An Indole-Rich Postbiotic Reduces Itching in Dogs: A Randomized, Double-Blinded Placebo-Controlled Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Intervention
2.4. Scratching Frequency Quantification via Accelerometer Device
2.5. Pruritus Visual Analog Scale Scoring
2.6. Skin and Coat Quality Assessment
2.7. Fecal Sampling
2.8. Gut Microbiome Sequencing
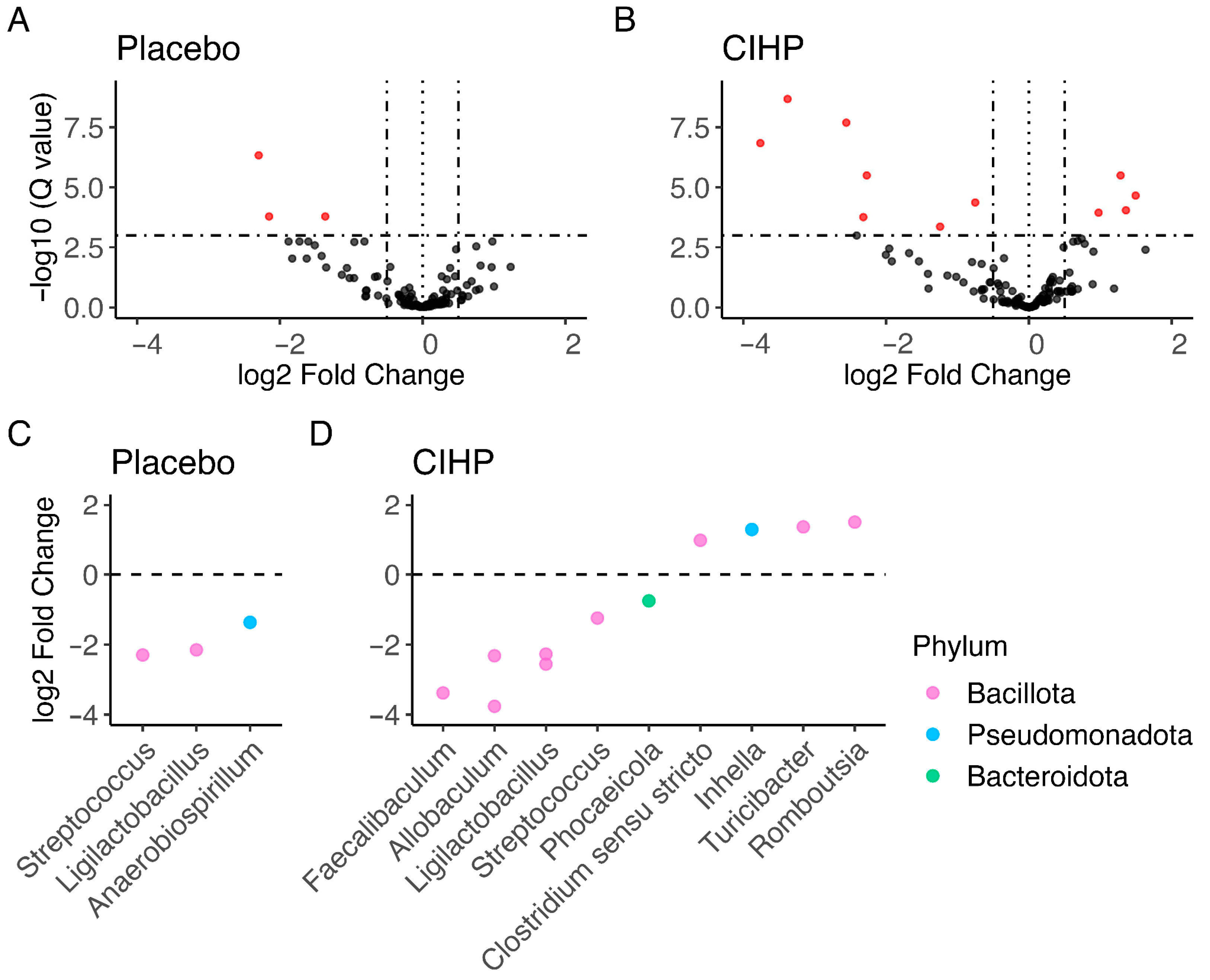
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bensignor, E. Canine atopic dermatitis. Bull. Acad. Natl. Med. 2010, 194, 1357–1364. [Google Scholar] [PubMed]
- Zanon, J.P.; Gomes, L.A.; Cury, G.M.M.; Teles, T.D.C.; Bicalho, A.P.D.C.V. Canine Atopic Dermatitis. Semin. Ciências Agrárias 2008, 29, 905. [Google Scholar] [CrossRef]
- Sauvé, F. Itch in Dogs and Cats. Can. Vet. J. 2023, 64, 686–690. [Google Scholar] [PubMed]
- Drechsler, Y.; Dong, C.; Clark, D.E.; Kaur, G. Canine Atopic Dermatitis: Prevalence, Impact, and Management Strategies. Vet. Med. Res. Rep. 2024, 15, 15–29. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, L.; Liu, F.; Xiong, X.; Ouyang, Y.; Deng, Y. Tryptophan, an Important Link in Regulating the Complex Network of Skin Immunology Response in Atopic Dermatitis. Front. Immunol. 2024, 14, 1300378. [Google Scholar] [CrossRef]
- Robinson, V. Canine Atopic Dermatitis. Companion Anim. 2023, 28, 2–9. [Google Scholar] [CrossRef]
- Marsella, R. Atopic Dermatitis in Domestic Animals: What Our Current Understanding Is and How This Applies to Clinical Practice. Vet. Sci. 2021, 8, 124. [Google Scholar] [CrossRef]
- Olivry, T.; Marsella, R.; Iwasaki, T.; Mueller, R. The International Task Force On Canine Atopic Dermatitis Validation of CADESI-03, a Severity Scale for Clinical Trials Enrolling Dogs with Atopic Dermatitis. Vet. Dermatol. 2007, 18, 78–86. [Google Scholar] [CrossRef]
- Vollset, I. Atopic Dermatitis in Norwegian Dogs. Nord. Vet. Med. 1985, 37, 97–106. [Google Scholar] [PubMed]
- Youn, H.Y.; Kang, H.S.; Bhang, D.H.; Kim, M.K.; Hwang, C.Y.; Han, H.R. Allergens Causing Atopic Diseases in Canine. J. Vet. Sci. 2002, 3, 335. [Google Scholar] [CrossRef]
- DeBoer, D.J. Canine Atopic Dermatitis: New Targets, New Therapies. J. Nutr. 2004, 134, 2056S–2061S. [Google Scholar] [CrossRef] [PubMed]
- Pucheu-Haston, C.M.; Santoro, D.; Bizikova, P.; Eisenschenk, M.N.C.; Marsella, R.; Nuttall, T. Review: Innate Immunity, Lipid Metabolism and Nutrition in Canine Atopic Dermatitis. Vet. Dermatol. 2015, 26, 104-e28. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.C.; Cunha, M.G.M.C.M.D.; Nunes-Pinheiro, D.C.S. Canine Atopic Dermatitis: Systemic Immunomodulatory Protocol Based on Clinical Phenotype. Ciênc. Rural 2023, 53, e20220068. [Google Scholar] [CrossRef]
- Pessôa, R.; Clissa, P.B.; Sanabani, S.S. The Interaction between the Host Genome, Epigenome, and the Gut–Skin Axis Microbiome in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 14322. [Google Scholar] [CrossRef]
- Craig, J.M. Atopic Dermatitis and the Intestinal Microbiota in Humans and Dogs. Vet. Med. Sci. 2016, 2, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Künstner, A.; Wohlers, I.; Olbrich, M.; Lenfers, T.; Osumi, T.; Shimazaki, Y.; Nishifuji, K.; Ibrahim, S.M.; Watson, A.; et al. A Comprehensive Analysis of Gut and Skin Microbiota in Canine Atopic Dermatitis in Shiba Inu Dogs. Microbiome 2023, 11, 232. [Google Scholar] [CrossRef]
- Rostaher, A.; Morsy, Y.; Favrot, C.; Unterer, S.; Schnyder, M.; Scharl, M.; Fischer, N.M. Comparison of the Gut Microbiome between Atopic and Healthy Dogs—Preliminary Data. Animals 2022, 12, 2377. [Google Scholar] [CrossRef]
- Swain, S.; Sahoo, P.; Biswal, S.; Sethy, K.; Panda, A.N.; Sahoo, N. Fecal Bacterial Microbiota Diversity Characterized for Dogs with Atopic Dermatitis: Its Alteration and Clinical Recovery after Meat-Exclusion Diet. Am. J. Vet. Res. 2025, 86, 1–9. [Google Scholar] [CrossRef]
