Lateralised Behavioural Responses of Chickens to a Threatening Human and a Novel Environment Indicate Fearful Emotions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Birds and Management
2.3. Behavioural Assessment
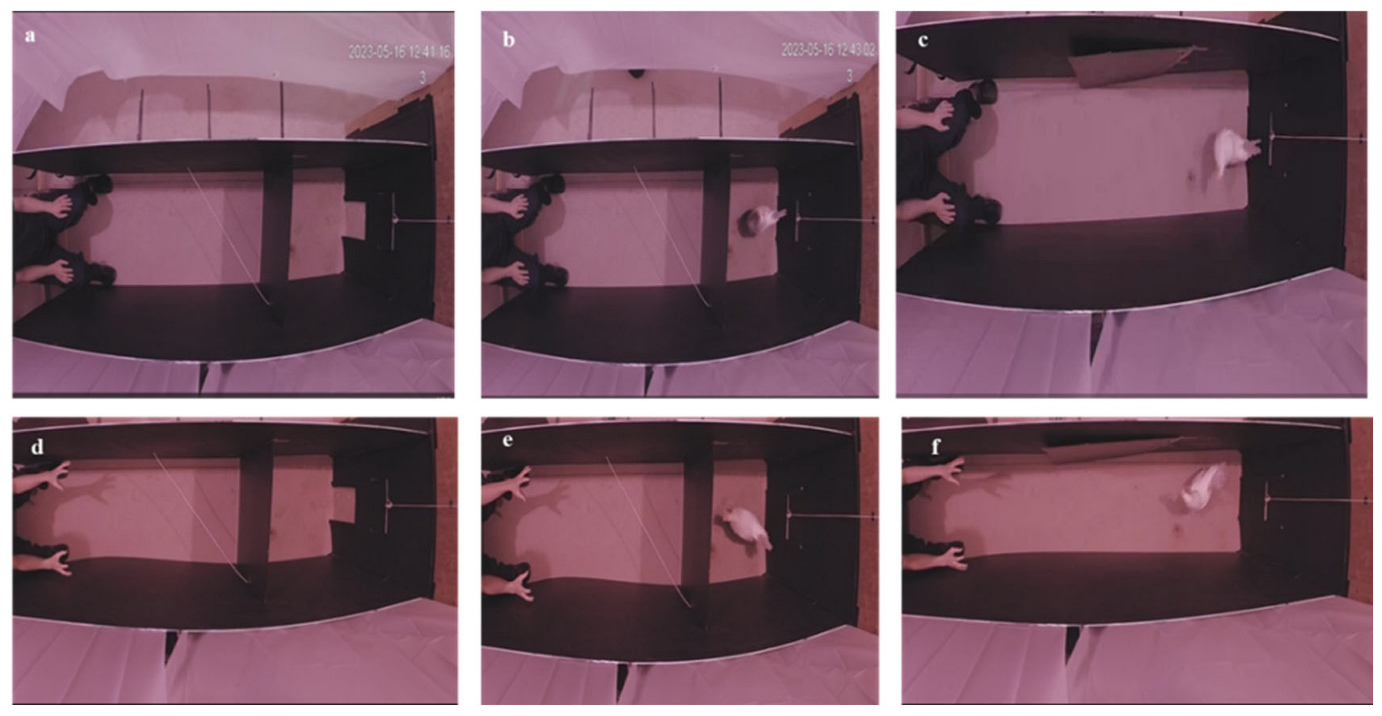
2.4. Arena and Adjacent Box
2.5. Familiarisation to the Arena Test
2.6. Human Condition Test
2.7. Statistical Analysis
2.7.1. Familiarisation to the Arena Test
Behaviour in the Box
Behaviour in the Arena
2.7.2. Human Condition Test
3. Results
3.1. Familiarisation to the Arena Test
3.1.1. Differences Between Birds’ Behaviours in the Box and the Arena
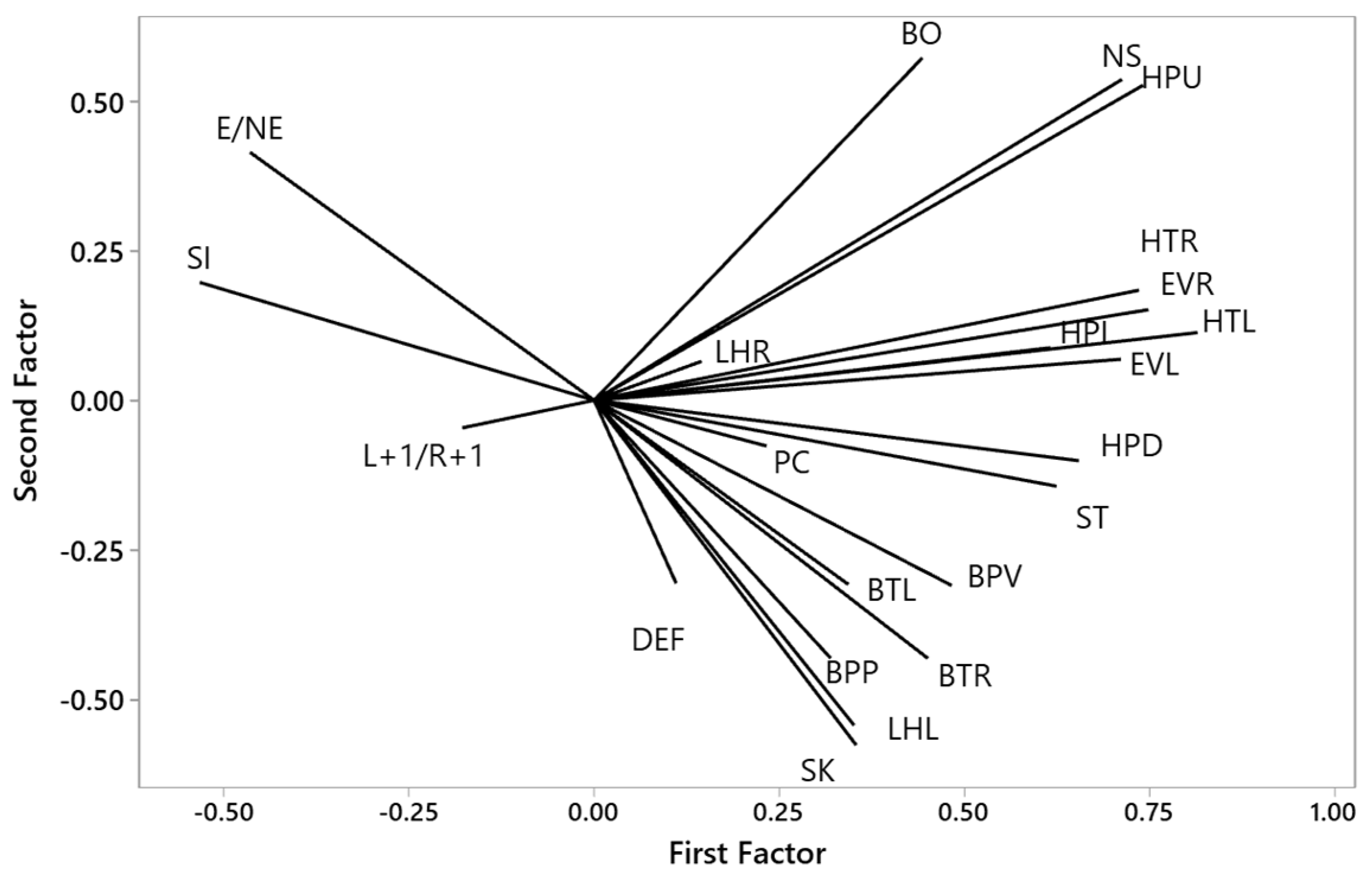
3.1.2. Principal Components of the Birds’ Behaviour in the Box
3.1.3. Behaviours Connected with Left Eye View in the Box
3.1.4. Principal Components of Birds’ Behaviour in the Arena
3.1.5. Behaviours Connected with Left Eye View in the Arena
3.2. Human Condition Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Component 1 | Component 2 | Component 3 | Component 4 |
|---|---|---|---|---|
| Emergence condition | 0.415 | |||
| Eye view left | 0.712 | |||
| Eye view right | 0.749 | |||
| L+1/R+1 | 0.664 | |||
| Level head | 0.617 | |||
| Head up | 0.742 | |||
| Head down | 0.655 | |||
| Neck stretch | 0.713 | |||
| Vocalisation | 0.573 | |||
| Head turn left | 0.816 | |||
| Head turn right | 0.737 | |||
| Body turn left | 0.344 | |||
| Body turn right | 0.451 | |||
| Body position vertical | 0.483 | |||
| Body position perpendicular | 0.320 | |||
| Left leg held | 0.523 | |||
| Standing | 0.625 | |||
| Sitting | 0.402 | |||
| Shaking | 0.529 | |||
| Eigen value | 6.260 | 2.519 | 1.958 | 1.767 |
| Proportion (%) of r2 | 0.285 (28.5) | 0.114 (11.4) | 0.089 (8.9) | 0.080 (8) |
Appendix B
| Variable | Component 1 | Component 3 | Component 4 | Component 5 |
|---|---|---|---|---|
| Eye view emerge | 0.560 | |||
| Eye view left | 0.800 | |||
| Eye view right | 0.646 | |||
| Level head | 0.512 | |||
| Head up | 0.477 | |||
| Head down | 0.510 | |||
| Neck stretch | 0.739 | |||
| Vocalisation | 0.749 | |||
| Head turn left | 0.586 | |||
| Head turn right | 0.518 | |||
| Body turn left | 0.821 | |||
| Body turn right | 0.667 | |||
| Body position vertical | 0.878 | |||
| Body position perpendicular | 0.650 | |||
| Left leg held | 0.764 | |||
| Right leg held | 0.673 | |||
| Location left | 0.751 | |||
| Location right | 0.492 | |||
| Location centre | 0.749 | |||
| Standing | 0.632 | |||
| Sitting | 0.452 | |||
| Pecking | 0.439 | |||
| Tail fanning | 0.480 | |||
| Eigen value | 7.251 | 2.143 | 1.903 | 1.747 |
| Proportion (%) of r2 | 0.259 (25.9) | 0.077 (7.7) | 0.068 (6.8) | 0.062 (6.2) |
References
- Fraser, D. Understanding animal welfare. Acta Vet. Scand. 2008, 50, S1. [Google Scholar] [CrossRef] [PubMed]
