Characterization and Evaluation of Lactic Acid Bacteria from Feline Milk for Probiotic Properties
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Purification
2.2.1. Isolation
2.2.2. Purification
2.3. Species Identification
2.4. Assessment of the Growth Kinetics
2.4.1. Growth Curve
2.4.2. Tolerance for Acid and Bile Salt
2.4.3. Tolerance for Simulating the GIT Environment
2.5. Assessment of Adhesion Capacity
2.5.1. Auto-Aggregation
2.5.2. Hydrophobicity Assay
2.5.3. Adhesion to Caco-2 Cell Line
2.6. Safety Assessment
2.6.1. Hemolytic Capacity
2.6.2. Antibiotic Susceptibility Assay
2.7. Assessment of Antipathogenic Activity
2.7.1. Antimicrobial Ability
2.7.2. Co-Aggregative Assay
2.8. Antioxidative Capacity
2.8.1. Tolerance for H2O2
2.8.2. Total Antioxidant Capacity
2.8.3. DPPH Radical Scavenging Ability
2.8.4. Reducing Capacity
2.9. Metabolite Assessment
2.9.1. Bile Salt Hydrolase (BSH) Production
2.9.2. Extracellular Polymeric Substances (EPS) Production
2.9.3. γ-aminobutyric Acid (GABA) Production
2.10. Statistical Analysis
3. Results
3.1. Species Identification
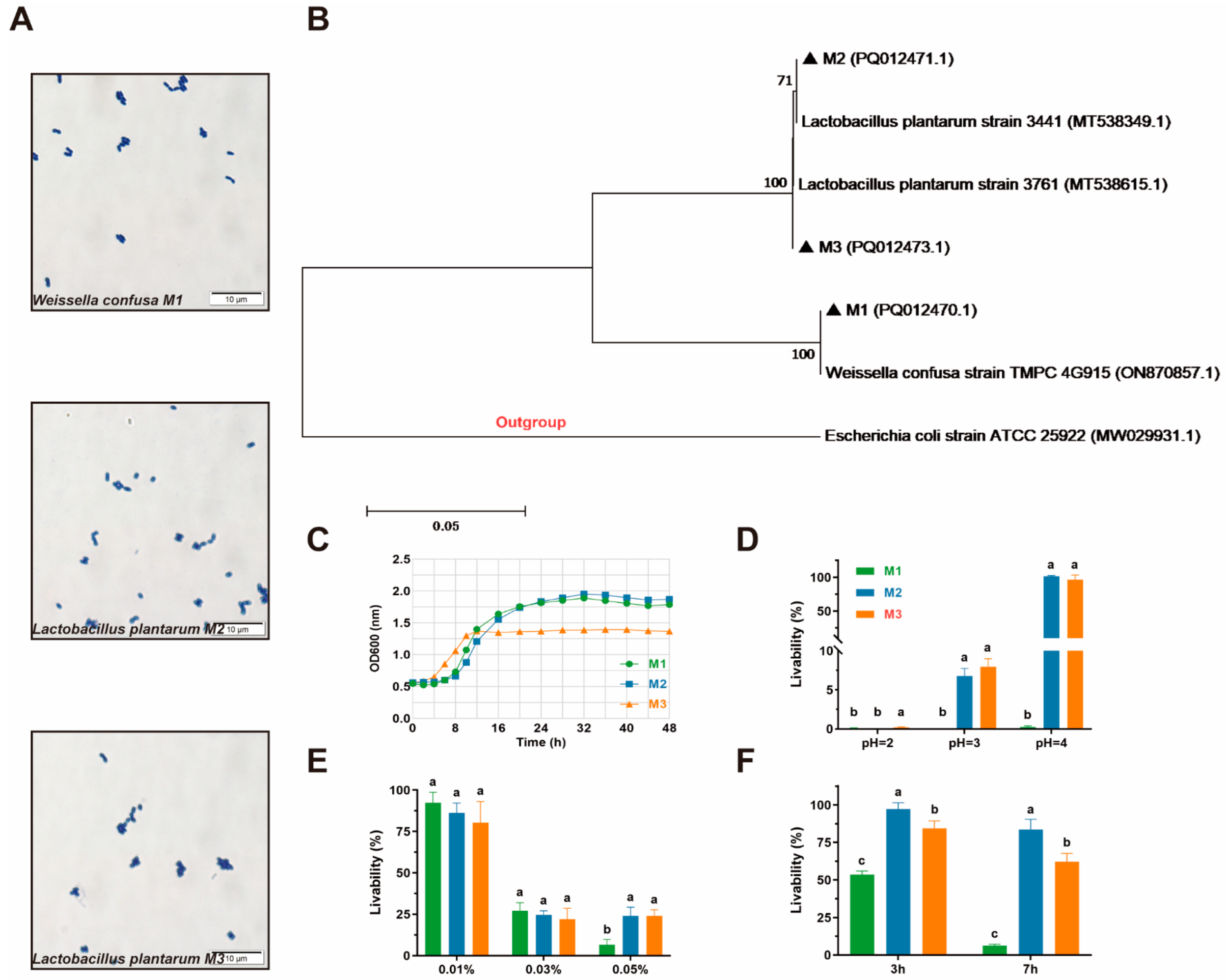
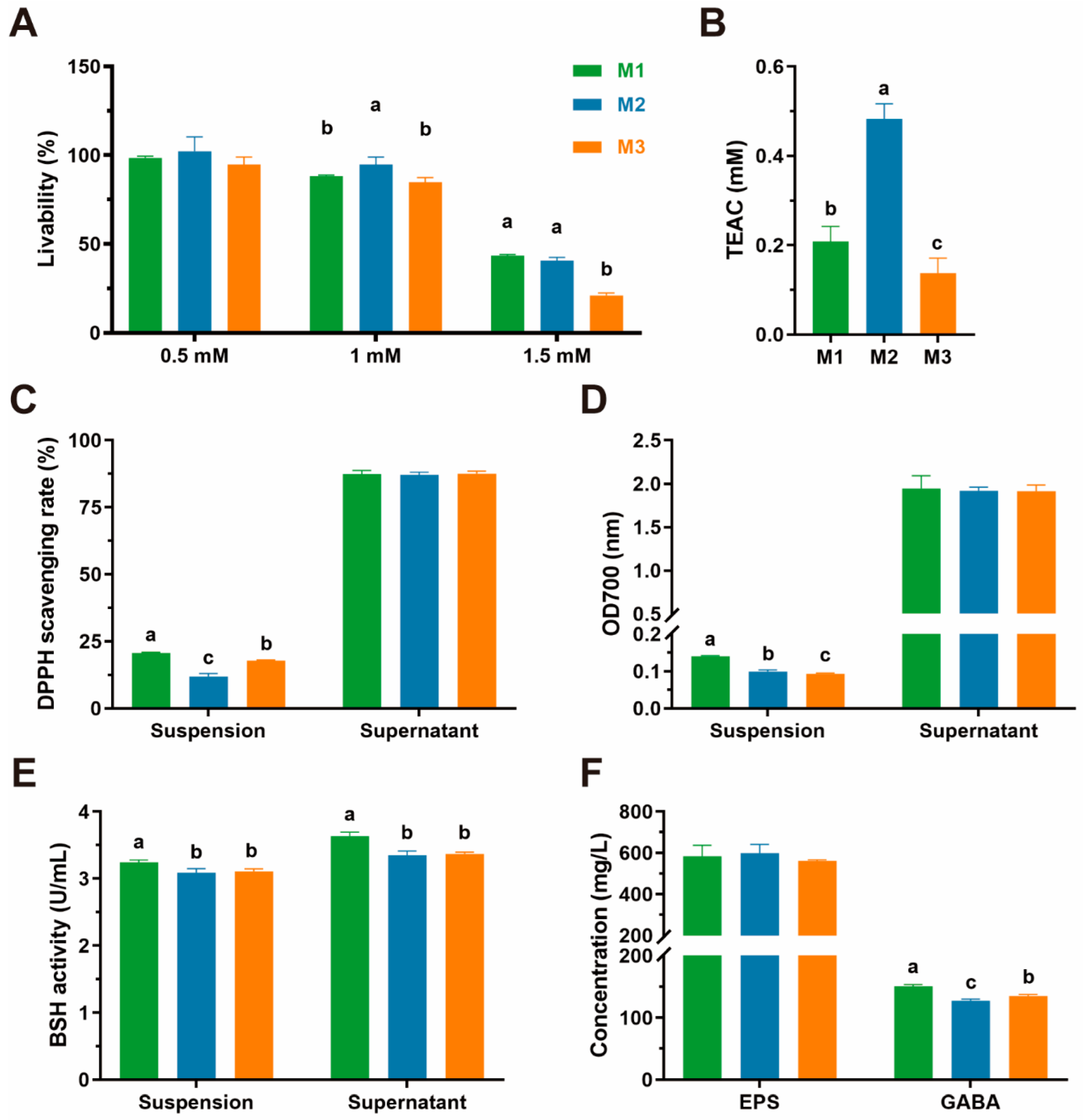
3.2. Growth Kinetics
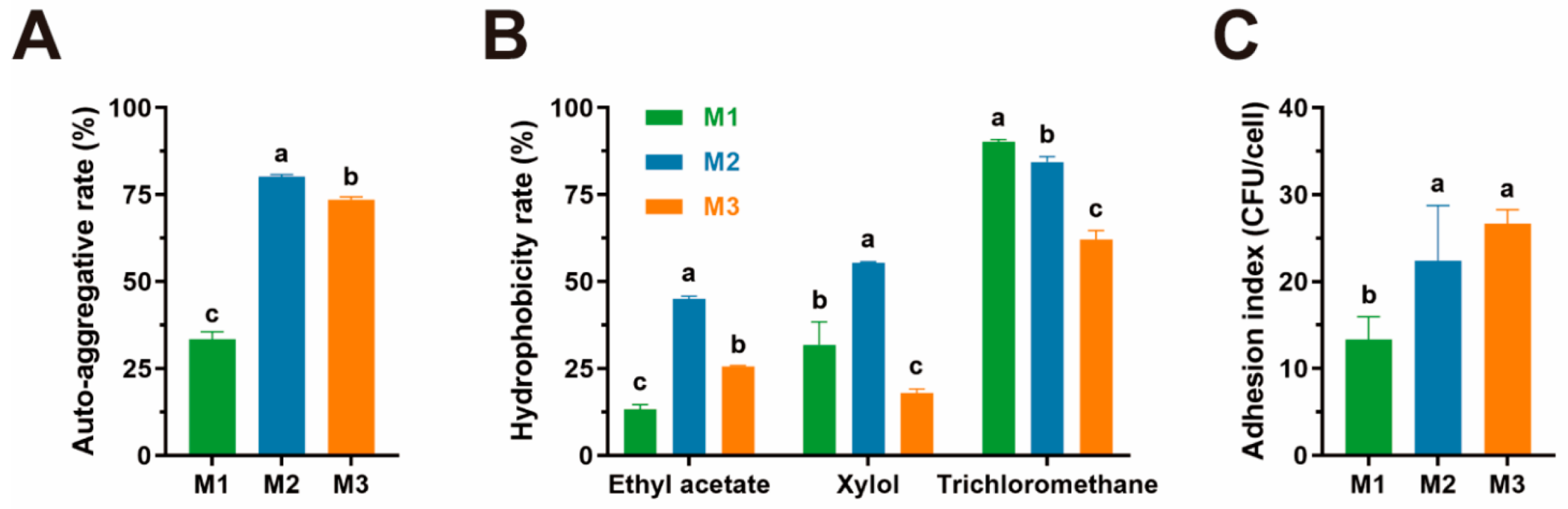
3.3. Adhesion Capacity
3.4. Safety Assessment
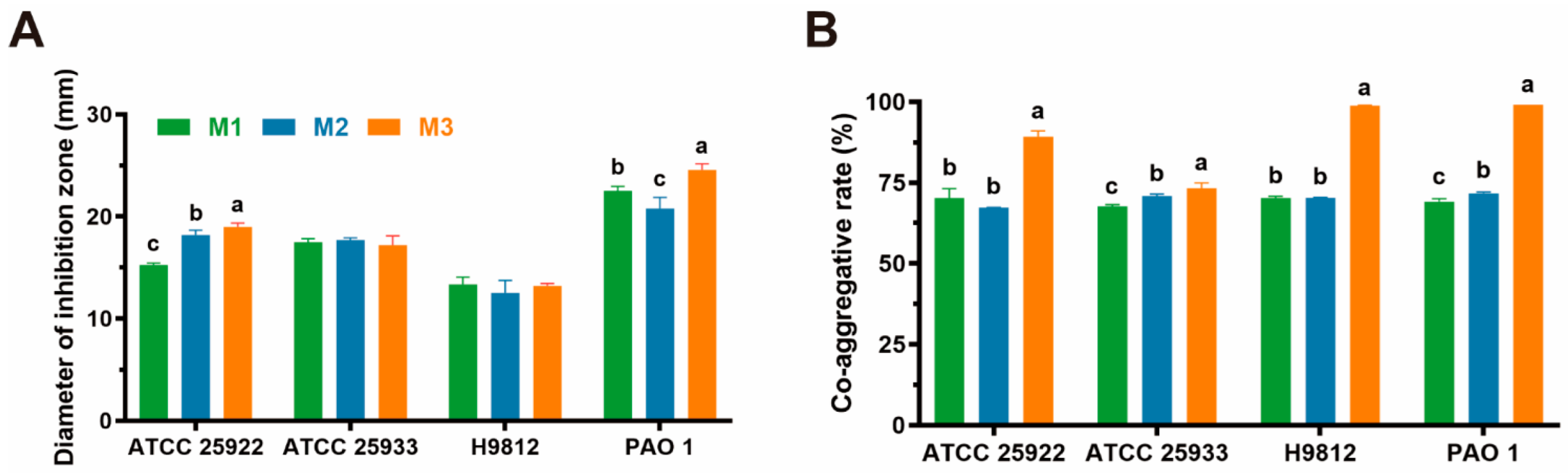
3.5. Antipathogenic Activity
3.6. Antioxidative Capacity
3.7. Metabolite Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J. Antimicrobial Resistance: An Antimicrobial/Diagnostic Stewardship and Infection Prevention Approach. Med. Clin. N. Am. 2018, 102, 819–829. [Google Scholar] [CrossRef]
