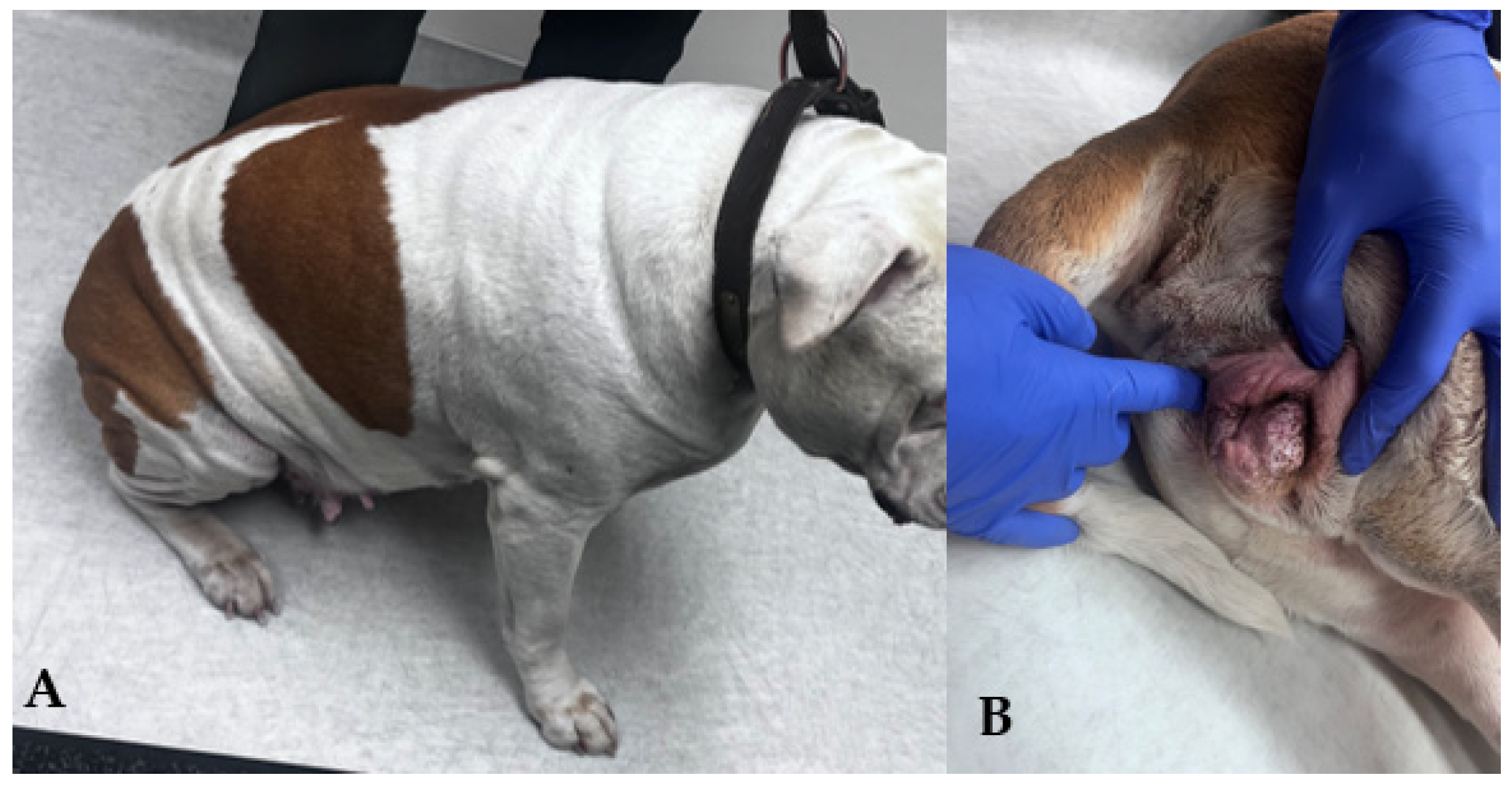
3.9. Perineal Conformation
3.10. Mammary Gland Conformation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSE | Breeding Soundness Examination |
| FCI | Fédération Cynologique Internationale |
| BCS | Body Condition Score |
| OFA | Orthopedic Foundation for Animals |
| CHIC | Canine Health Information Center |
| CAER | Companion Animal Eye Registry |
| DSD | Disorders of Sexual Differentiation |
| B. canis | Brucella canis |
| C-section | Cesarean section |
| GnRH | Gonadotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| fT4 | Free thyroxine |
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| PRDM | Progesterone-Related Diabetes Mellitus |
| IR | Insulin Resistance |
| DM | Diabetes Mellitus |
| GH | Growth Hormone |
| BOAS | Brachycephalic Obstructive Airway Syndrome |
| RFGS | Respiratory Function Grading Scheme |
| IVDD | Intervertebral Disc Disease |
| FGF4L2 | Fibroblast growth factor-4 |
| BAER | Brainstem Auditory-Evoked Response |
| DS | Dermoid Sinus |
| CSK | Chronic Superficial Keratitis |
References
- Menor-Campos, D.J. Ethical Concerns about Fashionable Dog Breeding. Animals 2024, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Wright, D. The Genetic Architecture of Domestication in Animals. Bioinform. Biol. Insights 2015, 9, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.B.; Evans, J.M.; Parker, H.G.; Kim, J.; Pearce-Kelling, S.; Whitaker, D.T.; Plassais, J.; Khan, Q.M.; Ostrander, E.A. Genetic analysis of the modern Australian labradoodle dog breed reveals an excess of the poodle genome. PLoS Genet. 2020, 16, e1008956. [Google Scholar] [CrossRef]
- Barstow, C.; Wilborn, R.R.; Johnson, A.K. Breeding soundness examination of the bitch. Vet. Clin. Small Anim. Pract. 2018, 48, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, E.S.; Fousse, S.L.; Crofton, A.E.; Hodge, T.E.; Gunther-Harrington, C.T.; Visser, L.C.; Stern, J.A. Congenital cardiac outflow tract abnormalities in dogs: Prevalence and pattern of inheritance from 2008 to 2017. Front. Vet. Sci. 2019, 6, 52. [Google Scholar] [CrossRef]
- Sruthi, S.; Prasanna, K.; George, A.; Sajitha, I.; Sudheesh, S.; Varuna, P.; Bharathi, R. Mammary Tumors in dogs: Age, Breed, Gender, Rearing, and Diet Factors. Indian Vet. J. 2024, 101, 34–38. [Google Scholar] [CrossRef]
- Moxon, R.; England, G.C.W. Fertility and whelping complications in bitches following correction of vaginal abnormalities. Vet. Rec. 2011, 168, 642. [Google Scholar] [CrossRef]