- Vasdal, G.; Moe, R.O.; de Jong, I.C.; Granquist, E.G. The relationship between measures of fear of humans and lameness in broiler chicken flocks. Animal 2018, 12, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Wemelsfelder, F.; Lawrence, A.B. Qualitative assessment of animal behaviour as an on-farm welfare-monitoring tool. Acta Agric. Scand. Sect. A-Anim. Sci. 2001, 51, 21–25. [Google Scholar] [CrossRef]
- McMillan, F.D. Mental Health and Well-Being in Animals, 2nd ed.; CABI: Wallingford, UK, 2020. [Google Scholar]
- Boissy, A. Fear and fearfulness in determining behavior. In Genetics and the Behavior of Domestic Animals; Grandin, T., Ed.; Academic Press: San Diego, CA, USA, 1998; pp. 67–111. [Google Scholar]
- Forkman, B.; Boissy, A.; Meunier-Salauen, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef]
- Steimer, T. The biology of fear- and anxiety-related behaviors. Dialogues Clin. Neurosci. 2002, 4, 231–249. [Google Scholar] [CrossRef]
- Sanyal, T.; Kumar, V.; Nag, T.C.; Jain, S.; Sreenivas, V.; Wadhwa, S. Prenatal loud music and noise: Differential impact on physiological arousal, hippocampal synaptogenesis and spatial behavior in one day-old chicks. PLoS ONE 2013, 8, e67347. [Google Scholar] [CrossRef]
- Bas Rodenburg, T.; de Haas, E.N. Of nature and nurture: The role of genetics and environment in behavioural development of laying hens. Curr. Opin. Behav. Sci. 2016, 7, 91–94. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; De Haas, E.N.; Lee, C. A review of environmental enrichment for laying hens during rearing in relation to their behavioral and physiological development. Poult. Sci. 2019, 98, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, L.; Whittle, R.; Jensen, P. Effects of commercial hatchery processing on short- and long-term stress responses in laying hens. Sci. Rep. 2019, 9, 2367. [Google Scholar] [CrossRef]
- Adolphs, R. The biology of fear. Curr. Biol. 2013, 23, R79–R93. [Google Scholar] [CrossRef]
- Mills, A.D.; Faure, J.M. Panic and hysteria in domestic-fowl–A review. In Social Stress in Domestic Animals; Zayan, R., Dantzer, R., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1990; pp. 248–272. [Google Scholar]
- Anderson, K.E.; Adams, A.W. Effects of cage versus floor rearing environments and cage floor mesh size on bone strength, fearfulness, and production of single comb White Leghorn hens. Poult. Sci. 1994, 73, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.M.; Knowles, T.G.; Nicol, C.J. The effect of rearing environment on feather pecking in young and adult laying hens. Appl. Anim. Behav. Sci. 2013, 148, 54–63. [Google Scholar] [CrossRef]
- De Haas, E.N.; Kemp, B.; Bolhuis, J.E.; Groothuis, T.; Rodenburg, T.B. Fear, stress, and feather pecking in commercial white and brown laying hen parent-stock flocks and their relationships with production parameters. Poult. Sci. 2013, 92, 2259–2269. [Google Scholar] [CrossRef]
- Hughes, R.N. Intrinsic exploration in animals: Motives and measurement. Behav. Process. 1997, 41, 213–226. [Google Scholar] [CrossRef]
