Hematological Changes and Immunomodulation of Neutrophil and Monocyte Populations in Shelter Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Material Collection
2.2. Hematological and Biochemical Analysis
2.3. Canine Peripheral Blood Mononuclear Cell Isolation and LPS Stimulation
2.4. Extracellular Staining
2.5. Apoptosis Assay
2.6. Flow Cytometry Analysis
2.7. Statistical Analysis
3. Results
3.1. Animals
3.2. Blood Samples—Hematology and Biochemistry
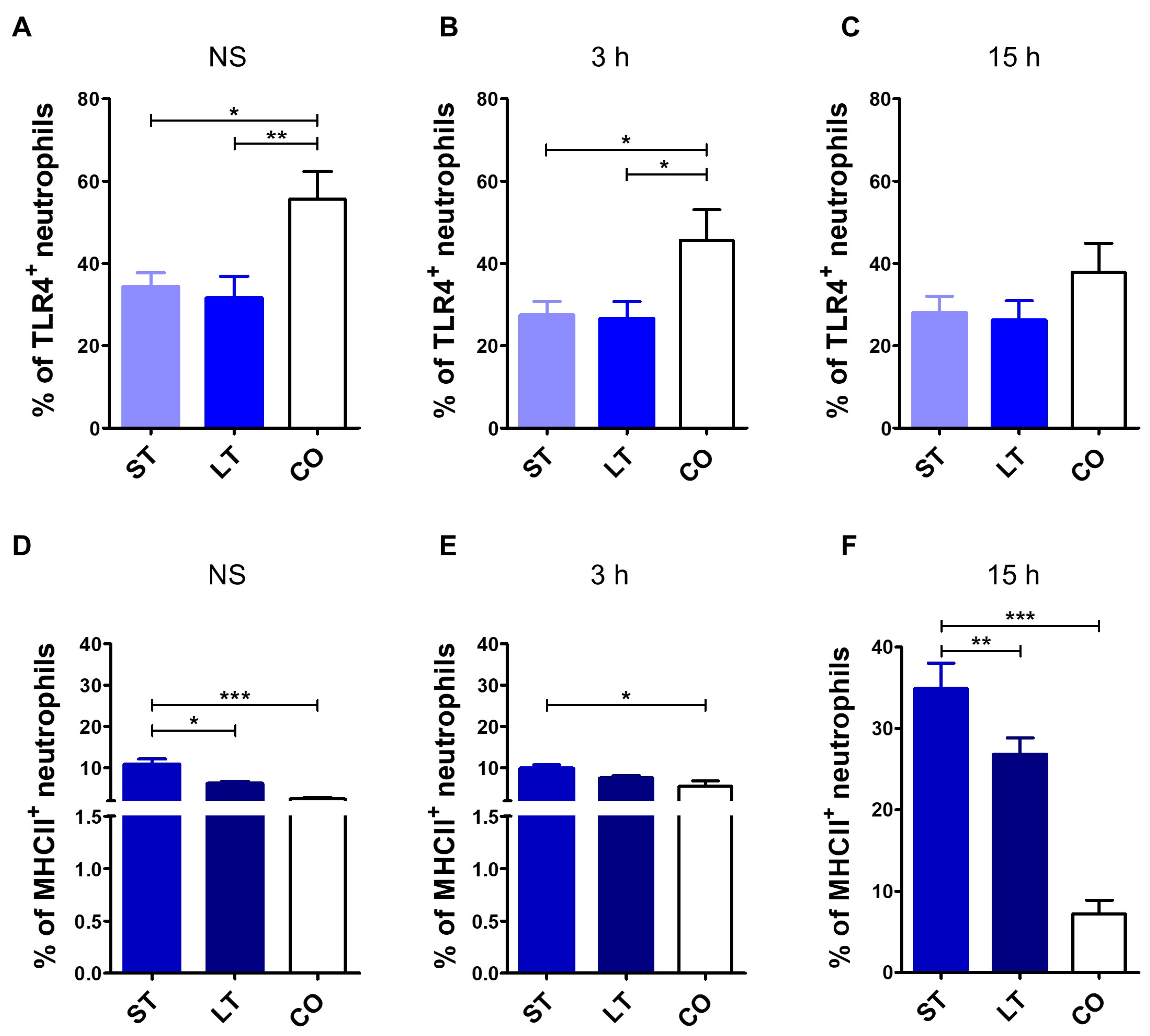
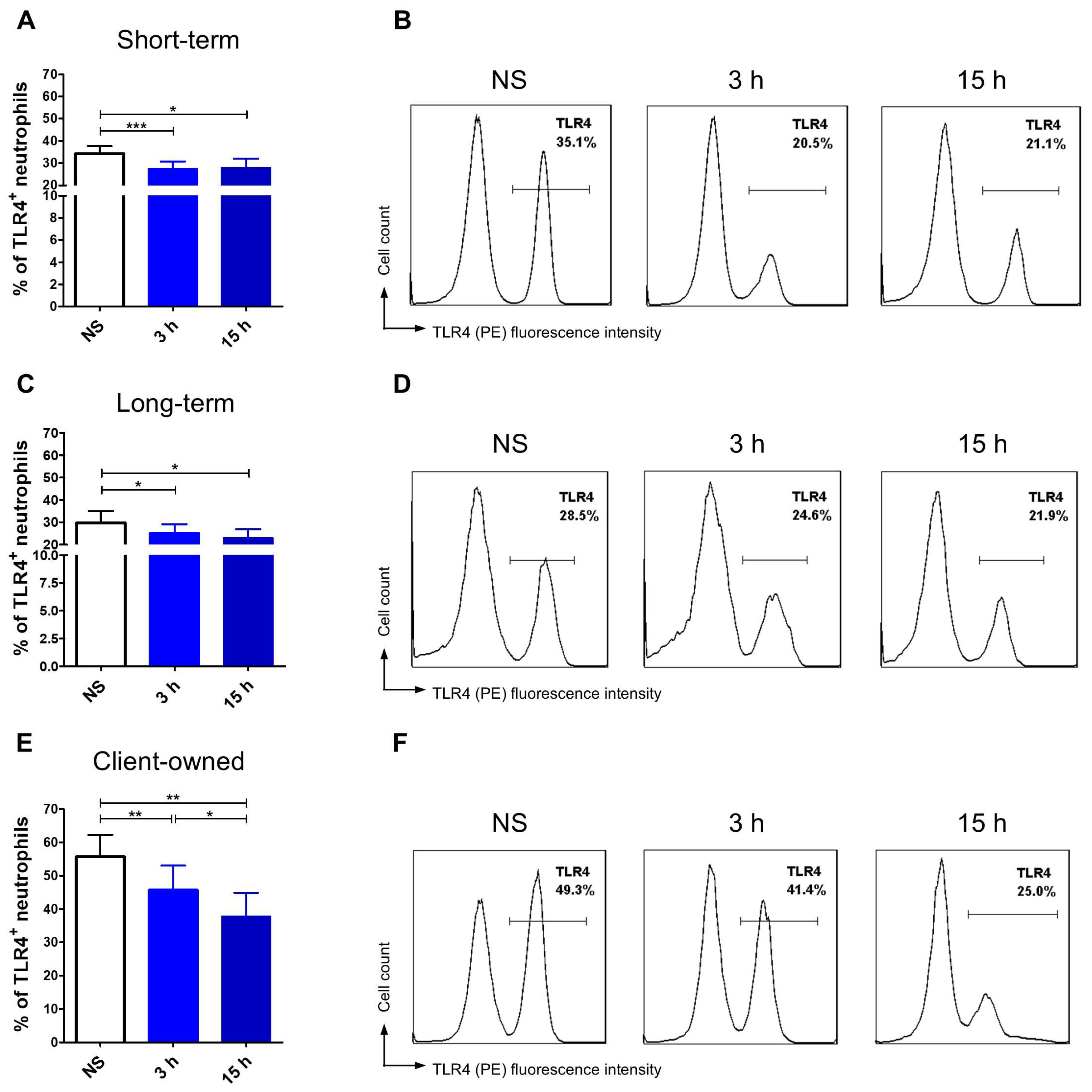
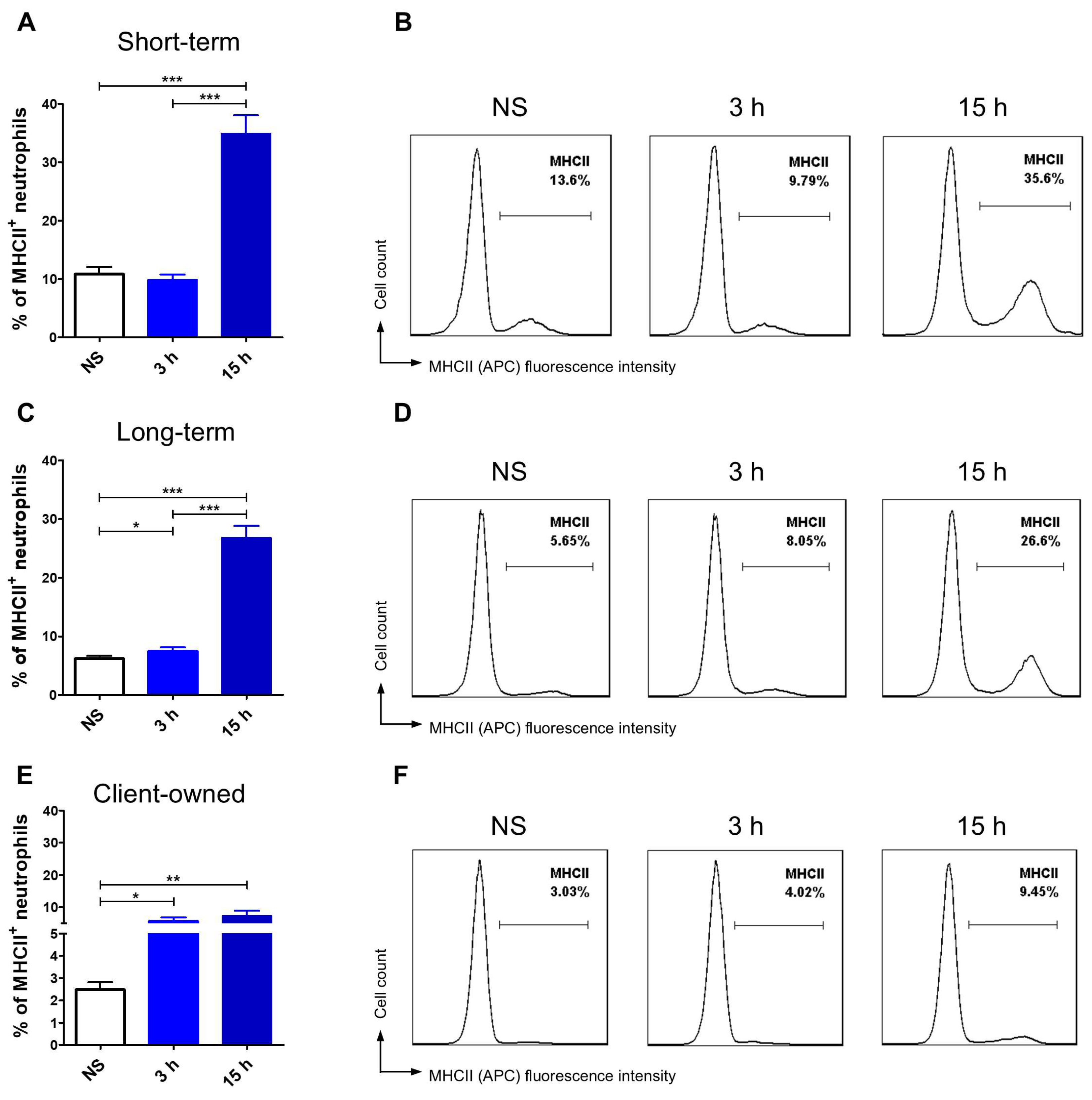
3.3. TLR4 and MHCII Expression Assessment
3.3.1. Neutrophils
3.3.2. Monocytes
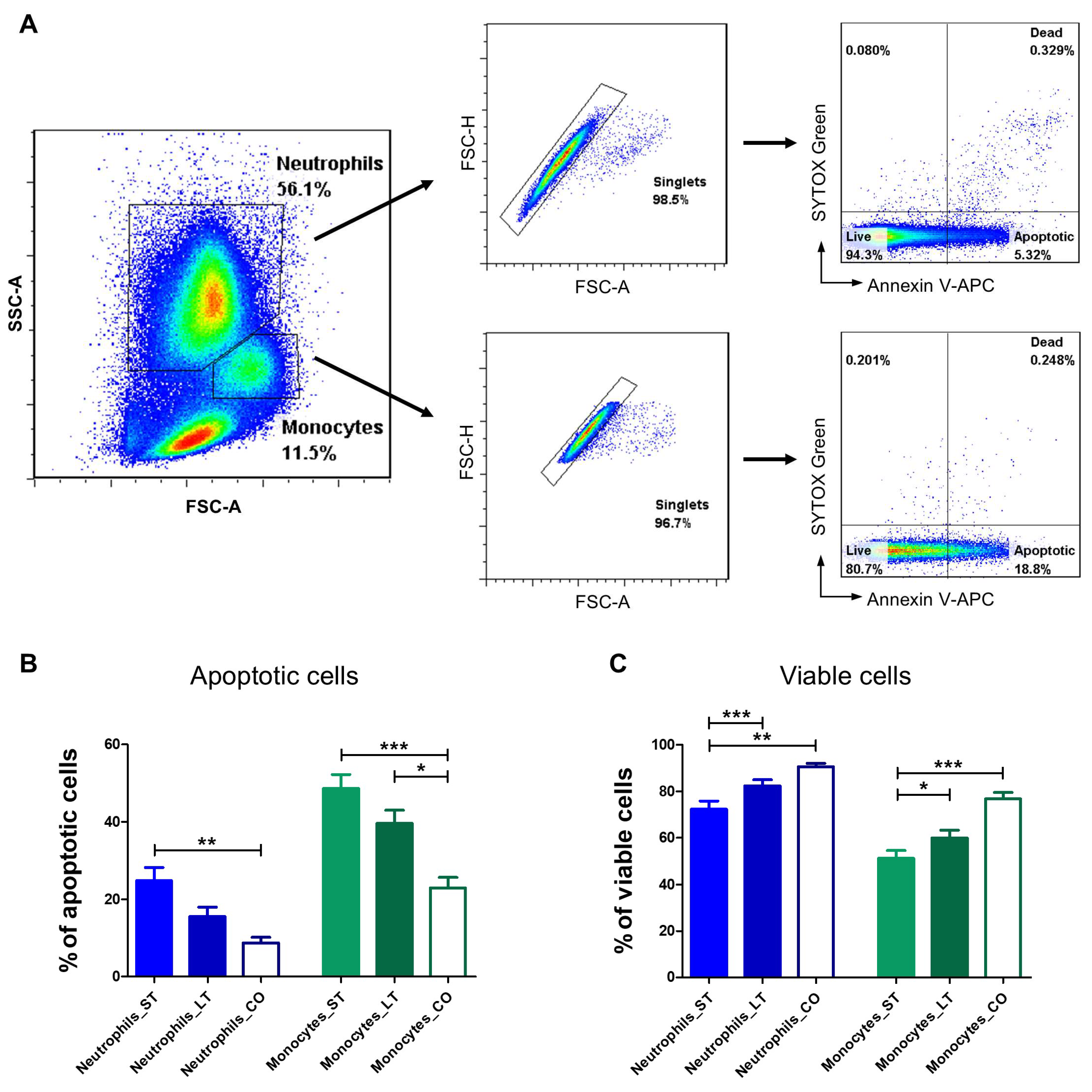
3.4. Apoptosis Evaluation
3.4.1. Neutrophils
3.4.2. Monocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhabhar, F.S. Effects of Stress on Immune Function: The Good, the Bad, and the Beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Dhabhar, F.S. The Short-Term Stress Response—Mother Nature’s Mechanism for Enhancing Protection and Performance under Conditions of Threat, Challenge, and Opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Miller, A.H.; Spencer, R.L.; hassett, J.; Kim, C.; Rhee, R.; Ciurea, D.; Dhabhar, F.; McEwen, B.; Stein, M. Effects of Selective Type I and II Adrenal Steroid Agonists on Immune Cell Distribution. Endocrinology 1994, 135, 1934–1944. [Google Scholar] [CrossRef]
- Barrett, T.J.; Corr, E.M.; Solingen, C.; van Schlamp, F.; Brown, E.J.; Koelwyn, G.J.; Lee, A.H.; Shanley, L.C.; Spruill, T.M.; Bozal, F.; et al. Chronic Stress Primes Innate Immune Responses in Mice and Humans. Cell Rep. 2021, 36, 109595. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liao, F.; Tian, Y.; Wang, Y.; Xia, F.; Wang, J. Investigating the Crosstalk between Chronic Stress and Immune Cells: Implications for Enhanced Cancer Therapy. Front. Neurosci. 2023, 17, 1321176. [Google Scholar] [CrossRef]