- Poth, T.; Breuer, W.; Walter, B.; Hecht, W.; Hermanns, W. Disorders of sex development in the dog—Adoption of a new nomenclature and reclassification of reported cases. Anim. Reprod. Sci. 2010, 121, 197–207. [Google Scholar] [CrossRef]
- Cosford, K.L. Brucella canis: An update on research and clinical management. Can. Vet. J. 2018, 59, 74. [Google Scholar]
- Fiszdon, K.; Kowalczyk, I. Litter size, puppy weight at birth and growth rates in different breeds of dogs. Ann. Waseda Univ. Life Sci. 2009, 46, 161–168. [Google Scholar]
- Loughin, C.A. Chiari-like malformation. Vet. Clin. Small Anim. Pract. 2016, 46, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, A.M.; Rusbridge, C.; Lappalainen, A.K.; Junnila, J.J.T.; Jokinen, T.S. Persistent fontanelles in Chihuahuas. Part I. Distribution and clinical relevance. J. Vet. Intern. Med. 2021, 35, 1834–1847. [Google Scholar] [CrossRef]
- Borge, K.S.; Tønnessen, R.; Nødtvedt, A.; Indrebø, A. Litter size at birth in purebred dogs—A retrospective study of 224 breeds. Theriogenology 2011, 75, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; Nødtvedt, A.; Lagerstedt, A.-S.; Egenvall, A. Incidence and Breed Predilection for Dystocia and Risk Factors for Cesarean Section in a Swedish Population of Insured Dogs. Vet. Surg. 2006, 35, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Axnér, E.; Rasmus, L.S.; Melangen, T. Factors affecting reproductive performance in the Swedish Bernese mountain dog. Acta Vet. Scand. 2022, 64, 28. [Google Scholar] [CrossRef]
- Galis, F.; Van Der Sluijs, I.; Van Dooren, T.J.M.; Metz, J.A.J.; Nussbaumer, M. Do large dogs die young? J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 119–126. [Google Scholar] [CrossRef]
- McMillan, K.M.; Bielby, J.; Williams, C.L.; Upjohn, M.M.; Casey, R.A.; Christley, R.M. Longevity of companion dog breeds: Those at risk from early death. Sci. Rep. 2024, 14, 531. [Google Scholar] [CrossRef]
- Sones, J.; Balogh, O. Body condition and fertility in dogs. Vet. Clin. Small Anim. Pract. 2023, 53, 1031–1045. [Google Scholar] [CrossRef]
- Gobello, C. Prepubertal and Pubertal Canine Reproductive Studies: Conflicting Aspects. Reprod. Domest. Anim. 2014, 49, e70–e73. [Google Scholar] [CrossRef]
- Chiang, C.-F.; Villaverde, C.; Chang, W.-C.; Fascetti, A.J.; Larsen, J.A. Prevalence, risk factors, and disease associations of overweight and obesity in dogs that visited the veterinary medical teaching hospital at the University of California, Davis from January 2006 to December 2015. Top. Companion Anim. Med. 2022, 48, 100640. [Google Scholar] [CrossRef]
