Evaluation of Cannabidiol Oil’s Effects on Sedation, Behavioral Responses to Handling, and Nociceptive Thresholds in Healthy Cats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Protocols
2.3. Quantification of CBD Concentrations in Plasma via In-Solution Digestion and Nanoscale Liquid Chromatography–Tandem Mass Spectrometry (nanoLC-MS/MS)
2.4. Bioinformatic Analysis of CBD Concentration in Plasma
3. Results
3.1. Behavioral Observations and Physiological Data
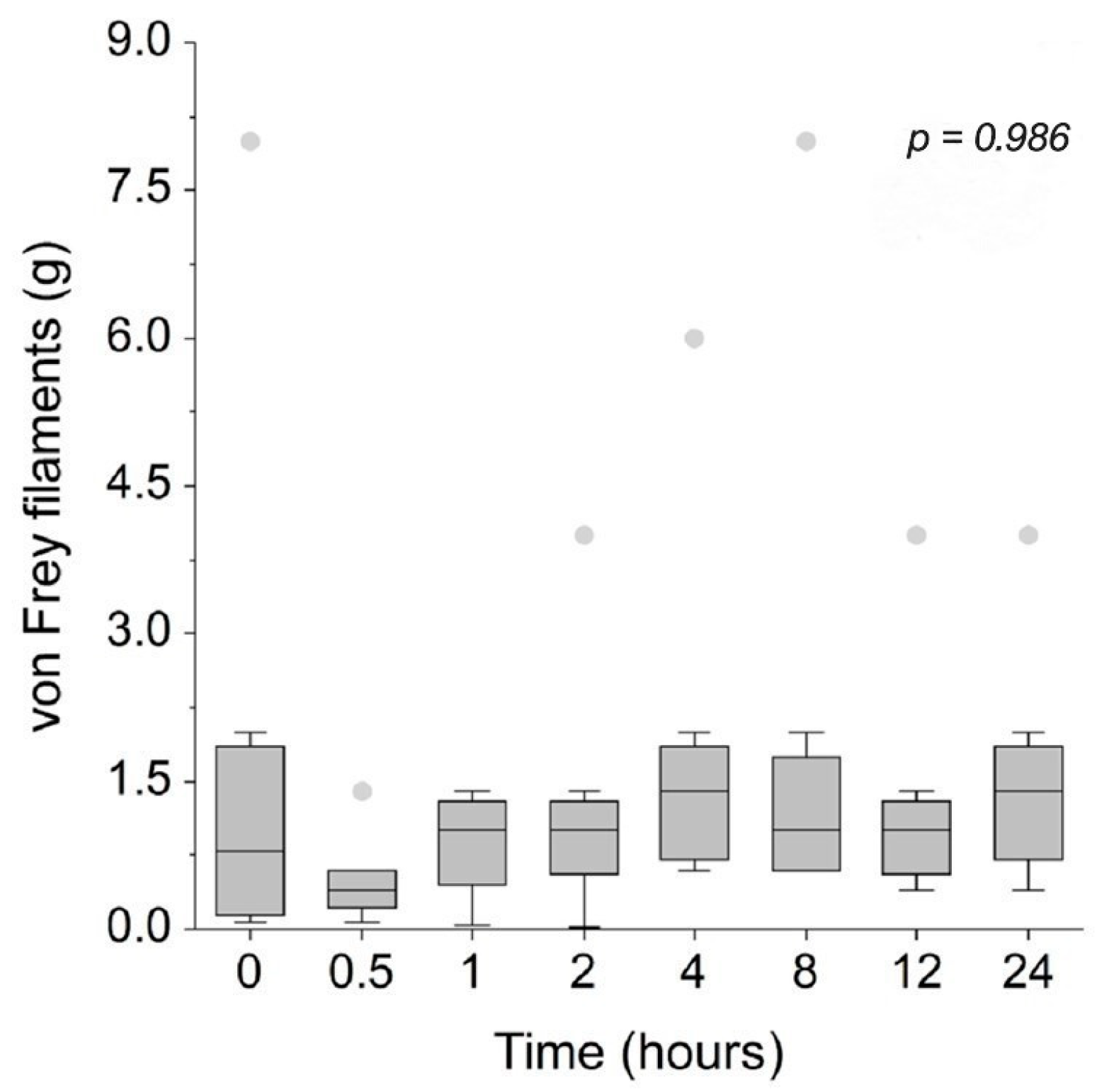
3.2. Mechanical Sensitivity and Nociceptive Thresholds
3.3. Plasma Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| ECS | Endocannabinoid system |
| CB1 | Cannabinoid receptors 1 |
| CB2 | Cannabinoid receptors 2 |
| 5-HT1A | Serotonin 5-HT1A receptor |
| TRPV1 | Transient receptor potential vanilloid 1 |
| THC | Tetrahydrocannabinol |
| nanoLC-MS/MS | Nanoscale liquid chromatography–tandem mass spectrometry |
| BUN | Blood urea nitrogen |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| DSH | Domestic shorthair |
| SD | Standard deviation |
| HR | Heart rate |
| RR | Respiratory rate |
| IQR | Interquartile range |
| ANOVA | Analysis of variance |
| FDR | False discovery rate |
| MS2 | Tandem mass spectrometry |
| THCA | Tetrahydrocannabinolic acid |
| CBDA | Cannabidiolic acid |
Appendix A
| Criteria | Score |
|---|---|
| Completely awake, able to stand and walk, normal posture | 0 |
| Stands but staggers when attempting to walk | 1 |
| Sternal recumbency, able to lift head up, unable to stand, may makes weak attempts to rise | 2 |
| Lateral recumbency, responds to gentle stroking, a moving toy, or handclapping (able to slightly lift the head, tail, or a limb) | 3 |
| Lateral recumbency, does not respond to gentle stroking, a moving toy, or handclapping | 4 |
References
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Kogan, L.R.; Hellyer, P.W.; Robinson, N.G. Consumers’ perceptions of hemp products for animals. J. Am. Holist. Vet. Med. Assoc. 2017, 42, 40–48. [Google Scholar]
- Alvarenga, I.C.; Panickar, K.S.; Hess, H.; McGrath, S. Scientific Validation of Cannabidiol for Management of Dog and Cat Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid receptors: An update on cell signaling, pathophysiological roles and therapeutic opportunities in neurological, cardiovascular, and inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2018, 160, 136–150. [Google Scholar] [CrossRef]