- Alotiby, A. Immunology of Stress: A Review Article. J. Clin. Med. 2024, 13, 6394. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.F.; Zachariae, R.; Bovbjerg, D.H. Psychological Stress and Antibody Response to Influenza Vaccination: A Meta-Analysis. Brain Behav. Immun. 2009, 23, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological Stress and Disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Katz, A.R.; Huntwork, M.P.; Kolls, J.K.; Hewes, J.L.; Ellsworth, C.R.; Clark, R.D.E.; Carlson, J.C. Impact of Psychological Stressors on Natural Killer Cell Function: A Comprehensive Analysis Based on Stressor Type, Duration, Intensity, and Species. Physiol. Behav. 2025, 288, 114734. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Gonzalez Herrero, M.E.; Kuehn, C. A Qualitative Mathematical Model of the Immune Response under the Effect of Stress. Chaos Interdiscip. J. Nonlinear Sci. 2021, 31, 061104. [Google Scholar] [CrossRef] [PubMed]
- Renner, V.; Schellong, J.; Bornstein, S.; Petrowski, K. Stress-Induced pro- and Anti-Inflammatory Cytokine Concentrations in Female PTSD and Depressive Patients. Transl. Psychiatry 2022, 12, 158. [Google Scholar] [CrossRef]
- Seiler, A.; Fagundes, C.P.; Christian, L.M. The Impact of Everyday Stressors on the Immune System and Health. In Stress Challenges and Immunity in Space: From Mechanisms to Monitoring and Preventive Strategies; Choukèr, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–92. ISBN 978-3-030-16996-1. [Google Scholar]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular Links between Endocrine, Nervous and Immune System during Chronic Stress. Brain Behav. 2021, 11, e01960. [Google Scholar] [CrossRef] [PubMed]