- Ménard, S.; Cerf-Bensussan, N.; Heyman, M. Multiple Facets of Intestinal Permeability and Epithelial Handling of Dietary Antigens. Mucosal Immunol. 2010, 3, 247–259. [Google Scholar] [CrossRef]
- Majamaa, H.; Isolauri, E. Evaluation of the Gut Mucosal Barrier: Evidence for Increased Antigen Transfer in Children with Atopic Eczema. J. Allergy Clin. Immunol. 1996, 97, 985–990. [Google Scholar] [CrossRef]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Pærregaard, A.; Michaelsen, K.F. Effect of Probiotics on Gastrointestinal Symptoms and Small Intestinal Permeability in Children with Atopic Dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, A.; Hesta, M.; Millet, S.; Janssens, G.P.J. Food Allergy in Dogs and Cats: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 259–273. [Google Scholar] [CrossRef]
- Ekici, Y.E.; Ok, M. Investigation of the Relationship between Atopic Dermatitis of Dogs and Intestinal Epithelial Damage. Vet. Med. Sci. 2024, 10, e1453. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Logan, A.C. Acne Vulgaris, Probiotics and the Gut-Brain-Skin Axis-Back to the Future? Gut Pathog. 2011, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Puttini, P.S.; Cassinotti, A.; Porro, G.B. Extraintestinal Manifestations of Inflammatory Bowel Disease. Dig. Liver Dis. 2008, 40, S253–S259. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rather, I.A.; Kim, H.; Kim, S.; Kim, T.; Jang, J.; Seo, J.; Lim, J.; Park, Y.-H. A Double-Blind, Placebo Controlled-Trial of a Probiotic Strain Lactobacillus Sakei Probio-65 for the Prevention of Canine Atopic Dermatitis. J. Microbiol. Biotechnol. 2015, 25, 1966–1969. [Google Scholar] [CrossRef]
- Petersen, E.; Skov, L.; Thyssen, J.; Jensen, P. Role of the Gut Microbiota in Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2018, 99, 5–11. [Google Scholar] [CrossRef]
- Tate, D.E.; Tanprasertsuk, J.; Jones, R.B.; Maughan, H.; Chakrabarti, A.; Khafipour, E.; Norton, S.A.; Shmalberg, J.; Honaker, R.W. A Randomized Controlled Trial to Evaluate the Impact of a Novel Probiotic and Nutraceutical Supplement on Pruritic Dermatitis and the Gut Microbiota in Privately Owned Dogs. Animals 2024, 14, 453. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A Comprehensive Review of the Application of Probiotics and Postbiotics in Oral Health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the Power of Postbiotics: A Revolutionary Approach to Nutrition for Humans and Animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Osumi, T.; Shimada, T.; Sakaguchi, M.; Tsujimoto, H. A Double-Blind, Placebo-Controlled Evaluation of Orally Administered Heat-Killed Enterococcus Faecalis FK-23 Preparation in Atopic Dogs. Vet. Dermatol. 2019, 30, 127-e36. [Google Scholar] [CrossRef]
- Beynen, A.C.; Saris, D.H.J.; Paap, P.M.; Altena, F.V.; Visser, E.A.; Middelkoop, J.; Jong, L.D.; Staats, M. Dietary Beta-1,3/1,6-Glucans Reduce Clinical Signs of Canine Atopy. Am. J. Anim. Vet. Sci. 2012, 6, 146–152. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Riaz, F.; Pan, F.; Wei, P. Aryl Hydrocarbon Receptor: The Master Regulator of Immune Responses in Allergic Diseases. Front. Immunol. 2022, 13, 1057555. [Google Scholar] [CrossRef]
- Vyhlídalová, B.; Krasulová, K.; Pečinková, P.; Marcalíková, A.; Vrzal, R.; Zemánková, L.; Vančo, J.; Trávníček, Z.; Vondráček, J.; Karasová, M.; et al. Gut Microbial Catabolites of Tryptophan Are Ligands and Agonists of the Aryl Hydrocarbon Receptor: A Detailed Characterization. Int. J. Mol. Sci. 2020, 21, 2614. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Fu, Y.; Yin, Y.; Xu, K. Modulating AHR Function Offers Exciting Therapeutic Potential in Gut Immunity and Inflammation. Cell Biosci. 2023, 13, 85. [Google Scholar] [CrossRef]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef]