- Nocheva, H.; Stoynev, N.; Vodenicharov, V.; Krastev, D.; Krastev, N.; Mileva, M. Cannabinoid and Serotonergic Systems: Unraveling the Pathogenetic Mechanisms of Stress-Induced Analgesia. Biomedicines 2024, 12, 235. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; E Ponde, D.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef]
- Kogan, L.R.; Hellyer, P.W.; Silcox, S.; Schoenfeld-Tacher, R. Canadian dog owners’ use and perceptions of cannabis products. Can. Vet. J. 2019, 60, 749–755. [Google Scholar]
- Kogan, L.; Schoenfeld-Tacher, R.; Hellyer, P.; Rishniw, M. US Veterinarians’ Knowledge, Experience, and Perception Regarding the Use of Cannabidiol for Canine Medical Conditions. Front. Vet. Sci. 2019, 5, 338. [Google Scholar] [CrossRef]
- Kogan, L.; Hellyer, P.; Downing, R. The use of cannabidiol-rich hemp oil extract to treat canine osteoarthritis-related pain: A pilot study. J. Am. Holistic Vet. Med. Assoc. 2020, 58, 11. [Google Scholar]
- Yu, C.H.; Rupasinghe, H.V. Cannabidiol-based natural health products for companion animals: Recent advances in the management of anxiety, pain, and inflammation. Res. Vet. Sci. 2021, 140, 38–46. [Google Scholar] [CrossRef]
- Gamble, L.-J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K.J.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Masataka, N. Possible effects of cannabidiol (CBD) administration on the vocal activity of healthy domestic dogs upon their temporary separation from caregivers. Heliyon 2024, 10, e25548. [Google Scholar] [CrossRef]
- Masataka, N. Is cannabidiol (CBD) effective to ease separation anxiety? Heliyon 2024, 10, e25851. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.M.; Kitts-Morgan, S.E.; Spangler, D.M.; McLeod, K.R.; Costa, J.H.C.; Harmon, D.L. The Impact of Feeding Cannabidiol (CBD) Containing Treats on Canine Response to a Noise-Induced Fear Response Test. Front. Vet. Sci. 2020, 7, 569565. [Google Scholar] [CrossRef]
- Corsetti, S.; Borruso, S.; Malandrucco, L.; Spallucci, V.; Maragliano, L.; Perino, R.; D’aGostino, P.; Natoli, E. Cannabis sativa L. may reduce aggressive behaviour towards humans in shelter dogs. Sci. Rep. 2021, 11, 2773. [Google Scholar] [CrossRef]