- Honigsbaum, M. Superbugs and Us. Lancet 2018, 391, 420. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Allerton, F.; Prior, C.; Bagcigil, A.F.; Broens, E.; Callens, B.; Damborg, P.; Dewulf, J.; Filippitzi, M.-E.; Carmo, L.P.; Gómez-Raja, J.; et al. Overview and Evaluation of Existing Guidelines for Rational Antimicrobial Use in Small-Animal Veterinary Practice in Europe. Antibiotics 2021, 10, 409. [Google Scholar] [CrossRef]
- Dickson, A.; Smith, M.; Smith, F.; Park, J.; King, C.; Currie, K.; Langdridge, D.; Davis, M.; Flowers, P. Understanding the Relationship between Pet Owners and Their Companion Animals as a Key Context for Antimicrobial Resistance-Related Behaviours: An Interpretative Phenomenological Analysis. Health Psychol. Behav. Med. 2019, 7, 45–61. [Google Scholar] [CrossRef]
- Smith, M.; King, C.; Davis, M.; Dickson, A.; Park, J.; Smith, F.; Currie, K.; Flowers, P. Pet Owner and Vet Interactions: Exploring the Drivers of AMR. Antimicrob. Resist. Infect. Control 2018, 7, 46. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO: Quebec, ON, Canada; WHO: Geneva, Switzerland, 2002; pp. 1–11. [Google Scholar]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Rubayet Ul Alam, A.S.M.; Jahid, I.K. Characterization and Evaluation of Lactic Acid Bacteria from Indigenous Raw Milk for Potential Probiotic Properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hou, K.; Zhao, J.; Wang, H. The Probiotic Properties of Lactic Acid Bacteria and Their Applications in Animal Husbandry. Curr. Microbiol. 2021, 79, 22. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jin, W.; Liu, S.