- Wazir, A.; O’Toole, E.A. Itching for Innovation: The Role of Aryl Hydrocarbon Receptor Agonists as a Future Therapy for Atopic Dermatitis. Clin. Exp. Dermatol. 2025, 50, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Bisson, W.H.; Lahoti, T.S.; Gowda, K.; Amin, S.G.; Patterson, A.D.; Perdew, G.H. Adaptation of the Human Aryl Hydrocarbon Receptor to Sense Microbiota-Derived Indoles. Sci. Rep. 2015, 5, 12689. [Google Scholar] [CrossRef]
- Puccetti, M.; Pariano, M.; Costantini, C.; Giovagnoli, S.; Ricci, M. Pharmaceutically Active Microbial AhR Agonists as Innovative Biodrugs in Inflammation. Pharmaceuticals 2022, 15, 336. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Perdew, G.H. The Aryl Hydrocarbon Receptor as a Mediator of Host-Microbiota Interplay. Gut Microbes 2020, 12, 1859812. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Boguniewicz, M.; Quintana, F.J.; Clark, R.A.; Gross, L.; Hirano, I.; Tallman, A.M.; Brown, P.M.; Fredericks, D.; Rubenstein, D.S.; et al. Tapinarof Validates the Aryl Hydrocarbon Receptor as a Therapeutic Target: A Clinical Review. J. Allergy Clin. Immunol. 2024, 154, 1–10. [Google Scholar] [CrossRef]
- Accioli, C.d.A.F.; da Silva, M.S.; Santos, B.A.M.C.; Rodrigues, C.R. Aryl Hydrocarbon Receptor as a Therapeutical Target of Environmentally Induced Skin Conditions. Mol. Pharmacol. 2023, 103, 255–265. [Google Scholar] [CrossRef]
- Silverberg, J.; Boguniewicz, M.; Rubenstein, D.; Tallman, A.; Brown, P. Tapinarof Cream Improved Itch in Two Phase 3 Trials of Moderate to Severe Atopic Dermatitis. Ann. Allergy. Asthma. Immunol. 2023, 131, S90. [Google Scholar] [CrossRef]
- Chambers, R.D.; Yoder, N.C.; Carson, A.B.; Junge, C.; Allen, D.E.; Prescott, L.M.; Bradley, S.; Wymore, G.; Lloyd, K.; Lyle, S. Deep Learning Classification of Canine Behavior Using a Single Collar-Mounted Accelerometer: Real-World Validation. Animals 2021, 11, 1549. [Google Scholar] [CrossRef]
- Hill, P.B.; Lau, P.; Rybnicek, J. Development of an Owner-Assessed Scale to Measure the Severity of Pruritus in Dogs. Vet. Dermatol. 2007, 18, 301–308. [Google Scholar] [CrossRef]
- Rybnícek, J.; Lau-Gillard, P.J.; Harvey, R.; Hill, P.B. Further Validation of a Pruritus Severity Scale for Use in Dogs. Vet. Dermatol. 2009, 20, 115–122. [Google Scholar] [CrossRef]
- Rees, C.A.; Bauer, J.E.; Burkholder, W.J.; Kennis, R.A.; Dunbar, B.L.; Bigley, C.E. Effects of Dietary Flax Seed and Sunflower Seed Supplementation on Normal Canine Serum Polyunsaturated Fatty Acids and Skin and Hair Coat Condition Scores. Vet. Dermatol. 2001, 12, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Devriendt, N.; Rodrigues, T.C.N.; Vandenabeele, S.; Favril, S.; Biscop, A.; Marynissen, S.; Broeckx, B.J.G.; Hofstra, I.; Mortier, F.; Bakker, E.de; et al. Validation of a Skin and Coat Scoring Protocol in Dogs. Vlaams Diergeneeskd. Tijdschr. 2021, 90, 227–230. [Google Scholar] [CrossRef]
- Kirby, N.A.; Hester, S.L.; Rees, C.A.; Kennis, R.A.; Zoran, D.L.; Bauer, J.E. Skin Surface Lipids and Skin and Hair Coat Condition in Dogs Fed Increased Total Fat Diets Containing Polyunsaturated Fatty Acids. J. Anim. Physiol. Anim. Nutr. 2009, 93, 505–511. [Google Scholar] [CrossRef]
- Edgar, R.C. UNOISE2: Improved Error-Correction for Illumina 16S and ITS Amplicon Sequencing 2016. Available online: https://onesearch.wesleyan.edu/discovery/fulldisplay?docid=cdi_unpaywall_primary_10_1101_081257&context=PC&vid=01CTW_WU:CTWWU&lang=en&search_scope=Everything&adaptor=Primo%20Central&query=null,,1,AND&facet=citing,exact,cdi_FETCH-LOGICAL-c606t-beeb33704b63fa20c49c55b024463ea6b762e19c6a707dd54a2711c16af97a8d3&offset=50 (accessed on 10 January 2025).