- Rozempolska-Rucinska, I.; Kibala, L.; Prochniak, T.; Zieba, G.; Lukaszewicz, M. Genetics of the Novel Object Test outcome in laying hens. Appl. Anim. Behav. Sci. 2017, 193, 73–76. [Google Scholar] [CrossRef]
- Powell, S.B.; Geyer, M.A.; Gallagher, D.; Paulus, M.P. The balance between approach and avoidance behaviors in a novel object exploration paradigm in mice. Behav. Brain Res. 2004, 152, 341–349. [Google Scholar] [CrossRef]
- Jones, R.B. Fear and adaptability in poultry: Insights, implications and imperatives. Worlds Poult. Sci. J. 1996, 52, 131–174. [Google Scholar] [CrossRef]
- Agnvall, B.; Ali, A.; Olby, S.; Jensen, P. Red Junglefowl (Gallus gallus) selected for low fear of humans are larger, more dominant and produce larger offspring. Animal 2014, 8, 1498–1505. [Google Scholar] [CrossRef]
- Butterworth, A.; Arnould, C.; Fiks van Niekerk, T.; Veissier, I.; Keeling, L.; van Overbeke, G.; Bedaux, V. Welfare Quality® Assessment for Poultry (Broilers, Laying Hens); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Brantsæter, M.; Tahamtani, F.M.; Nordgreen, J.; Sandberg, E.; Hansen, T.B.; Rodenburg, T.B.; Moe, R.O.; Janczak, A.M. Access to litter during rearing and environmental enrichment during production reduce fearfulness in adult laying hens. Appl. Anim. Behav. Sci. 2017, 189, 49–56. [Google Scholar] [CrossRef]
- Duncan, I.J.H. Reactions of poultry to human beings. In Social Stress in Domestic Animals; Zayan, R., Dantzer, R., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1990; pp. 121–131. [Google Scholar]
- Healy, S. Spatial Representation in Animals; Oxford University Press: New York, NY, USA, 1998; pp. 1–188. [Google Scholar]
- Cheng, K.; Newcombe, N.S. Is There a Geometric Module for Spatial Orientation? Squaring Theory and Evidence. Psychon. Bull. Rev. 2005, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Landau, B.; Lakusta, L. Spatial Representation across Species: Geometry, Language, and Maps. Curr. Opin. Neurobiol. 2009, 19, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.T.; Naguib, M.; Trillmich, F.; Schrader, L. The effects of short term enrichment on learning in chickens from a laying strain (Gallus gallus domesticus). Appl. Anim. Behav. Sci. 2006, 101, 318–327. [Google Scholar] [CrossRef]
- Nicol, C.J. The Behavioural Biology of Chickens; CABI: Wallingford, UK, 2015; pp. 1–200. [Google Scholar] [CrossRef]
- Garnham, L.; Løvlie, H. Sophisticated fowl: The complex behaviour and cognitive skills of chickens and red junglefowl. Behav. Sci. 2018, 8, 13. [Google Scholar] [CrossRef]
- Marino, L. Thinking chickens: A review of cognition, emotion, and behavior in the domestic chicken. Anim. Cogn. 2017, 20, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P. Domestication—From behaviour to genes and back again. Appl. Anim. Behav. Sci. 2006, 97, 3–15. [Google Scholar] [CrossRef]
- Nicol, C. Farm animal cognition. Anim. Sci. 1996, 62, 375–391. [Google Scholar] [CrossRef]
- Abeyesinghe, S.M.; Nicol, C.J.; Hartnell, S.J.; Wathes, C.M. Can domestic fowl, Gallus gallus domesticus, show self-control? Anim. Behav. 2005, 70, 1–11. [Google Scholar] [CrossRef]