-J.; Jiao, Z.; Li, X. Probiotics, Prebiotics, and Postbiotics in Health and Disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, S.; Zhou, Z.; Yang, J.; Liu, H.; Zhong, Z.; Fu, H.; Ren, Z.; Shen, L.; Cao, S.; et al. Screening and Evaluation of Lactic Acid Bacteria with Probiotic Potential from Local Holstein Raw Milk. Front. Microbiol. 2022, 13, 918774. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and Probiotics in Canine and Feline Welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Li, Y.; Liu, K.; Zhang, C.; Li, G. Complete Genome Sequence and Probiotic Properties of Pediococcus Acidilactici CLP03 Isolated from Healthy Felis Catus. Probiotics Antimicrob. Proteins 2023, 17, 903–917. [Google Scholar] [CrossRef]
- Chiang, M.-L.; Chen, H.-C.; Chen, K.-N.; Lin, Y.-C.; Lin, Y.-T.; Chen, M.-J. Optimizing Production of Two Potential Probiotic Lactobacilli Strains Isolated from Piglet Feces as Feed Additives for Weaned Piglets. Asian-Australas. J. Anim. Sci. 2015, 28, 1163–1170. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P.; Singh, B.R. Selection and Characterization of Probiotic Lactic Acid Bacteria and Its Impact on Growth, Nutrient Digestibility, Health and Antioxidant Status in Weaned Piglets. PLoS ONE 2018, 13, e0192978. [Google Scholar] [CrossRef]
- Jose, N.M.; Bunt, C.R.; McDowell, A.; Chiu, J.Z.S.; Hussain, M.A. Short Communication: A Study of Lactobacillus Isolates’ Adherence to and Influence on Membrane Integrity of Human Caco-2 Cells. J. Dairy Sci. 2017, 100, 7891–7896. [Google Scholar] [CrossRef]