- Marchi, P.H.; Vendramini, T.H.A.; Perini, M.P.; Zafalon, R.V.A.; Amaral, A.R.; Ochamotto, V.A.; Da Silveira, J.C.; Dagli, M.L.Z.; Brunetto, M.A. Obesity, inflammation, and cancer in dogs: Review and perspectives. Front. Vet. Sci. 2022, 9, 1004122. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.T. Canine hypothyroidism: A review of aetiology and diagnosis. N. Z. Vet. J. 2011, 59, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A. Thyroid issues in reproduction. Clin. Tech. Small Anim. Pract. 2002, 17, 129–132. [Google Scholar] [CrossRef]
- Panciera, D.L. Conditions associated with canine hypothyroidism. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 935–950. [Google Scholar]
- Wilborn, R.R.; Maxwell, H.S. Clinical approaches to infertility in the bitch. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 457–468, v. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid hormones and female reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef]
- Rao, M.; Yang, Z.; Su, C.; Zhao, Z.; Wan, R.; Liu, J.; Yao, Y.; Su, Z.; Wang, K.; Tang, L.; et al. Paternal Subclinical Hypothyroidism Affects the Clinical Outcomes of In Vitro Fertilization/Intracytoplasmic Sperm Injection. Thyroid 2021, 31, 12–22. [Google Scholar] [CrossRef]
- Antonov, A. Pregnancy toxaemia in a French bulldog due to extremely large fetal number: A case report. Bulg. J. Vet. Med. 2023, 1, 128–132. [Google Scholar] [CrossRef]
- Nak, D.; Nak, Y.; Shahzad, A. Pregnancy toxemia in a golden retriever bitch, a case report. Adv. Anim. Vet. Sci. 2020, 8, 1184–1187. [Google Scholar] [CrossRef]
- Mongini, A.; Van Saun, R.J. Pregnancy toxemia in sheep and goats. Vet. Clin. Food Anim. Pract. 2023, 39, 275–291. [Google Scholar] [CrossRef]
- Fall, T.; Hedhammar, A.; Wallberg, A.; Fall, N.; Ahlgren, K.M.; Hamlin, H.H.; Lindblad-Toh, K.; Andersson, G.; Kämpe, O. Diabetes mellitus in elkhounds is associated with diestrus and pregnancy. J. Vet. Intern. Med. 2010, 24, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Fall, T.; Johansson Kreuger, S.; Juberget, A.; Bergström, A.; Hedhammar, A. Gestational diabetes mellitus in 13 dogs. J. Vet. Intern. Med. 2008, 22, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Pöppl, A.G.; Mottin, T.S.; González, F.H. Diabetes mellitus remission after resolution of inflammatory and progesterone-related conditions in bitches. Res. Vet. Sci. 2013, 94, 471–473. [Google Scholar] [CrossRef]