- Wang, Q.; Cole, J.R. Updated RDP Taxonomy and RDP Classifier for More Accurate Taxonomic Classification. Microbiol. Resour. Announc. 2024, 13, e0106323. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef]
- Kotnik, T. Quality of Life of Allergic Dogs Treated with Allergen-Specific Immunotherapy—A Retrospective Study. Vet. Sci. 2023, 10, 72. [Google Scholar] [CrossRef]
- Linek, M.; Favrot, C. Impact of Canine Atopic Dermatitis on the Health-Related Quality of Life of Affected Dogs and Quality of Life of Their Owners. Vet. Dermatol. 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Noli, C.; Colombo, S.; Cornegliani, L.; Ghibaudo, G.; Persico, P.; Vercelli, A.; Galzerano, M. Quality of Life of Dogs with Skin Disease and of Their Owners. Part 2: Administration of a Questionnaire in Various Skin Diseases and Correlation to Efficacy of Therapy. Vet. Dermatol. 2011, 22, 344–351. [Google Scholar] [CrossRef]
- Harvey, N.D.; Craigon, P.J.; Shaw, S.C.; Blott, S.C.; England, G.C.W. Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus. Animals 2019, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.D.; Hutchison, J.; Ruch-Gallie, R.; Kogan, L.; New, J.C., Jr.; Kass, P.H.; Scarlett, J.M. Behavioral Reasons for Relinquishment of Dogs and Cats to 12 Shelters. J. Appl. Anim. Welf. Sci. 2000, 3, 93–106. [Google Scholar] [CrossRef]
- Serpell, J.A. Evidence for an Association between Pet Behavior and Owner Attachment Levels. Appl. Anim. Behav. Sci. 1996, 47, 49–60. [Google Scholar] [CrossRef]
- Volk, J.O.; Felsted, K.E.; Thomas, J.G.; Siren, C.W. Executive Summary of the Bayer Veterinary Care Usage Study. J. Am. Vet. Med. Assoc. 2011, 238, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Lem, M. Barriers to Accessible Veterinary Care. Can. Vet. J. 2019, 60, 891–893. [Google Scholar]
- Morris, A.; Wu, H.; Morales, C. Barriers to Care in Veterinary Services: Lessons Learned From Low-Income Pet Guardians’ Experiences at Private Clinics and Hospitals During COVID-19. Front. Vet. Sci. 2021, 8, 764753. [Google Scholar] [CrossRef]