- Everds, N.E.; Snyder, P.W.; Bailey, K.L.; Bolon, B.; Creasy, D.M.; Foley, G.L.; Rosol, T.J.; Sellers, T. Interpreting Stress Responses during Routine Toxicity Studies: A Review of the Biology, Impact, and Assessment. Toxicol. Pathol. 2013, 41, 560–614. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kitaoka, S.; Kawano, Y.; Ishii, S.; Suzuki, T.; Wakahashi, K.; Kato, T.; Katayama, Y.; Furuyashiki, T. Repeated Social Defeat Stress Induces Neutrophil Mobilization in Mice: Maintenance after Cessation of Stress and Strain-Dependent Difference in Response. Br. J. Pharmacol. 2021, 178, 827–844. [Google Scholar] [CrossRef]
- Schäfer, I.; Rehbein, S.; Holtdirk, A.; Kottmann, T.; Klein, R.; Müller, E.; Thoren-Tolling, K. Diagnostic Cut-off Values for the Urinary Corticoid:Creatinine Ratio for the Diagnosis of Canine Cushing’s Syndrome Using an Automated Chemiluminescent Assay. Veter. Clin. Pathol. 2023, 52, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Arranz, L.; Guayerbas, N.; De la Fuente, M. Impairment of Several Immune Functions in Anxious Women. J. Psychosom. Res. 2007, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Machida, K. Effects of Life Events and Stress on Neutrophil Functions in Elderly Men. Immun. Ageing 2012, 9, 13. [Google Scholar] [CrossRef]
- Mills, D.; Karagiannis, C.; Zulch, H. Stress--Its Effects on Health and Behavior: A Guide for Practitioners. Vet. Clin. N. Am. Small Anim. Pr. 2014, 44, 525–541. [Google Scholar] [CrossRef]
- Protopopova, A. Effects of Sheltering on Physiology, Immune Function, Behavior, and the Welfare of Dogs. Physiol. Behav. 2016, 159, 95–103. [Google Scholar] [CrossRef]
- Raudies, C.; Waiblinger, S.; Arhant, C. Characteristics and Welfare of Long-Term Shelter Dogs. Animals 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.B. Using Hypothalamic-Pituitary-Adrenal Measures for Assessing and Reducing the Stress of Dogs in Shelters: A Review. Appl. Anim. Behav. Sci. 2013, 149, 1–12. [Google Scholar] [CrossRef]