- Wang, J.; Pu, Y.; Zeng, Y.; Chen, Y.; Zhao, W.; Niu, L.; Chen, B.; Yang, Z.; Wu, L.; Pan, K.; et al. Multi-Functional Potential of Five Lactic Acid Bacteria Strains Derived from Giant Panda (Ailuropoda Melanoleuca). Probiotics Antimicrob. Proteins 2023, 15, 668–681. [Google Scholar] [CrossRef]
- Hof, H.; Christen, A.; Hacker, J. Comparative Therapeutic Activities of Ciprofloxacin, Amoxicillin, Ceftriaxone and Co-Trimoxazole in a New Model of Experimental Infection with Escherichia coli. Infection 1986, 14, 190–194. [Google Scholar] [CrossRef]
- El-Shaer, S.; Shaaban, M.; Barwa, R.; Hassan, R.H.E. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement M100-S23. In Proceedings of the First International Conference of Pharmaceutical Sciences, Mansoura, Egypt, 9–11 April 2017. [Google Scholar]
- Hua, Q.; Chen, C.; Tel Zur, N.; Wang, H.; Wu, J.; Chen, J.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Metabolomic Characterization of Pitaya Fruit from Three Red-Skinned Cultivars with Different Pulp Colors. Plant Physiol. Biochem. 2018, 126, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Chang, F.J. Antioxidative Effect of Intestinal Bacteria Bifidobacterium Longum ATCC 15708 and Lactobacillus Acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Li, Y.; Bumann, M.; Raftis, E.J.; Casey, P.G.; Cooney, J.C.; Walsh, M.A.; O’Toole, P.W. Allelic Variation of Bile Salt Hydrolase Genes in Lactobacillus Salivarius Does Not Determine Bile Resistance Levels. J. Bacteriol. 2009, 191, 5743–5757. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Cui, T.; Zeng, J.; Chen, L.; Zhang, W.; Xu, X.; Cheng, L.; Li, M.; Li, J.; Zhou, X.; et al. Molecule Targeting Glucosyltransferase Inhibits Streptococcus Mutans Biofilm Formation and Virulence. Antimicrob. Agents Chemother. 