- Kanwal, S.; Singh, S.K.; Soman, S.P.; Choudhury, S.; Kumari, P.; Ram, P.K.; Garg, S.K. Expression of Barrier Proteins in the Skin Lesions and Inflammatory Cytokines in Peripheral Blood Mononuclear Cells of Atopic Dogs. Sci. Rep. 2021, 11, 11418. [Google Scholar] [CrossRef]
- Calesso, J.R.; Marques, V.S.; de Carvalho, O.V.; Costa-Val, A.P. Correlation between Clinical Efficacy on Pruritus and Serum Interleukin-31 Levels in Dogs with Atopic Dermatitis Treated with Lokivetmab. Pol. J. Vet. Sci. 2023, 26, 231–238. [Google Scholar] [CrossRef]
- Lee, S.; Yun, T.; Koo, Y.; Chae, Y.; Lee, D.; Choi, D.; Choi, Y.; Kim, H.; Yang, M.-P.; Kang, B.-T. Clinical Efficacy of Oclacitinib and Lokivetmab in Dogs with Canine Atopic Dermatitis. J. Vet. Clin. 2021, 38, 127–134. [Google Scholar] [CrossRef]
- Ohshima-Terada, Y.; Higuchi, Y.; Kumagai, T.; Hagihara, A.; Nagata, M. Complementary Effect of Oral Administration of Lactobacillus Paracasei K71 on Canine Atopic Dermatitis. Vet. Dermatol. 2015, 26, 350-e75. [Google Scholar] [CrossRef]
- Lee, K.-I.; Yun, T.; Ham, J.; Lee, W.-K.; Kang, J.-H.; Yang, M.-P.; Kang, B.-T. Clinical Trial of Oral Administration of Bifidobacterium Longum in Dogs with Atopic Dermatitis. Korean J. Vet. Res. 2020, 60, 19–24. [Google Scholar] [CrossRef]
- Yamazaki, C.; Rosenkrantz, W.; Griffin, C. Pilot Evaluation of Enterococcus Faecium SF68 as Adjunctive Therapy for Oclacitinib-Responsive Adult Atopic Dermatitis in Dogs. J. Small Anim. Pract. 2019, 60, 499–506. [Google Scholar] [CrossRef]
- Marchegiani, A.; Fruganti, A.; Spaterna, A.; Dalle Vedove, E.; Bachetti, B.; Massimini, M.; Di Pierro, F.; Gavazza, A.; Cerquetella, M. Impact of Nutritional Supplementation on Canine Dermatological Disorders. Vet. Sci. 2020, 7, 38. [Google Scholar] [CrossRef]