- Gunter, L.M.; Feuerbacher, E.N.; Gilchrist, R.J.; Wynne, C.D.L. Evaluating the Effects of a Temporary Fostering Program on Shelter Dog Welfare. PeerJ 2019, 7, e6620. [Google Scholar] [CrossRef] [PubMed]
- Rooney, N.J.; Gaines, S.A.; Bradshaw, J.W.S. Behavioural and Glucocorticoid Responses of Dogs (Canis familiaris) to Kennelling: Investigating Mitigation of Stress by Prior Habituation. Physiol. Behav. 2007, 92, 847–854. [Google Scholar] [CrossRef]
- Sandri, M.; Colussi, A.; Perrotta, M.G.; Stefanon, B. Salivary Cortisol Concentration in Healthy Dogs Is Affected by Size, Sex, and Housing Context. J. Vet. Behav. 2015, 10, 302–306. [Google Scholar] [CrossRef]
- Dudley, E.S.; Schiml, P.A.; Hennessy, M.B. Effects of Repeated Petting Sessions on Leukocyte Counts, Intestinal Parasite Prevalence, and Plasma Cortisol Concentration of Dogs Housed in a County Animal Shelter. J. Am. Vet. Med. Assoc. 2015, 247, 1289–1298. [Google Scholar] [CrossRef]
- Menor-Campos, D.J.; Molleda-Carbonell, J.M.; López-Rodríguez, R. Effects of Exercise and Human Contact on Animal Welfare in a Dog Shelter. Vet. Rec. 2011, 169, 388. [Google Scholar] [CrossRef]
- Coppola, C.L.; Grandin, T.; Enns, R.M. Human Interaction and Cortisol: Can Human Contact Reduce Stress for Shelter Dogs? Physiol. Behav. 2006, 87, 537–541. [Google Scholar] [CrossRef]
- Laflamme, D. Development and Validation of a Body Condition Score System for Dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Chun, J.L.; Bang, H.T.; Ji, S.Y.; Jeong, J.Y.; Kim, M.; Kim, B.; Lee, S.D.; Lee, Y.K.; Reddy, K.E.; Kim, K.H. A Simple Method to Evaluate Body Condition Score to Maintain the Optimal Body Weight in Dogs. J. Anim. Sci. Technol. 2019, 61, 366–370. [Google Scholar] [CrossRef]
- German, A.J.; Holden, S.L.; Moxham, G.L.; Holmes, K.L.; Hackett, R.M.; Rawlings, J.M. A Simple, Reliable Tool for Owners to Assess the Body Condition of Their Dog or Cat1–3. J. Nutr. 2006, 136, 2031S–2033S. [Google Scholar] [CrossRef] [PubMed]