- Johnson, C.A. High-risk pregnancy and hypoluteoidism in the bitch. Theriogenology 2008, 70, 1424–1430. [Google Scholar] [CrossRef]
- Johnson, C.A. Glucose homeostasis during canine pregnancy: Insulin resistance, ketosis, and hypoglycemia. Theriogenology 2008, 70, 1418–1423. [Google Scholar] [CrossRef]
- Palerme, J.-S.; Zellner, E.; Leonard, S.; Viall, A.K.; Berger, D.J. Characterization of recessed vulvas in dogs. J. Am. Vet. Med. Assoc. 2021, 259, 744–748. [Google Scholar] [CrossRef]
- Pöppl, Á.G.; Lopes, J.L.X.; Nogueira, T.B.; da Silva, D.I.; Machado, B.d.S. Progesterone-related diabetes mellitus in the bitch: Current knowledge, the role of Pyometra, and relevance in practice. Animals 2024, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Saif, R.; Iftekhar, A.; Asif, F.; Alghanem, M.S. Dog coat colour genetics: A review. Adv. Life Sci. 2020, 7, 215–224. [Google Scholar] [CrossRef]
- Charon, K.M.; Lipka, K.R. The effect of a coat colour-associated genes polymorphism on animal health—A review. Ann. Anim. Sci. 2015, 15, 3. [Google Scholar] [CrossRef]
- Brancalion, L.; Haase, B.; Wade, C.M. Canine coat pigmentation genetics: A review. Anim. Genet. 2022, 53, 3–34. [Google Scholar] [CrossRef]
- Strain, G.M. The Genetics of Deafness in Domestic Animals. Front. Vet. Sci. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Varga, L.; Lénárt, X.; Zenke, P.; Orbán, L.; Hudák, P.; Ninausz, N.; Pelles, Z.; Szőke, A. Being Merle: The Molecular Genetic Background of the Canine Merle Mutation. Genes 2020, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Asher, L.; Diesel, G.; Summers, J.F.; McGreevy, P.D.; Collins, L.M. Inherited defects in pedigree dogs. Part 1: Disorders related to breed standards. Vet. J. 2009, 182, 402–411. [Google Scholar] [CrossRef]
- Bannasch, D.; Safra, N.; Young, A.; Karmi, N.; Schaible, R.; Ling, G. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet. 2008, 4, e1000246. [Google Scholar] [CrossRef] [PubMed]
- Trimble, H.; Keeler, C. The Inheritance of ’ High Uric Acid Excretion ’in Dogs. J. Hered. 1938, 29, 281–289. [Google Scholar] [CrossRef]
- Sampson, E. Overview of Backcross Project: Normal Uric Acid in Dalmatians. 2011. Available online: https://www.vettimes.co.uk/app/uploads/wp-post-to-pdf-enhanced-cache/1/overview-of-backcross-project-normal-uric-acid-in-dalmatians.pdf (accessed on 8 July 2025).