- Smith, C.L.; Johnson, J. The chicken challenge: What contemporary studies of fowl mean for science and ethics. Between Species 2012, 15, 75–102. [Google Scholar] [CrossRef]
- Mendl, M.; Paul, E.S. Assessing affective states in animals. In Mental Health and Well-Being in Animals, 2nd ed.; McMillan, F.D., Ed.; CABI: Wallingford, UK, 2020; pp. 328–344. [Google Scholar]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef]
- Goursot, C.; Düpjan, S.; Puppe, B.; Leliveld, L.M.C. Affective styles and emotional lateralization: A promising framework for animal welfare research. Appl. Anim. Behav. Sci. 2021, 237, 105279. [Google Scholar] [CrossRef]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Philos. Trans. R. Soc. B 2009, 364, 943–954. [Google Scholar] [CrossRef]
- Leliveld, L.M.C.; Langbein, J.; Puppe, B. The emergence of emotional lateralization: Evidence in non-human vertebrates and implications for farm animals. Appl. Anim. Behav. Sci. 2013, 145, 1–14. [Google Scholar] [CrossRef]
- Versace, E.; Vallortigara, G. Forelimb preferences in human beings and other species: Multiple models for testing hypotheses on lateralization. Front. Psychol. 2015, 6, 233. [Google Scholar] [CrossRef]
- Vallortigara, G.; Versace, E. Laterality at the neural, cognitive, and behavioral levels. In APA Handbook of Comparative Psychology: Basic Concepts, Methods, Neural Substrate, and Behavior; Call, J., Burghardt, M., Pepperber, I.M., Snowdon, C.T., Zentall, T., Eds.; American Psychological Association: Washington, DC, USA, 2017; pp. 557–577. [Google Scholar]
- Vallortigara, G.; Rogers, L.J. A function for the bicameral mind. Cortex 2020, 124, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Rogers, L.; Vallortigara, G. Lateralized Brain Functions. Methods in Human and Non-Human Species Neuromethods Series, 1st ed.; Humana: New York, NY, USA, 2017; p. 710. [Google Scholar]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left & right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef]
- Cozzutti, C.; Vallortigara, G. Hemispheric Memories for the Content and Position of Food Caches in the Domestic Chick. Behav. Neurosci. 2001, 115, 305–313. [Google Scholar] [CrossRef]
- Deng, C.; Rogers, L.J. Differential Contributions of the Two Visual Pathways to Functional Lateralization in Chicks. Behav. Brain Res. 1997, 87, 173–182. [Google Scholar] [CrossRef]
- Deng, C.; Rogers, L.J. Bilaterally Projecting Neurons in the Two Visual Pathways of Chicks. Brain Res. 1998, 794, 281–290. [Google Scholar] [CrossRef]
- Rogers, L.J. Development and function of lateralization in the avian brain. Brain Res. Bull. 2008, 76, 235–244. [Google Scholar] [CrossRef]
- DeKalb, Undated. DeKalb White Commercial Management Guide, North American Version. Available online: https://www.dekalb-poultry.com/documents/1827/Dekalb_White_CS_management_guide__North_American_Version_L2221-1.pdf (accessed on 17 March 2025).