- Szopa, I.M.; Granica, M.; Bujak, J.K.; Łabędź, A.; Błaszczyk, M.; Paulos, C.M.; Majchrzak-Kuligowska, K. Effective Activation and Expansion of Canine Lymphocytes Using a Novel Nano-Sized Magnetic Beads Approach. Front. Immunol. 2021, 12, 604066. [Google Scholar] [CrossRef]
- Szopa, I.M.; Majchrzak-Kuligowska, K.; Pingwara, R.; Kulka, M.; Taşdemir, M.; Gajewska, M. A New Method of Canine CD4+ T Lymphocyte Differentiation Towards the Th17 Phenotype with Analysis of Properties and Mitochondrial Activity. Int. J. Mol. Sci. 2025, 26, 4946. [Google Scholar] [CrossRef]
- DeClue, A.E.; Axiak-Bechtel, S.; Cowan, C.F.; Zhang, Y.; Amorim, J.; Halpin, R.; Melillo, G.N.; Hagan, C. Transportation and Routine Veterinary Interventions Alter Immune Function in the Dog. Top. Companion Anim. Med. 2020, 39, 100408. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.W. Veterinary Hematology: A Diagnostic Guide and Color Atlas; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011; ISBN 978-1-4377-0173-9. [Google Scholar]
- Cook, A.M.; Bauer, N.; Neiger, R.; Peppler, C.; Moritz, A. Neutropenia in dogs: Etiology and prognostic factors. Tieraerztliche Prax. Ausg. Kleintiere Heimtiere 2016, 44, 307–315. [Google Scholar] [CrossRef]
- Piotti, P.; Pierantoni, L.; Albertini, M.; Pirrone, F. Inflammation and Behavior Changes in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pr. 2024, 54, 1–16. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; Van Hooff, J.A.R.A.M.; De Vries, H.W.; Mol, J.A. Chronic Stress in Dogs Subjected to Social and Spatial Restriction. I. Behavioral Responses. Physiol. Behav. 1999, 66, 233–242. [Google Scholar] [CrossRef]
- Stephen, J.M.; Ledger, R.A. An Audit of Behavioral Indicators of Poor Welfare in Kenneled Dogs in the United Kingdom. J. Appl. Anim. Welf. Sci. 2005, 8, 79–95. [Google Scholar] [CrossRef]