- Laukner, A.; Truchet, L.; Manukjan, G.; Schulze, H.; Langbein-Detsch, I.; Mueller, E.; Leeb, T.; Kehl, A. Effects of Cocoa Genotypes on Coat Color, Platelets and Coagulation Parameters in French Bulldogs. Genes 2021, 12, 1092. [Google Scholar] [CrossRef]
- Davis, N. First UK Hairless French Bulldog Litter Prompts ‘Extreme Breeding’ Concerns. The Guardian. 2022. Available online: https://www.proquest.com/newspapers/first-uk-hairless-french-bulldog-litter-prompts/docview/2623317516/se-2?accountid=8421 (accessed on 8 July 2025).
- Hillbertz, N.H. Inheritance of dermoid sinus in the Rhodesian ridgeback. J. Small Anim. Pract. 2005, 46, 71–74. [Google Scholar] [CrossRef]
- Bell, J.S.; Cavanagh, K.E.; Tiley, L.P.; Smith, F.W.K. Veterinary Medical Guide to Dog and Cat Breeds; Teton News Media Jackson: Jackson, WY, USA, 2012. [Google Scholar]
- Fatone, G.; Brunetti, A.; Lamagna, F.; Potena, A. Dermoid sinus and spinal malformations in a Yorkshire terrier: Diagnosis and follow-up. J. Small Anim. Pract. 1995, 36, 178–180. [Google Scholar] [CrossRef]
- Cornegliani, L.; Jommi, E.; Vercelli, A. Dermoid sinus in a golden retriever. J. Small Anim. Pract. 2001, 42, 514–516. [Google Scholar] [CrossRef]
- Booth, M.J. Atypical dermoid sinus in a chow chow dog. J. S. Afr. Vet. Assoc. 1998, 69, 102–104. [Google Scholar] [CrossRef]
- Distl, O. Prevalence and segregation analysis of dermoid sinus in Rhodesian Ridgebacks. Vet. J. 2022, 280, 105803. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.; Bengtsson, F.; Killander, J.; Franco, F.; Lundeheim, N.; Varga, C.; Båge, R.; Morrell, J.M. Fiber quality and fertility in male alpacas in the Cusco region of Peru. Front. Vet. Sci. 2024, 11, 1421593. [Google Scholar] [CrossRef] [PubMed]
- Ekenstedt, K.J.; Crosse, K.R.; Risselada, M. Canine Brachycephaly: Anatomy, Pathology, Genetics and Welfare. J. Comp. Pathol. 2020, 176, 109–115. [Google Scholar] [CrossRef]
- Mitze, S.; Barrs, V.R.; Beatty, J.A.; Hobi, S.; Bęczkowski, P.M. Brachycephalic obstructive airway syndrome: Much more than a surgical problem. Vet. Q. 2022, 42, 213–223. [Google Scholar] [CrossRef]
- Sebbag, L.; Sanchez, R.F. The pandemic of ocular surface disease in brachycephalic dogs: The brachycephalic ocular syndrome. Vet. Ophthalmol. 2023, 26, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, P.S.; Mach, R.; Nolte, I.; Freise, F.; Levicar, C.; Merhof, K.; Bach, J.-P. Breed-specific values for vertebral heart score (VHS), vertebral left atrial size (VLAS), and radiographic left atrial dimension (RLAD) in pugs without cardiac disease, and their relationship to Brachycephalic Obstructive Airway Syndrome (BOAS). PLoS ONE 2022, 17, e0274085. [Google Scholar] [CrossRef]
- Hobi, S.; Barrs, V.R.; Bęczkowski, P.M. Dermatological Problems of Brachycephalic Dogs. Animals 2023, 13, 2016. [Google Scholar] [CrossRef]
- Kriss, R. Most Popular Dog Breeds. American Kennel Club. Available online: https://www.akc.org/most-popular-breeds/ (accessed on 8 July 2025).