- DeBoer, D.J.; Hillier, A. The ACVD Task Force on Canine Atopic Dermatitis (XV): Fundamental Concepts in Clinical Diagnosis. Vet. Immunol. Immunopathol. 2001, 81, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.R.; Rest, J.R.; Seewald, W. A Prospective Study of the Clinical Findings, Treatment and Histopathology of 44 Cases of Pyotraumatic Dermatitis. Vet. Dermatol. 2004, 15, 369–376. [Google Scholar] [CrossRef]
- Jung, J.; Nam, E.; Park, S.; Han, S.; Hwang, C. Clinical Use of a Ceramide-Based Moisturizer for Treating Dogs with Atopic Dermatitis. J. Vet. Sci. 2013, 14, 199–205. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the Fecal Microbiota and Serum Metabolite Profiles in Dogs with Idiopathic Inflammatory Bowel Disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef]
- De Lima, D.C.; Souza, C.M.M.; Nakamura, N.; Mesa, D.; De Oliveira, S.G.; Félix, A.P. Dietary Supplementation with Bacillus Subtilis C-3102 Improves Gut Health Indicators and Fecal Microbiota of Dogs. Anim. Feed Sci. Technol. 2020, 270, 114672. [Google Scholar] [CrossRef]
- Koziol, S.A.; Oba, P.M.; Soto-Diaz, K.; Steelman, A.J.; Suchodolski, J.S.; Eckhardt, E.R.M.; Swanson, K.S. Effects of a Lactobacillus Fermentation Product on the Fecal Characteristics, Fecal Microbial Populations, Immune Function, and Stress Markers of Adult Dogs. J. Anim. Sci. 2023, 101, skad160. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Daristotle, L.; Quesnell, R.; Reinhart, G.A.; Frantz, N.Z. Altered Fecal Microbiota, IgA, and Fermentative End-Products in Adult Dogs Fed Prebiotics and a Nonviable Lactobacillus Acidophilus. J. Anim. Sci. 2021, 99, skab347. [Google Scholar] [CrossRef] [PubMed]
- Hooda, S.; Minamoto, Y.; Suchodolski, J.S.; Swanson, K.S. Current State of Knowledge: The Canine Gastrointestinal Microbiome. Anim. Health Res. Rev. 2012, 13, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.A.; Park, B.; Scarsella, E.; Jospin, G.; Entrolezo, Z.; Jarett, J.K.; Martin, A.; Ganz, H.H. Species-Level Characterization of the Core Microbiome in Healthy Dogs Using Full-Length 16S rRNA Gene Sequencing. Front. Vet. Sci. 2024, 11, 1405470. [Google Scholar] [CrossRef]