- Owczarczak-Garstecka, S.C.; Burman, O.H.P. Can Sleep and Resting Behaviours Be Used as Indicators of Welfare in Shelter Dogs (Canis Lupus familiaris)? PLoS ONE 2016, 11, e0163620. [Google Scholar] [CrossRef]
- Troìa, R.; Agnoli, C.; Calipa, S.; Segalina, S.; Murgia, E.; Gruarin, M.; Dondi, F.; Giunti, M. Evaluation of the Delta Neutrophil Index from an Automated Blood Cell Analyser in Septic Dogs. Vet. J. 2017, 230, 13–19. [Google Scholar] [CrossRef]
- Muthusamy, N.; Caligiuri, M.A. The Structure of Lymphocytes and Plasma Cells. In Williams Hematology, 9e; Kaushansky, K., Lichtman, M.A., Prchal, J.T., Levi, M.M., Press, O.W., Burns, L.J., Caligiuri, M., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Vaure, C.; Liu, Y. A Comparative Review of Toll-Like Receptor 4 Expression and Functionality in Different Animal Species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Kanzler, H.; Barrat, F.J.; Hessel, E.M.; Coffman, R.L. Therapeutic Targeting of Innate Immunity with Toll-like Receptor Agonists and Antagonists. Nat. Med. 2007, 13, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Ramakers, B.P.; Kamp, V.M.; Loi, A.L.T.; Lam, S.W.; Hietbrink, F.; Leenen, L.P.; Tool, A.T.; Pickkers, P.; Koenderman, L. Functional Heterogeneity and Differential Priming of Circulating Neutrophils in Human Experimental Endotoxemia. J. Leukoc. Biol. 2010, 88, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Holling, T.M.; Schooten, E.; van Den Elsen, P.J. Function and Regulation of MHC Class II Molecules in T-Lymphocytes: Of Mice and Men. Hum. Immunol. 2004, 65, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils Acquire the Capacity for Antigen Presentation to Memory CD4+ T Cells in Vitro and Ex Vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Schobesberger, M.; Summerfield, A.; Doherr, M.G.; Zurbriggen, A.; Griot, C. Canine Distemper Virus-Induced Depletion of Uninfected Lymphocytes Is Associated with Apoptosis. Vet. Immunol. Immunopathol. 2005, 104, 33–44. [Google Scholar] [CrossRef]