- Mills, G. French bulldogs now the UK’s top breed. Vet. Rec. 2018, 182, 705. [Google Scholar]
- Fasanella, F.J.; Shivley, J.M.; Wardlaw, J.L.; Givaruangsawat, S. Brachycephalic airway obstructive syndrome in dogs: 90 cases (1991–2008). J. Am. Vet. Med. Assoc. 2010, 237, 1048–1051. [Google Scholar] [CrossRef]
- Packer, R.; Hendricks, A.; Burn, C. Do dog owners perceive the clinical signs related to conformational inherited disorders as ‘normal’for the breed? A potential constraint to improving canine welfare. Anim. Welf. 2012, 21, 81–93. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Pooch, A.S.; Liu, H. A genetic assessment of the English bulldog. Canine Genet. Epidemiol. 2016, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lederhouse, C. Health Screening Test Rolled Out for Brachycephalic Dog Breeds. Available online: https://www.avma.org/news/health-screening-test-rolled-out-brachycephalic-dog-breeds (accessed on 8 July 2025).
- Moon, P.F.; Erb, H.N.; Ludders, J.W.; Gleed, R.D.; Pascoe, P.J. Perioperative management and mortality rates of dogs undergoing cesarean section in the United States and Canada. J. Am. Vet. Med. Assoc. 1998, 213, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Vullo, C.; Meligrana, M.C.T.; Tambella, A.M.; Dini, F.; Palumbo Piccionello, A.; Catone, G. Anesthetic management during cesarean section in English bulldogs. J. Life Sci. 2014, 8, 58–64. [Google Scholar]
- Palanova, A. Collie eye anomaly: A review. Vet. Med. 2015, 60, 345–350. [Google Scholar] [CrossRef]
- MacMillan, A.; Lipton, D. Heritability of multifocal retinal dysplasia in American Cocker Spaniels. J. Am. Vet. Med. Assoc. 1978, 172, 568–572. [Google Scholar] [CrossRef]
- Iwabe, S.; Dufour, V.L.; Guzmán, J.M.; Holle, D.M.; Cohen, J.A.; Beltran, W.A.; Aguirre, G.D. Focal/multifocal and geographic retinal dysplasia in the dog—In vivo retinal microanatomy analyses. Vet. Ophthalmol. 2020, 23, 292–304. [Google Scholar] [CrossRef]
- Rodarte-Almeida, A.C.V.; Petersen-Jones, S.; Langohr, I.M.; Occelli, L.; Dornbusch, P.T.; Shiokawa, N.; Montiani-Ferreira, F. Retinal dysplasia in American pit bull terriers–Phenotypic characterization and breeding study. Vet. Ophthalmol. 2016, 19, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.C.; Meyer-Lindenberg, A. Progression and complications of canine cataracts for different stages of development and aetiologies. J. Small Anim. Pract. 2018, 59, 616–624. [Google Scholar] [CrossRef]
- Shastry, B.S. Persistent hyperplastic primary vitreous: Congenital malformation of the eye. Clin. Experiment. Ophthalmol. 2009, 37, 884–890. [Google Scholar] [CrossRef]
- Bjerkås, E.; Ekesten, B.; Farstad, W. Pectinate ligament dysplasia and narrowing of the iridocorneal angle associated with glaucoma in the English Springer Spaniel. Vet. Ophthalmol. 2002, 5, 49–54. [Google Scholar] [CrossRef]
- Ali, K.M.; Mostafa, A.A. Lens-related ocular emergencies (LROE) in dogs: Treatment and visual outcome after late presentation of 90 eyes. Ir. Vet. J. 2023, 76, 12. [Google Scholar] [CrossRef]