- Archer, G.S.; Mench, J.A. Natural incubation patterns and the effects of exposing eggs to light at various times during incubation on post-hatch fear and stress responses in broiler (meat) chickens. Appl. Anim. Behav. Sci. 2014, 152, 44–51. [Google Scholar] [CrossRef]
- Ericsson, M.; Henriksen, R.; Belteky, J.; Sundman, A.S.; Shionoya, K.; Jensen, P. Long-term and transgenerational effects of stress experienced during different life phases in chickens (Gallus gallus). PLoS ONE 2016, 11, e0153879. [Google Scholar] [CrossRef]
- Archer, G.S.; Mench, J.A. Exposing avian embryos to light affects post-hatch anti-predator fear responses. Appl. Anim. Behav. Sci. 2017, 186, 80–84. [Google Scholar] [CrossRef]
- Kremer, L.; Klein Holkenborg, S.E.J.; Reimert, I.; Bolhuis, J.E.; Webb, L.E. The Nuts and Bolts of Animal Emotion. Neurosci. Biobehav. Rev. 2020, 113, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Quino, M.; Gautam, A.; Gilpin, M.; Still, K.; Landers, D.; Baker-Cook, B. The impact of multiple exposures and movement on the fear response of poultry. Poult. Sci. 2025, 104, 104594. [Google Scholar] [CrossRef]
- Romero, L.M. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Blas, J.; Bortolotti, G.R.; Tella, J.L.; Baos, R.; Marchant, T.A. Stress response during development predicts fitness in a wild, long-lived bird. Proc. Natl. Acad. Sci. USA 2007, 104, 8880–8884. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; Hinch, G.N.; Downing, J.A.; Lee, C. Fear and coping styles of outdoor-preferring, moderate-out door and indoor-preferring free-range laying hens. Appl. Anim. Behav. Sci. 2016, 185, 73–77. [Google Scholar] [CrossRef]
- Manns, M. Hemispheric Specialization. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; SpringerNature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Rogers, L.J.; Anson, J.M. Lateralisation of function in the chicken fore-brain. Pharm. Biochem. Behav. 1979, 10, 679–686. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–633. [Google Scholar] [CrossRef]
- Rogers, L.J. Evolution of hemispheric specialisation: Advantages and disadvantages. Brain Lang. 2000, 73, 236–253. [Google Scholar] [CrossRef]
- Rogers, L.J.; Zappia, J.V.; Bullock, S.P. Testosterone and eye-brain asymmetry for copulation in chickens. Experientia 1985, 41, 1447–1449. [Google Scholar] [CrossRef]
- Morandi-Raikova, A.; Mayer, U. Selective activation of the right hippocampus during navigation by spatial cues in domestic chicks (Gallus gallus). Neurobiol. Learn. Memory 2021, 177, 107344. [Google Scholar] [CrossRef]
- Deng, C.; Rogers, L.J. Social recognition and approach in the chick: Lateralization and effect of visual experience. Anim. Behav. 2002, 63, 697–706. [Google Scholar] [CrossRef]
- Daisley, J.N.; Mascalzoni, E.; Salva, O.R.; Rugani, R.; Regolin, L. Lateralization of social cognition in the domestic chicken (Gallus gallus). Phil. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 965–981. [Google Scholar] [CrossRef]
- Rosa Salva, O.; Mascalzoni, E.; Regolin, L.; Vallortigara, G. Cerebral and behavioural asymmetries in animal social recognition. Comp. Cogn. Behav. Rev. 2012, 7, 110–138. [Google Scholar] [CrossRef]
- Rugani, R.; Rosa Salva, O.; Regolin, L.; Vallortigara, G. Brain asymmetry modulates perception of biological motion in newborn chicks (Gallus gallus). Behav. Brain Res. 2015, 290, 1–7. [Google Scholar] [CrossRef]
- Rosa Salva, O.; Regolin, L.; Vallortigara, G. Chicks discriminate human gaze with their right hemisphere. Behav. Brain Res. 2007, 177, 15–21. [Google Scholar] [CrossRef]
- Rogers, L.J. Light experience and asymmetry of brain function in chickens. Nature 1982, 297, 223–225. [Google Scholar] [CrossRef]
- Giljov, A.; Karenina, K.; Malashichev, Y. Facing each other: Mammal mothers and infants prefer the position favouring right hemisphere processing. Biol. Lett. 2018, 14, 20170707. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Quaranta, A. Lateralized emotional functioning in domestic animals. Appl. Anim. Behav. Sci. 2021, 237, 105282. [Google Scholar] [CrossRef]
- Mendl, M.; Paul, E.S. Consciousness, emotion and animal welfare: Insights from cognitive science. Anim. Welf. 2004, 13, 17–25. [Google Scholar] [CrossRef]
- Royet, J.P.; Plailly, J. Lateralization of olfactory processes. Chem. Senses 2004, 29, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Eye and ear preferences. In Lateralized Brain Functions—Methods in Human and Non-Human Species, 1st ed.; Rogers, L.J., Vallortigara, G., Eds.; Neuromethods series; Humana: New York, NY, USA, 2017; p. 710. [Google Scholar]