- Fang, Z.; Pan, T.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Limosilactobacillus Reuteri Attenuates Atopic Dermatitis via Changes in Gut Bacteria and Indole Derivatives from Tryptophan Metabolism. Int. J. Mol. Sci. 2022, 23, 7735. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Y.; Zhang, G.; Cai, S.; Ma, Y.; Yang, L.; Wang, Y.; Yu, H.; Qiao, S.; Zeng, X. Microbiota-Derived Indoles Alleviate Intestinal Inflammation and Modulate Microbiome by Microbial Cross-Feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-Lactic Acid Associated with Bifidobacterium-Dominated Microbiota Significantly Decreases Inflammation in Intestinal Epithelial Cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Huang, Z.-B.; Hu, Z.; Lu, C.-X.; Luo, S.-D.; Chen, Y.; Zhou, Z.-P.; Hu, J.-J.; Zhang, F.-L.; Deng, F.; Liu, K.-X. Gut Microbiota-Derived Indole 3-Propionic Acid Partially Activates Aryl Hydrocarbon Receptor to Promote Macrophage Phagocytosis and Attenuate Septic Injury. Front. Cell. Infect. Microbiol. 2022, 12, 1015386. [Google Scholar] [CrossRef]
- Heath-Pagliuso, S.; Rogers, W.J.; Tullis, K.; Seidel, S.D.; Cenijn, P.H.; Brouwer, A.; Denison, M.S. Activation of the Ah Receptor by Tryptophan and Tryptophan Metabolites. Biochemistry 1998, 37, 11508–11515. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-Propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium Species as Probiotics: Potentials and Challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, C.; Gao, J. Extensive Summary of the Important Roles of Indole Propionic Acid, a Gut Microbial Metabolite in Host Health and Disease. Nutrients 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Plant, J.D. Repeatability and Reproducibility of Numerical Rating Scales and Visual Analogue Scales for Canine Pruritus Severity Scoring. Vet. Dermatol. 2007, 18, 294–300. [Google Scholar] [CrossRef] [PubMed]