- Clements, P.J.M.; Sargan, D.R.; Gould, D.J.; Petersen-Jones, S.M. Recent advances in understanding the spectrum of canine generalised progressive retinal atrophy. J. Small Anim. Pract. 1996, 37, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, R.; Cabral, L.; Gooch, L.; Bedford, P.; Boulton, M. Retinal pigment epithelial dystrophy in Briard dogs. Res. Vet. Sci. 1996, 60, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Karamatic, S.; Goode, R.; Bageswaran, N.; Willet, C.E.; Samaha, G.; Ferguson, R.; Mazrier, H.; Wade, C.M. Genome-Wide Association Analysis for Chronic Superficial Keratitis in the Australian Racing Greyhound. Genes 2022, 13, 1328. [Google Scholar] [CrossRef] [PubMed]
- Lackmann, F.; Forterre, F.; Brunnberg, L.; Loderstedt, S. Epidemiological study of congenital malformations of the vertebral column in French bulldogs, English bulldogs and pugs. Vet. Rec. 2022, 190, e509. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; ter Haar, G.; De Decker, S. Congenital malformations of the lumbosacral vertebral column are common in neurologically normal French Bulldogs, English Bulldogs, and Pugs, with breed-specific differences. Vet. Radiol. Ultrasound 2019, 60, 400–408. [Google Scholar] [CrossRef]
- Volta, A.; Gnudi, G.; Morgan, J.P.; Bonazzi, M.; Manfredi, S.; Bottarelli, E.; Zanichelli, S.; Bertoni, G. Radiographic features of pelvis and hip joint development of English Bulldogs. Vet. Comp. Orthop. Traumatol. 2010, 23, 19–27. [Google Scholar] [CrossRef]
- Sullivan, S.; Redden, D.; Hardeng, F.; Sundqvist, M.; Kutzler, M. The relationship between radiographic disc calcification score and FGF4L2 genotype in dachshunds. J. Vet. Intern. Med. 2025, 39, e17281. [Google Scholar] [CrossRef]
- Haworth, K.; Putt, W.; Cattanach, B.; Breen, M.; Binns, M.; Lingaas, F.; Edwards, Y.H. Canine homolog of the T-box transcription factor T; failure of the protein to bind to its DNA target leads to a short-tail phenotype. Mamm. Genome 2001, 12, 212–218. [Google Scholar] [CrossRef]
- Indrebø, A.; Langeland, M.; Juul, H.M.; Skogmo, H.K.; Rengmark, A.H.; Lingaas, F. A study of inherited short tail and taillessness in Pembroke Welsh corgi. J. Small Anim. Pract. 2008, 49, 220–224. [Google Scholar] [CrossRef]
- Hytönen, M.K.; Grall, A.; Hédan, B.; Dréano, S.; Seguin, S.J.; Delattre, D.; Thomas, A.; Galibert, F.; Paulin, L.; Lohi, H.; et al. Ancestral T-Box Mutation Is Present in Many, but Not All, Short-Tailed Dog Breeds. J. Hered. 2008, 100, 236–240. [Google Scholar] [CrossRef]
- Niskanen, J.E.; Reunanen, V.; Salonen, M.; Bannasch, D.; Lappalainen, A.K.; Lohi, H.; Hytönen, M.K. Canine DVL2 variant contributes to brachycephalic phenotype and caudal vertebral anomalies. Hum. Genet. 2021, 140, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- United Kennel Club. Offical Standard of the Dachshund. 2018. Available online: https://www.ukcdogs.com/dachshund (accessed on 8 July 2025).
- Schlensker, E.; Distl, O. Heritability of hemivertebrae in the French bulldog using an animal threshold model. Vet. J. 2016, 207, 188–189. [Google Scholar] [CrossRef]
- Eneroth, A.; Linde-Forsberg, C.; Uhlhorn, M.; Hall, M. Radiographic pelvimetry for assessment of dystocia in bitches: A clinical study in two terrier breeds. J. Small Anim. Pract. 1999, 40, 257–264. [Google Scholar] [CrossRef]
- Linde-Forsberg, C. Pelvimetry to Diagnose Dystocia in the Bitch. 2003. Available online: https://www.vin.com/apputil/content/defaultadv1.aspx?id=3850086&pid=8768 (accessed on 8 July 2025).