2016, 60, 126–135. [Google Scholar] [CrossRef]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the Probiotic Properties of New Lactobacillus and Bifidobacterium Strains and Their in Vitro Effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus Plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Guo, X.-H.; Kim, J.-M.; Nam, H.-M.; Park, S.-Y.; Kim, J.-M. Screening Lactic Acid Bacteria from Swine Origins for Multistrain Probiotics Based on in Vitro Functional Properties. Anaerobe 2010, 16, 321–326. [Google Scholar] [CrossRef]
- Gao, W.; Jing, H.; Qiu, B.; Zhang, S.; Zhang, J.; Xu, L.; Ba, F.; Xie, S.; Liu, X.-M.; Li, L.; et al. Effects of Biofilm Formation on Gastrointestinal Tolerance, Mucoadhesion and Transcriptomic Responses of Probiotics. Food Sci. Nutr. 2025, 13, e70206. [Google Scholar] [CrossRef]
- Kim, K.-T.; Kim, J.-W.; Kim, S.-I.; Kim, S.; Nguyen, T.H.; Kang, C.-H. Antioxidant and Anti-Inflammatory Effect and Probiotic Properties of Lactic Acid Bacteria Isolated from Canine and Feline Feces. Microorganisms 2021, 9, 1971. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.C.; Langa, R.L.S.; Aiyegoro, O.A.; Okoh, A.I. Safety Evaluation and Colonisation Abilities of Four Lactic Acid Bacteria as Future Probiotics. Probiotics Antimicrob. Proteins 2019, 11, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Sirichokchatchawan, W.; Pupa, P.; Praechansri, P.; Am-In, N.; Tanasupawat, S.; Sonthayanon, P.; Prapasarakul, N. Autochthonous Lactic Acid Bacteria Isolated from Pig Faeces in Thailand Show Probiotic Properties and Antibacterial Activity against Enteric Pathogenic Bacteria. Microb. Pathog. 2018, 119, 208–215. [Google Scholar] [CrossRef]