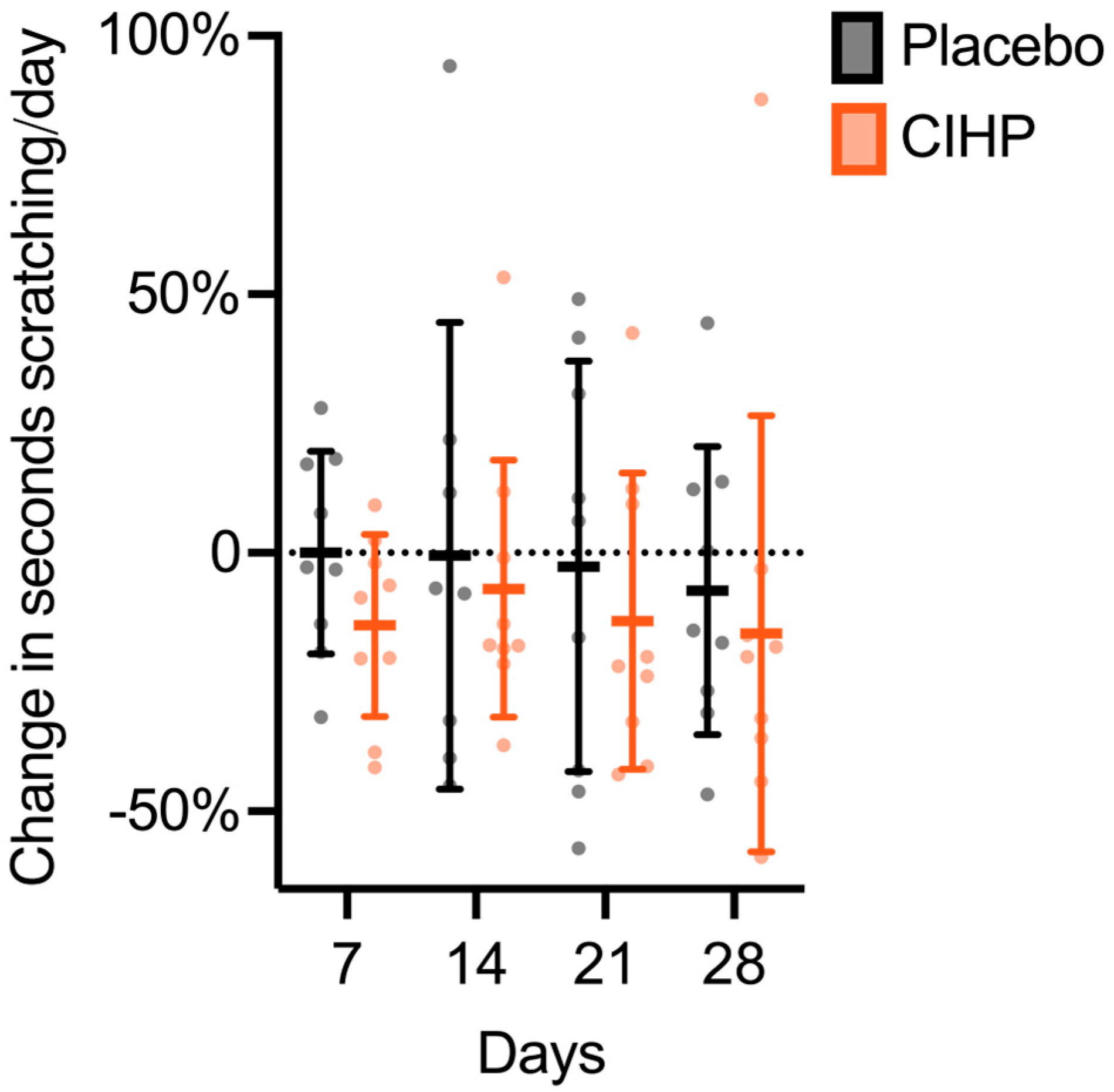
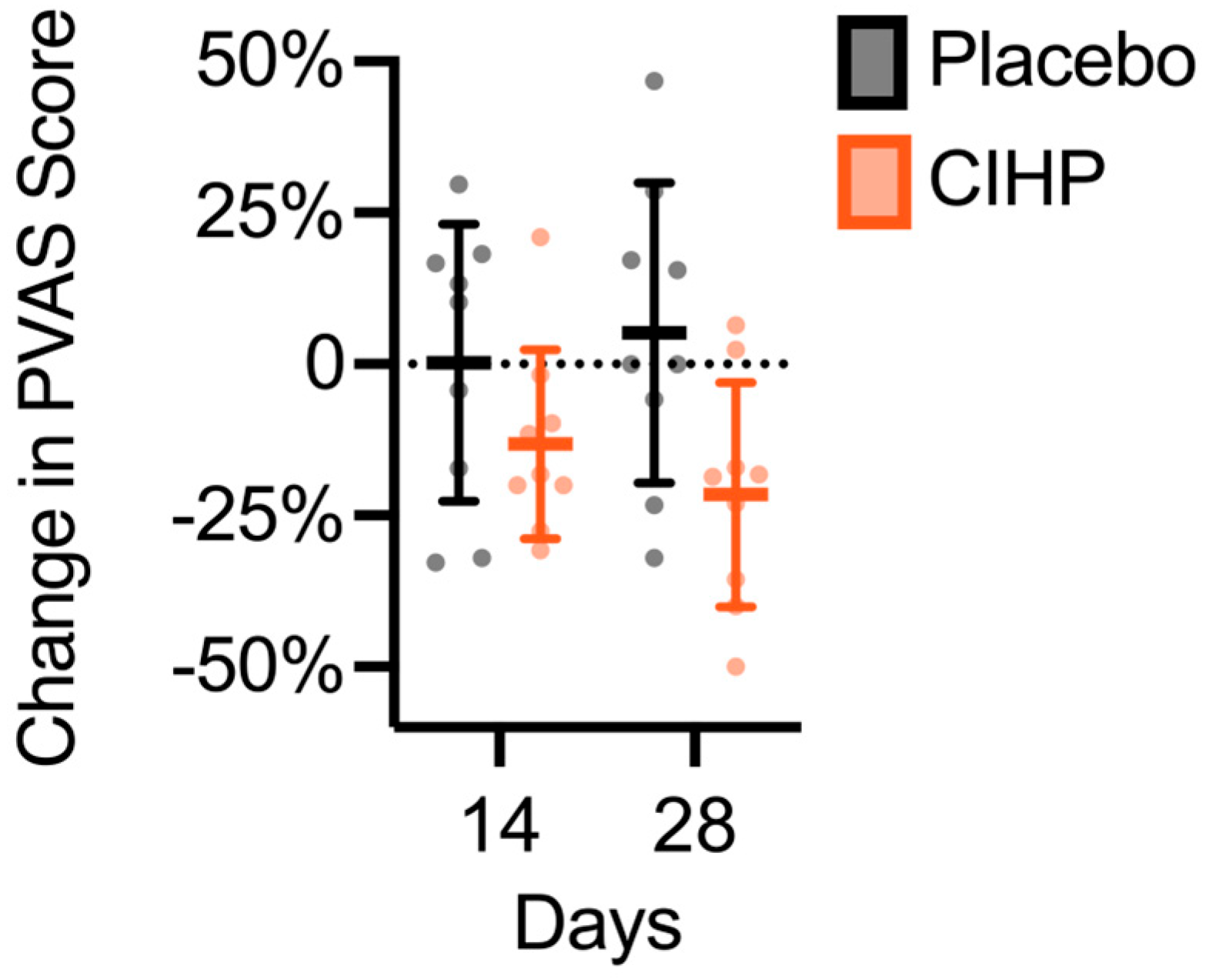
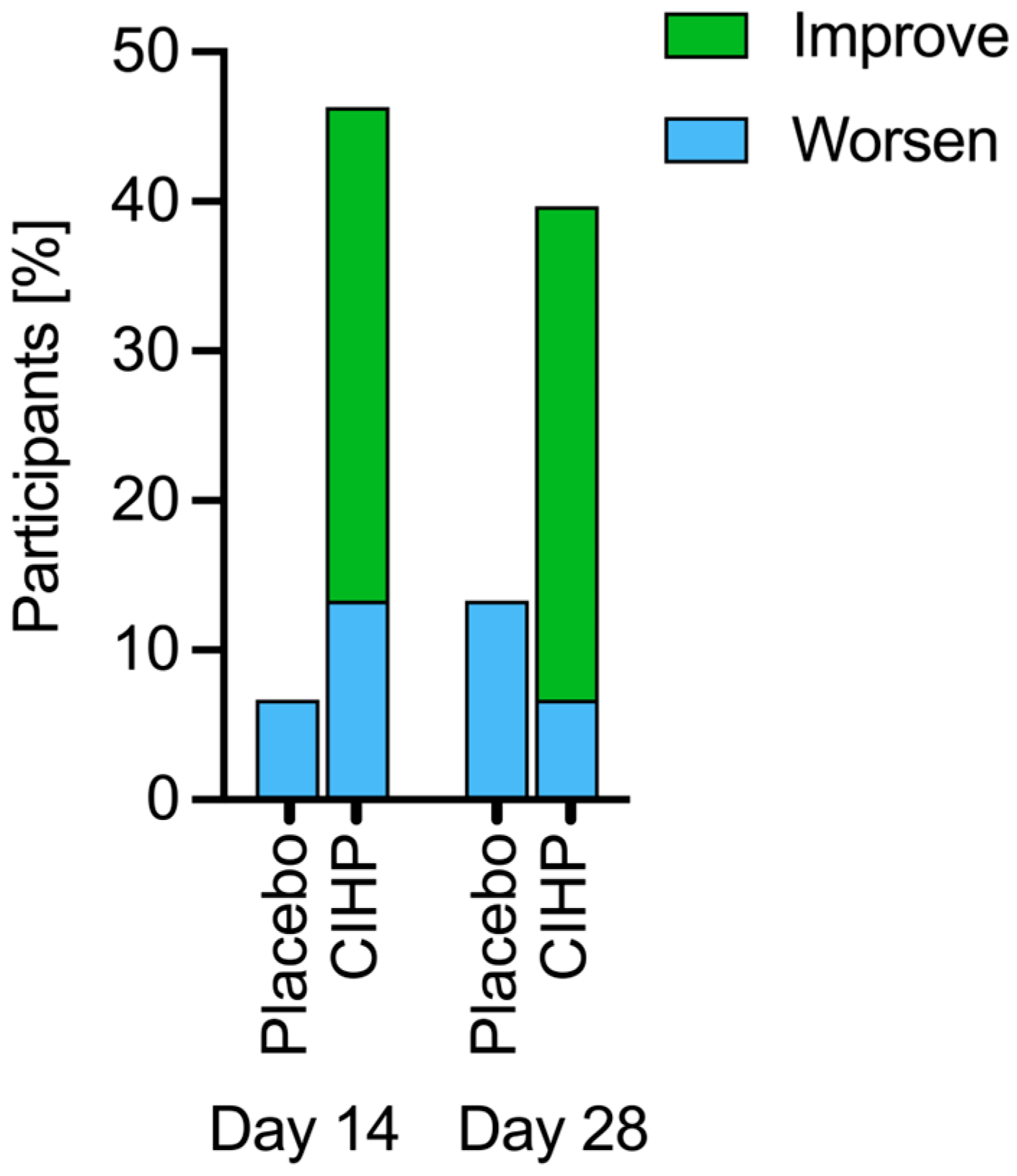
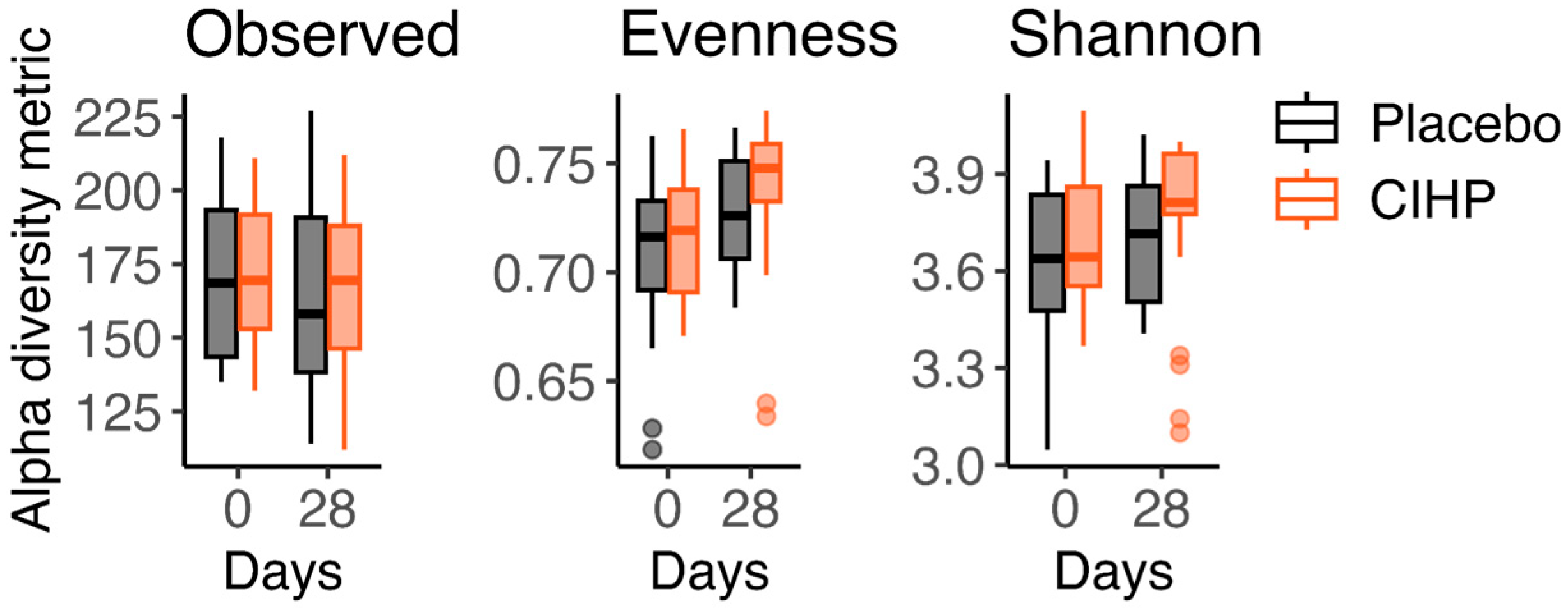
| Timepoint | ||||||
|---|---|---|---|---|---|---|
| Group | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Scratching [s/day] | CIHP | 151 ± 78 | 144 ± 92 | 136 ± 80 | 132 ± 80 | 128 ± 77 |
| Placebo | 184 ± 52 | 185 ± 70 | 196 ± 140 | 192 ± 116 | 180 ± 96 | |
| Sex ratio [female:male] | CIHP | 2:8 | 2:7 | 2:8 | 2:7 | 2:7 |
| Placebo | 4:5 | 4:5 | 3:5 | 4:5 | 4:5 | |
| Participants * [n] | CIHP | 10 | 9 | 10 | 9 | 9 |
| Placebo | 9 | 9 | 8 | 9 | 9 | |
| Group | Measure | Observed | Evenness | Shannon |
|---|---|---|---|---|
| Placebo | p-value | 0.12 | 0.015 | 0.092 |
| % change | −6.2 | 1.4 | 2.1 | |
| CIHP | p-value | 0.21 | 0.00095 | 0.043 |
| % change | 0.0 | 4.0 | 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sordillo, A.; Heldrich, J.; Turcotte, R.; Sheth, R.U. An Indole-Rich Postbiotic Reduces Itching in Dogs: A Randomized, Double-Blinded Placebo-Controlled Study. Animals 2025, 15, 2019. https://doi.org/10.3390/ani15142019
Sordillo A, Heldrich J, Turcotte R, Sheth RU. An Indole-Rich Postbiotic Reduces Itching in Dogs: A Randomized, Double-Blinded Placebo-Controlled Study. Animals. 2025; 15(14):2019. https://doi.org/10.3390/ani15142019
Chicago/Turabian StyleSordillo, Aylesse, Jonna Heldrich, Raphaël Turcotte, and Ravi U. Sheth. 2025. "An Indole-Rich Postbiotic Reduces Itching in Dogs: A Randomized, Double-Blinded Placebo-Controlled Study" Animals 15, no. 14: 2019. https://doi.org/10.3390/ani15142019
APA StyleSordillo, A., Heldrich, J., Turcotte, R., & Sheth, R. U. (2025). An Indole-Rich Postbiotic Reduces Itching in Dogs: A Randomized, Double-Blinded Placebo-Controlled Study. Animals, 15(14), 2019. https://doi.org/10.3390/ani15142019