- Dawkins, M. What are birds looking at? Head movements and eye use in chickens. Anim. Behav. 2002, 63, 991–998. [Google Scholar] [CrossRef]
- Rogers, L.J.; Andrew, R.J. Frontal and lateral visual field use by chicks after treatment of testosterone. Anim. Behav. 1989, 38, 394–405. [Google Scholar] [CrossRef]
- Vallortigara, G.; Cozzutti, C.; Tommasi, L.; Rogers, L.J. How birds use their eyes: Opposite left-right specialization for the lateral and frontal visual hemifield in the domestic chick. Curr. Biol. 2001, 11, 29–33. [Google Scholar] [CrossRef]
- Dharmaretnam, M.; Andrew, R.J. Age- and stimulus-specific use of the right and left eyes by domestic chick. Anim. Behav. 1994, 48, 1395–1406. [Google Scholar] [CrossRef]
- Vallortigara, G. Comparative neuropsychology of the dual brain: A stroll through left and right animals’ perceptual worlds. Brain Lang. 2000, 73, 189–219. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Quaranta, A. Lateralized functions in the dog brain. Symmetry 2017, 9, 71. [Google Scholar] [CrossRef]
- Isparta, S.; Demirbas, Y.S.; Bars, Z.; Kul, B.C.; Güntürkün, O.; Ocklenburg, S.; Pereira, G.D.G. The relationship between problem-solving ability and laterality in cats. Behav. Brain Res. 2020, 391, 112691. [Google Scholar] [CrossRef]
- Cameron, R.; Rogers, L.J. Hand preference of the common marmoset (Callithrix jacchus): Problem solving and responses in a novel setting. J. Comp. Psychol. 1999, 113, 149–157. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Asymmetry of flight and escape turning responses in horses. Laterality 2007, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Siniscalchi, M.; Vallortigara, G. Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr. Biol. 2007, 17, R199–R201. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Lusito, R.; Vallortigara, G.; Quaranta, A. Seeing left-or right asymmetric tail wagging produces different emotional responses in dogs. Curr. Biol. 2013, 23, 2279–2282. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Pergola, G.; Quaranta, A. Detour behaviour in attack-trained dogs: Left-turners perform better than right-turners. Laterality 2013, 18, 282–293. [Google Scholar] [CrossRef]
- Barnard, S.; Matthews, L.; Messori, S.; Podaliri-Vulpiani, M.; Ferri, N. Laterality as an indicator of emotional stress in ewes and lambs during a separation test. Anim. Cogn. 2016, 19, 207–214. [Google Scholar] [CrossRef]
- Goursot, C.; Düpjan, S.; Kanitz, E.; Tuchscherer, A.; Puppe, B.; Leliveld, L.M. Assessing animal individuality: Links between personality and laterality in pigs. Curr. Zool. 2019, 65, 541–551. [Google Scholar] [CrossRef]
- Batt, L.S.; Batt, M.S.; Baguley, J.A.; McGreevy, P.D. The relationships between motor lateralization, salivary cortisol concentrations and behavior in dogs. J. Vet. Behav. 2009, 4, 216–222. [Google Scholar] [CrossRef]
- McDowell, L.J.; Wells, D.L.; Hepper, P.G.; Dempster, M. Lateral bias and temperament in the domestic cat (Felis silvestris). J. Comp. Psychol. 2016, 130, 313–320. [Google Scholar] [CrossRef]
- Barnard, S.; Wells, D.L.; Hepper, P.G. Laterality as a predictor of coping strategies in dogs entering a rescue shelter. Symmetry 2018, 10, 538. [Google Scholar] [CrossRef]
- Branson, N.J.; Rogers, L.J. Relationship between paw preference strength and noise phobia in Canis familiaris. J. Comp. Psychol. 2006, 120, 176–183. [Google Scholar] [CrossRef]
- Barnard, S.; Wells, D.L.; Hepper, P.G.; Milligan, A.D. Association between lateral bias and personality traits in the domestic dog (Canis familiaris). J. Comp. Psychol. 2017, 131, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, Y.S.; Isparta, S.; Ozturk, H.; Safak, E.; Emre, B.; Piskin, I.; Kaya, U.; Sagmanligil, V.; Akgul, B.; Pereira, G.D.G. Functional cerebral asymmetry in dogs living under different environmental conditions. Behav. Process. 2019, 165, 4–8. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G.; Milligan, A.D.S.; Barnard, S. Stability of motor bias in the domestic dog, Canis familiaris. Behav. Process. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Rogers, L.J. Cognition and animal welfare. Wiley Interdiscip. Rev. Cogn. Sci. 2010, 1, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Franklin, W.E.; Lima, S.L. Laterality in avian vigilance: Do sparrows have a favourite eye? Anim. Behav. 2001, 62, 879–885. [Google Scholar] [CrossRef]