- Adams, M.R. Safety of Industrial Lactic Acid Bacteria. J. Biotechnol. 1999, 68, 171–178. [Google Scholar] [CrossRef]
- Jose, N.M.; Bunt, C.R.; Hussain, M.A. Comparison of Microbiological and Probiotic Characteristics of Lactobacilli Isolates from Dairy Food Products and Animal Rumen Contents. Microorganisms 2015, 3, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Al Kassaa, I.; Hamze, M.; Hober, D.; Chihib, N.-E.; Drider, D. Identification of Vaginal Lactobacilli with Potential Probiotic Properties Isolated from Women in North Lebanon. Microb. Ecol. 2014, 67, 722–734. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-Specific Probiotics Properties of Lactobacillus Fermentum, Lactobacillus Plantarum and Lactobacillus Brevis Isolates from Brazilian Food Products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef]
- Xia, Y.; Qin, S.; Shen, Y. Probiotic Potential of Weissella Strains Isolated from Horse Feces. Microb. Pathog. 2019, 132, 117–123. [Google Scholar] [CrossRef]
- Kiousi, D.E.; Efstathiou, C.; Tzampazlis, V.; Plessas, S.; Panopoulou, M.; Koffa, M.; Galanis, A. Genetic and Phenotypic Assessment of the Antimicrobial Activity of Three Potential Probiotic Lactobacilli against Human Enteropathogenic Bacteria. Front. Cell. Infect. Microbiol. 2023, 13, 1127256. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Rohani, M. Antibiotic Resistance and the Alternatives to Conventional Antibiotics: The Role of Probiotics and Microbiota in Combating Antimicrobial Resistance. Microbiol. Res. 2023, 267, 127275. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Rojas, M.F.; Reinheimer, J.A.; Binetti, A.G. Lactobacillus Fermentum: Could EPS Production Ability Be Responsible for Functional Properties? Food Microbiol. 2020, 90, 103465. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, H.; Lang, Y.; Zhao, Y.; Sun, H.; Zhang, P.; Ma, X.; Han, J.; Wang, Q.; Zhou, J.; et al. The Effects of Acute GABA Treatment on the Functional Connectivity and Network Topology of Cortical Cultures. Neurochem. Res. 2017, 42, 1394–1402. [Google Scholar] [CrossRef]
- Hamet, M.F.; Piermaria, J.A.; Abraham, A.G. Selection of EPS-Producing Lactobacillus Strains Isolated from Kefir Grains and Rheological Characterization of the Fermented Milks. LWT—Food Sci. Technol. 2015, 63, 129–135. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Shu, G.; Ma, W. Screening of Gamma-Aminobutyric Acid-Producing Lactic Acid Bacteria and Its Application in Monascus-Fermented Rice Production. Acta Sci. Pol. Technol. Aliment. 2020, 19, 387–394. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, Prebiotics and Amelioration of Diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
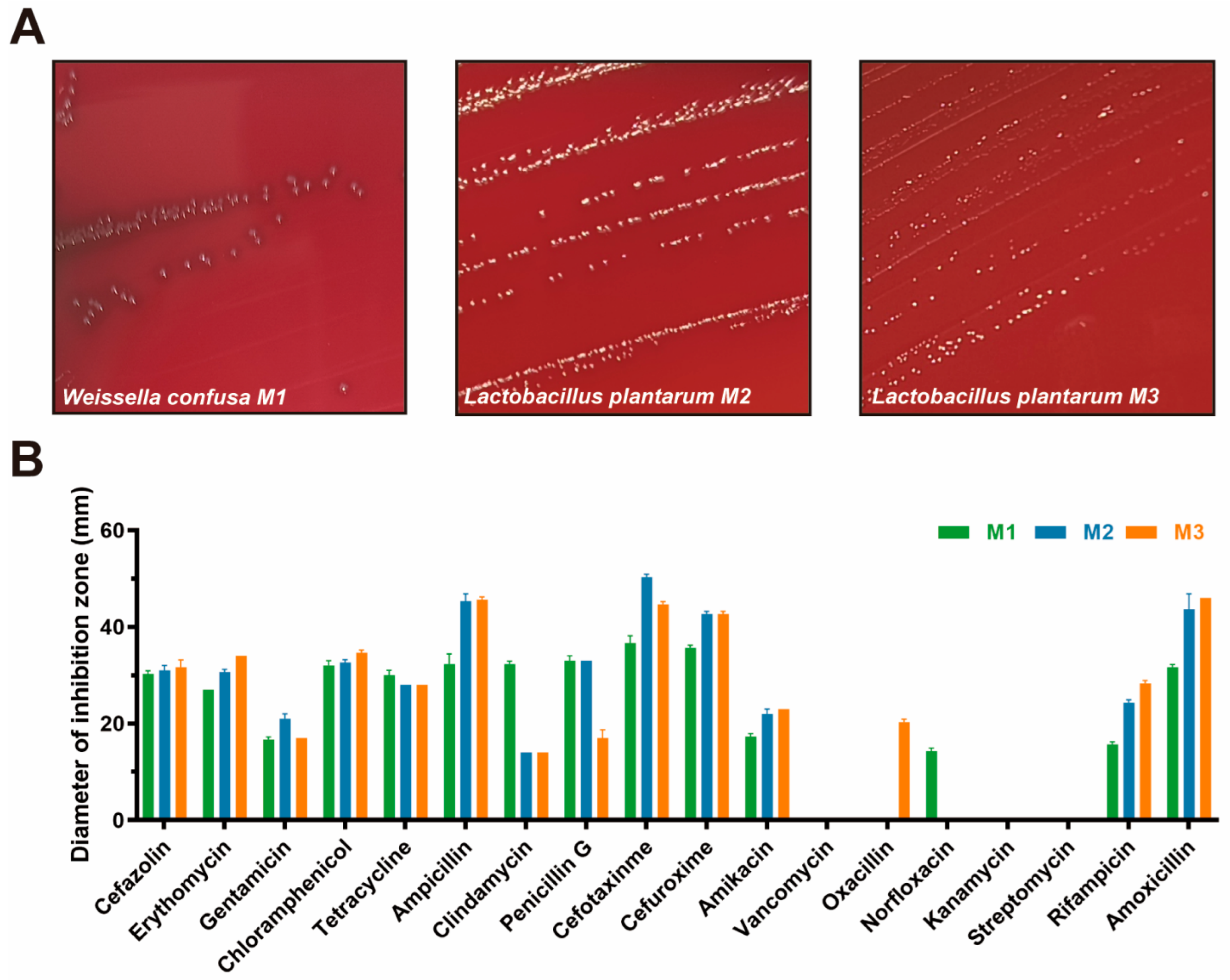
| Strain | Antibiotic Susceptibility | Sensitive Rate (S + I, %) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KZ | E | GM | C | TE | AMP | DA | P | CTX | CXM | AK | VA | OX | NOR | KM | S | RD | AML | ||
| M1 | S | S | I | S | S | S | S | S | S | S | I | R | R | R | R | R | I | S | 72.22 |