- Dascanio, J.J. Perineal Conformation Evaluation. In Equine Reproductive Procedures; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 17–19. [Google Scholar]
- Mathews, K.G. Surgery of the canine vagina and vulva. Vet. Clin. Small Anim. Pract. 2001, 31, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.A. The anatomy of the mammary gland of the dog and cat. J. Small Anim. Pract. 1966, 7, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, J.W.; de Lahunta, A. Miller’s Anataomy of the Dog; Elsevier: Amsterday, The Netherlands, 2013. [Google Scholar]
- Morrison, W.B. Canine and feline mammary tumors. In Cancer in Dogs and Cats Medical and Surgical Management; Morrison, W.B., Ed.; Teton NewMedia Jackson: Jackson, WY, USA, 2002; p. 565. [Google Scholar]
- Earnhardt-San, A.L.; Gray, K.A.; Knauer, M.T. Genetic parameter estimates for teat and mammary traits in commercial sows. Animals 2023, 13, 2400. [Google Scholar] [CrossRef]
- Dal Zotto, R.; De Marchi, M.; Dalvit, C.; Cassandro, M.; Gallo, L.; Carnier, P.; Bittante, G. Heritabilities and Genetic Correlations of Body Condition Score and Calving Interval with Yield, Somatic Cell Score, and Linear Type Traits in Brown Swiss Cattle. J. Dairy Sci. 2007, 90, 5737–5743. [Google Scholar] [CrossRef]




| Increased Risk for Diabetes Mellitus | Decreased Risk of Diabetes Mellitus |
|---|---|
| Australian Terrier | Airedale Terrier |
| Bichon Frise | Basset Hound |
| Border Collie | Beagle |
| Border Terrier | Boston Terrier |
| Cairn Terrier | Boxer |
| Cavalier King Charles Spaniel | Brittany Spaniel |
| English Setter | Bulldog |
| English Springer Spaniel | Cocker Spaniel |
| Finish Spitz | Collie |
| Fox Terrier | Dalmatian |
| Irish Setter | Doberman Pinscher |
| Keeshond | English Pointer |
| Miniature and Standard Schnauzer | English Setter |
| Poodle | German Shepherd Dog |
| Samoyed | German Short-Hair Pointer |
| Siberian Husky | Golden Retriever |
| Swedish Elkhound | Great Dane |
| Swedish Lapphund | Greyhound |
| Tibetan Terrier | Irish Setter |
| West Highlander White Terrier | Norwegian Elkhound |
| Yorkshire Terrier | Old English Sheepdog |
| Severity of Tail Malformations | Number of Nonmalformed Caudal Vertebra |
|---|---|
| Nonmalformed | No malformed caudal vertebra |
| Minimal malformations | >4 nonmalformed caudal vertebra |
| Moderate malformations | 2–3 nonmalformed caudal vertebra |
| Severe malformations | 0–1 nonmalformed caudal vertebra |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, D.W.; Kvernum, J.; Macias, N.; Waqas, M.S.; Ciccarelli, M. Incorporating Morphological Evaluations into Breeding Soundness Examinations for Female Dogs. Animals 2025, 15, 2045. https://doi.org/10.3390/ani15142045
Schwartz DW, Kvernum J, Macias N, Waqas MS, Ciccarelli M. Incorporating Morphological Evaluations into Breeding Soundness Examinations for Female Dogs. Animals. 2025; 15(14):2045. https://doi.org/10.3390/ani15142045
Chicago/Turabian StyleSchwartz, Dane Wells, Jonah Kvernum, Naomie Macias, Muhammed Salman Waqas, and Michela Ciccarelli. 2025. "Incorporating Morphological Evaluations into Breeding Soundness Examinations for Female Dogs" Animals 15, no. 14: 2045. https://doi.org/10.3390/ani15142045
APA StyleSchwartz, D. W., Kvernum, J., Macias, N., Waqas, M. S., & Ciccarelli, M. (2025). Incorporating Morphological Evaluations into Breeding Soundness Examinations for Female Dogs. Animals, 15(14), 2045. https://doi.org/10.3390/ani15142045