- Rogers, L.J. Advantages and disadvantages of lateralization. In Comparative Vertebrate Lateralization; Rogers, L.J., Andrew, R.J., Eds.; Cambridge University Press: New York, NY, USA, 2002; pp. 126–153. [Google Scholar]
- Dharmaretnam, M.; Rogers, L.J. Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain. Res. 2005, 162, 62–70. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Back to basics of antipredator vigilance: Can nonvigilant animals detect attack? Anim. Behav. 1999, 58, 537–543. [Google Scholar] [CrossRef]
- Zimmerman, P.H.; Buijs, S.A.F.; Bolhuis, J.E.; Keeling, L.J. Behavior of domestic fowl in anticipation of positive and negative stimuli. Anim. Behav. 2011, 81, 569–577. [Google Scholar] [CrossRef]
- Golfidis, A.; Kriengwatana, B.P.; Mounir, M.; Norton, T. An Interactive Feeder to Induce and Assess Emotions from Vocalisations of Chickens. Animals 2024, 14, 1386. [Google Scholar] [CrossRef]
- Barnard, C. Ethical regulation and animal science: Why animal behaviour is special. Anim. Behav. 2007, 74, 5–13. [Google Scholar] [CrossRef]
- Neethirajan, S.; Reimert, I.; Kemp, B. Measuring Farm Animal Emotions—Sensor-Based Approaches. Sensors 2021, 21, 553. [Google Scholar] [CrossRef]


| Behaviour | Description |
|---|---|
| L+1/R+1 | Ratio of left eye view (L) plus one to right eye view (R) plus one |
| Eye view left (EVL) * | Using left eye to view arena or human |
| Eye view right (EVR) * | Using right eye to view arena or human |
| Eye view before emerging (EVE) *# | Eye used to view arena just before emerging |
| Vocalisation (BO) | Opens the beak to give sound |
| Head position level (HPL) | Position of head in relation to the body |
| Head position up (HPU) | Position of head up in relation to the body |
| Head position down (HPD) | Position of head down in relation to the body |
| Head turn left (HTL) * | Turning head to left side |
| Head turn right (HTR) * | Turning head to right side |
| Body turn left (BTL) * | Turning whole body to the left side |
| Body turn right (BTR) * | Turning whole body to the right side |
| Body position vertical (BPV) | Position of body vertical in relation to floor |
| Body position perpendicular (BPP) | Position of body perpendicular in relation to floor |
| Neck stretch (NS) | Stretching neck with body stationary |
| Side of arena (location right = LCR) # | Bird present on right wall side of arena |
| Side of arena (location left = LCL) # | Birds present on left wall side of arena |
| Side of arena (location centre = LCC) # | The bird stands or passes in the centre of arena |
| Leg held left (LHL) * | Paused left leg when lifted before it is replaced again on ground or taking a step |
| Leg held right (LHR) * | Paused right leg when lifted before it is replaced again on ground or taking a step |
| Standing (ST) | Standing stationary without movement, includes head or body turn or other activities |
| Sitting (SI) | Sitting with bent legs underneath |
| Pecking (PC) | Pecking on ground or wall with beak |
| Shaking (SK) | Shaking body with ruffling of feathers |
| Defecation (DEF) | Defecation |
| Preening (PR) | Using a beak to groom self |
| Tail fanning (TF)# | Spreading tail feathers into a fan |
| Dustbathing (DB)# | Spreading wings, lowering body to ground and scratching ground or body with legs followed by shaking |
| Variable * | Box vs. Arena | Median | N ≤ Median | N > Median | X2 | p-Value |
|---|---|---|---|---|---|---|
| Left eye view | Box | 2.00 | 68 | 31 | 7.14 | 0.008 |
| Arena | 3.00 | 12 | 17 | |||
| Right eye view | Box | 2.00 | 44 | 55 | 1.66 | 0.197 |
| Arena | 2.00 | 9 | 20 | |||
| L+1/R+1 | Box | 1.00 | 51 | 48 | 0.40 | 0.526 |
| Arena | 1.25 | 13 | 16 | |||
| Level head | Box | 1.00 | 73 | 26 | 12.75 | <0.0001 |
| Arena | 2.00 | 11 | 18 | |||
| Head up | Box | 0.00 | 56 | 43 | 0.02 | 0.894 |
| Arena | 0.00 | 16 | 13 | |||
| Head down | Box | 0.00 | 62 | 37 | 13.37 | <0.0001 |
| Arena | 2.00 | 7 | 22 | |||
| Neck stretch | Box | 0.00 | 58 | 41 | 0.97 | 0.325 |
| Arena | 1.00 | 14 | 15 | |||
| Vocalisation | Box | 0.00 | 76 | 23 | 3.81 | 0.051 |
| Arena | 0.00 | 27 | 2 | |||
| Head turn left | Box | 1.00 | 69 | 30 | 7.74 | 0.005 |
| Arena | 2.00 | 12 | 17 | |||
| Head turn right | Box | 1.00 | 68 | 31 | 13.27 | <0.0001 |