| M2 | S | S | S | S | S | S | R | S | S | S | S | S | R | R | R | R | S | S | 72.22 |
| M3 | S | S | I | S | S | S | R | I | S | S | S | S | R | I | R | R | S | S | 77.78 |
| Strain | Diameter of Inhibition Zone (mm) | |||
|---|---|---|---|---|
| E. coli ATCC 25922 | Staphylococcus aureus ATCC 25923 | Salmonella H9812 | P. aeruginosa PAO 1 | |
| M1 | 15.27 ± 0.16 II | 17.62 ± 0.27 III | 12.99 ± 0.27 II | 22.49 ± 0.45 IV |
| M2 | 18.25 ± 0.46 III | 17.64 ± 0.15 III | 12.32 ± 1.17 II | 21.12 ± 0.90 IV |
| M3 | 19.20 ± 0.16 III | 17.65 ± 0.50 III | 13.29 ± 0.19 II | 24.31 ± 0.45 IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Wang, J.; Liu, Y.; Zhong, Z.; Liu, H.; Zhou, Z.; Peng, G. Characterization and Evaluation of Lactic Acid Bacteria from Feline Milk for Probiotic Properties. Animals 2025, 15, 1990. https://doi.org/10.3390/ani15131990
Zheng H, Wang J, Liu Y, Zhong Z, Liu H, Zhou Z, Peng G. Characterization and Evaluation of Lactic Acid Bacteria from Feline Milk for Probiotic Properties. Animals. 2025; 15(13):1990. https://doi.org/10.3390/ani15131990
Chicago/Turabian StyleZheng, Haohong, Jiali Wang, Yunjiang Liu, Zhijun Zhong, Haifeng Liu, Ziyao Zhou, and Guangneng Peng. 2025. "Characterization and Evaluation of Lactic Acid Bacteria from Feline Milk for Probiotic Properties" Animals 15, no. 13: 1990. https://doi.org/10.3390/ani15131990
APA StyleZheng, H., Wang, J., Liu, Y., Zhong, Z., Liu, H., Zhou, Z., & Peng, G. (2025). Characterization and Evaluation of Lactic Acid Bacteria from Feline Milk for Probiotic Properties. Animals, 15(13), 1990. https://doi.org/10.3390/ani15131990