- Noseykina, E.M.; Schepetkin, I.A.; Atochin, D.N. Molecular Mechanisms for Regulation of Neutrophil Apoptosis under Normal and Pathological Conditions. J. Evol. Biochem. Physiol. 2021, 57, 429–450. [Google Scholar] [CrossRef]
- Akgul, C.; Moulding, D.A.; Edwards, S.W. Molecular Control of Neutrophil Apoptosis. FEBS Lett. 2001, 487, 318–322. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Słotwińska, M.; Stachura, A.; Marmurowska-Michałowska, H.; Dubas-Ślemp, H.; Bojarska-Junak, A.; Kandefer-Szerszeń, M. Accelerated Apoptosis of Blood Leukocytes and Oxidative Stress in Blood of Patients with Major Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 686–694. [Google Scholar] [CrossRef]
- Maianski, N.A.; Maianski, A.N.; Kuijpers, T.W.; Roos, D. Apoptosis of Neutrophils. Acta Haematol. 2003, 111, 56–66. [Google Scholar] [CrossRef]
- Sarjan, H.N.; Yajurvedi, H.N. Chronic Stress Induced Duration Dependent Alterations in Immune System and Their Reversibility in Rats. Immunol. Lett. 2018, 197, 31–43. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Units | Values | |||
|---|---|---|---|---|---|
| Short-Term | Long-Term | Client-Owned | Reference Ranges | ||
| RBC | T/L | 7 ± 0.74 | 7.26 ± 0.48 | 7.3 ± 0.5 | 5.2–7.9 |
| HGB | g/dL | 15.72 ± 1.51 | 16.57 ± 1.17 | 15.83 ± 1.27 | 12.4–19.2 |
| HCT | % | 44.23 ± 4.59 | 46.09 ± 3.07 | 45.37 ± 3.5 | 35.0–52.0 |
| MCV | fL | 63.84 ± 2.48 | 63.68 ± 2.53 | 63.78 ± 3.2 | 60.0–71.0 |
| MCHC | g/dL | 35.92 ± 1.02 | 36.97 ± 2.55 | 36.87 ± 2.7 | 34.4–38.1 |
| MCH | pg | 22.8 ± 0.61 | 23.1 ± 0.74 | 23.9 ± 0.87 | 21.9–26.3 |
| PLT | G/L | 221.58 ± 72.05 | 258.4 ± 52.73 | 284 ± 56.34 | 108–562 |
| WBC | G/L | 10.7 ± 2.25 | 9.41 ± 1.73 | 10 ± 2.15 | 6.00–17.00 |
| NEU | G/L | 6.57 ± 2.35 | 5.4 ± 1.19 | 6.1 ± 1.42 | 2.90–13.60 |