| Arena | 2.00 | 9 | 20 | |||
| Body turn left | Box | 0.00 | 96 | 3 | 13.88 | <0.0001 |
| Arena | 0.00 | 22 | 7 | |||
| Body turn right | Box | 0.00 | 91 | 8 | 2.06 | 0.151 |
| Arena | 0.00 | 24 | 5 | |||
| Body position vertical | Box | 1.00 | 94 | 5 | 6.98 | 0.008 |
| Arena | 1.00 | 23 | 6 | |||
| Body position perpendicular | Box | 0.00 | 81 | 18 | 3.49 | 0.062 |
| Arena | 0.00 | 19 | 10 | |||
| Standing | Box | 0.00 | 54 | 45 | 27.36 | <0.0001 |
| Arena | 1.00 | 0 | 29 | |||
| Sitting | Box | 1.00 | 31 | 68 | 38.42 | <0.0001 |
| Arena | 0.00 | 28 | 1 | |||
| Pecking | Box | 0.00 | 93 | 6 | 8.03 | 0.005 |
| Arena | 0.00 | 22 | 7 | |||
| Shaking | Box | 0.00 | 98 | 1 | 3.40 | 0.065 |
| Arena | 0.00 | 27 | 2 | |||
| Defecation | Box | 0.00 | 98 | 1 | 3.40 | 0.065 |
| Arena | 0.00 | 27 | 2 | |||
| Left leg held ** | Box | 0.00 | 99 | 0 | 6.94 | 0.008 |
| Arena | 0.00 | 27 | 2 |
| Variable | Human Condition | Median | N ≤ Median | N > Median | X2 | p-Value |
|---|---|---|---|---|---|---|
| L+1/R+1 | Neutral | 0.83 | 25 | 12 | 5.14 | 0.023 |
| Threatening | 1.25 | 22 | 29 | |||
| Left eye view | Neutral | 3.00 | 29 | 8 | 6.00 | 0.014 |
| Threatening | 4.00 | 27 | 24 | |||
| Right eye view | Neutral | 4.00 | 15 | 22 | 5.05 | 0.025 |
| Threatening | 3.00 | 33 | 18 | |||
| Level head | Neutral | 1.00 | 26 | 11 | 0.85 | 0.358 |
| Threatening | 1.00 | 31 | 20 | |||
| Head up | Neutral | 2.00 | 24 | 13 | 2.18 | 0.140 |
| Threatening | 3.00 | 25 | 26 | |||
| Head down | Neutral | 1.00 | 25 | 12 | 1.04 | 0.309 |
| Threatening | 1.00 | 29 | 22 | |||
| Neck stretch | Neutral | 1.00 | 21 | 16 | 0.13 | 0.723 |
| Threatening | 1.00 | 27 | 24 | |||
| Vocalisation | Neutral | 0.00 | 33 | 4 | 3.65 | 0.056 |
| Threatening | 0.00 | 37 | 14 | |||
| Head turn left | Neutral | 2.00 | 23 | 14 | 0.00 | 0.956 |
| Threatening | 2.00 | 32 | 19 | |||
| Head turn right | Neutral | 2.00 | 22 | 15 | 1.66 | 0.197 |
| Threatening | 2.00 | 37 | 14 | |||
| Body turn left | Neutral | 0.00 | 27 | 10 | 0.14 | 0.708 |
| Threatening | 0.00 | 39 | 12 | |||
| Body turn right | Neutral | 0.00 | 28 | 9 | 0.28 | 0.596 |
| Threatening | 0.00 | 41 | 10 | |||
| Body position vertical | Neutral | 1.00 | 8 | 29 | 4.42 | 0.036 |
| Threatening | 1.00 | 22 | 29 | |||
| Body position perpendicular | Neutral | 1.00 | 15 | 22 | 3.71 | 0.054 |
| Threatening | 1.00 | 11 | 40 | |||
| Standing | Neutral | 1.00 | 5 | 32 | 7.31 | 0.007 |
| Threatening | 1.00 | 0 | 51 | |||
| Sitting | Neutral | 0.00 | 31 | 6 | 5.95 | 0.015 |
| Threatening | 0.00 | 50 | 1 | |||
| Left leg held | Neutral | 0.00 | 31 | 6 | 0.03 | 0.860 |
| Threatening | 0.00 | 42 | 9 | |||
| Right leg held | Neutral | 0.00 | 27 | 10 | 0.14 | 0.708 |
| Threatening | 0.00 | 39 | 12 | |||
| Location left | Neutral | 0.00 | 25 | 12 | 1.90 | 0.168 |
| Threatening | 0.00 | 27 | 24 | |||
| Location right | Neutral | 1.00 | 16 | 21 | 1.59 | 0.207 |
| Threatening | 0.00 | 29 | 22 | |||
| Location centre | Neutral | 1.00 | 18 | 19 | 0.58 | 0.446 |
| Threatening | 0.00 | 29 | 22 | |||
| Pecking | Neutral | 0.00 | 29 | 8 | 0.00 | 0.995 |
| Threatening | 0.00 | 40 | 11 | |||
| Shaking | Neutral | 0.00 | 32 | 5 | 0.00 | 0.977 |
| Threatening | 0.00 | 44 | 7 | |||
| Preening | Neutral | 0.00 | 36 | 1 | 0.05 | 0.818 |
| Threatening | 0.00 | 50 | 1 | |||
| Defecation | Neutral | 0.00 | 32 | 5 | 1.51 | 0.219 |
| Threatening | 0.00 | 48 | 3 | |||
| Dust bathing | Neutral | 0.00 | 36 | 1 | 0.05 | 0.818 |
| Threatening | 0.00 | 50 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goma, A.A.; Phillips, C.J.C. Lateralised Behavioural Responses of Chickens to a Threatening Human and a Novel Environment Indicate Fearful Emotions. Animals 2025, 15, 2023. https://doi.org/10.3390/ani15142023
Goma AA, Phillips CJC. Lateralised Behavioural Responses of Chickens to a Threatening Human and a Novel Environment Indicate Fearful Emotions. Animals. 2025; 15(14):2023. https://doi.org/10.3390/ani15142023
Chicago/Turabian StyleGoma, Amira A., and Clive J. C. Phillips. 2025. "Lateralised Behavioural Responses of Chickens to a Threatening Human and a Novel Environment Indicate Fearful Emotions" Animals 15, no. 14: 2023. https://doi.org/10.3390/ani15142023
APA StyleGoma, A. A., & Phillips, C. J. C. (2025). Lateralised Behavioural Responses of Chickens to a Threatening Human and a Novel Environment Indicate Fearful Emotions. Animals, 15(14), 2023. https://doi.org/10.3390/ani15142023