| LYM | G/L | 2.49 ± 0.85 | 2.72 ± 0.88 | 2.5 ± 0.75 | 1.10–5.30 |
| MON | G/L | 0.8 ± 0.35 | 0.57 ± 0.12 | 0.7 ± 0.20 | 0.40–1.60 |
| EOS | G/L | 0.62 ± 0.48 | 0.47 ± 0.16 | 0.7 ± 0.49 | 0.10–3.10 |
| BAS | % | 0.48 ± 0.26 | 0.29 ± 0.16 | 0.37 ± 0.18 | 0,00–1,00 |
| ALT | U/L | 39.74 ± 11.73 | 38.47 ± 11.06 | 35.67 ± 10.76 | <60.0 |
| AST | U/L | 32.04 ± 10.01 | 35.53 ± 8.47 | 34.56 ± 7.64 | <45.0 |
| ALP | U/L | 46.75 ± 22.09 | 59.80 ± 15.24 | 49.78 ± 16.74 | <155.0 |
| Urea | mg/dL | 27.08 ± 7.61 | 26.73 ± 6.22 | 29.23 ± 5.93 | 20.0–50.0 |
| Creatinine | mg/dL | 0.82 ± 0.18 | 0.91 ± 0.13 | 0.87 ± 0.19 | 0.5–1.7 |
| Total Protein | mg/dL | 6.84 ± 0.46 | 6.81 ± 0.37 | 6.9 ± 0.31 | 5.5–7.5 |
| Albumin | g/dL | 3.88 ± 0.71 | 3.91 ± 0.12 | 3.89 ± 0.36 | 3.3–5.6 |
| Globulin | g/dL | 3.1 ± 0.50 | 2.91 ± 0.33 | 3.2 ± 0.43 | 2.1–4.5 |
| Lipase | U/L | 70 ± 0.24 | 83 ± 0.54 | 78 ± 0.96 | <120 |
| Cortisol | μg/dL | 1 ± 0.22 | 1 ± 0.30 | 1 ± 0.28 | 1–6 |
| CRP | mg/L | <10 | <10 | <10 | <20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulka, M.; Szopa, I.M.; Klockiewicz, M. Hematological Changes and Immunomodulation of Neutrophil and Monocyte Populations in Shelter Dogs. Animals 2025, 15, 1988. https://doi.org/10.3390/ani15131988
Kulka M, Szopa IM, Klockiewicz M. Hematological Changes and Immunomodulation of Neutrophil and Monocyte Populations in Shelter Dogs. Animals. 2025; 15(13):1988. https://doi.org/10.3390/ani15131988
Chicago/Turabian StyleKulka, Marek, Iwona Monika Szopa, and Maciej Klockiewicz. 2025. "Hematological Changes and Immunomodulation of Neutrophil and Monocyte Populations in Shelter Dogs" Animals 15, no. 13: 1988. https://doi.org/10.3390/ani15131988
APA StyleKulka, M., Szopa, I. M., & Klockiewicz, M. (2025). Hematological Changes and Immunomodulation of Neutrophil and Monocyte Populations in Shelter Dogs. Animals, 15(13), 1988. https://doi.org/10.3390/ani15131988