- Deabold, K.A.; Schwark, W.S.; Wolf, L.; Wakshlag, J.J. Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats. Animals 2019, 9, 832. [Google Scholar] [CrossRef]
- E Kulpa, J.; Paulionis, L.J.; Eglit, G.M.; Vaughn, D.M. Safety and tolerability of escalating cannabinoid doses in healthy cats. J. Feline Med. Surg. 2021, 23, 1162–1175. [Google Scholar] [CrossRef]
- Wang, T.; Zakharov, A.; Gomez, B.; Lyubimov, A.; Trottier, N.L.; Schwark, W.S.; Wakshlag, J.J. Serum Cannabinoid 24 h and 1 Week Steady State Pharmacokinetic Assessment in Cats Using a CBD/CBDA Rich Hemp Paste. Front. Vet. Sci. 2022, 9, 895368. [Google Scholar] [CrossRef]
- Rozental, A.J.; Gustafson, D.L.; Kusick, B.R.; Bartner, L.R.; Castro, S.C.; McGrath, S. Pharmacokinetics of escalating single-dose administration of cannabidiol to cats. J. Vet. Pharmacol. Ther. 2023, 46, 25–33. [Google Scholar] [CrossRef]
- Coltherd, J.C.; Bednall, R.; Bakke, A.M.; Ellerby, Z.; Newman, C.; Watson, P.; Logan, D.W.; Holcombe, L.J. Healthy cats tolerate long-term daily feeding of Cannabidiol. Front. Vet. Sci. 2024, 10, 1324622. [Google Scholar] [CrossRef]
- Bookout, W.; Dziwenka, M.; Valm, K.; Kovacs-Nolan, J. Safety study of cannabidiol products in healthy dogs. Front. Vet. Sci. 2024, 11, 1349590. [Google Scholar] [CrossRef]
- Abyadeh, M.; Meyfour, A.; Gupta, V.; Moghaddam, M.Z.; Fitzhenry, M.J.; Shahbazian, S.; Salekdeh, G.H.; Mirzaei, M. Recent Advances of Functional Proteomics in Gastrointestinal Cancers- a Path towards the Identification of Candidate Diagnostic, Prognostic, and Therapeutic Molecular Biomarkers. Int. J. Mol. Sci. 2020, 21, 8532. [Google Scholar] [CrossRef]
- Abyadeh, M.; Gupta, V.; Liu, X.; Rossio, V.; Mirzaei, M.; Cornish, J.; Paulo, J.A.; Haynes, P.A. Proteome-Wide Profiling Using Sample Multiplexing of a Human Cell Line Treated with Cannabidiol (CBD) and Tetrahydrocannabinol (THC). Proteomes 2023, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Bindukumar, B.; Mahajan, S.; Reynolds, J.; Hu, Z.; Sykes, D.; Aalinkeel, R.; Schwartz, S. Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Res. 2008, 1191, 1–11. [Google Scholar] [CrossRef][Green Version]
- Slingsby, L.S.; Lane, E.C.; Mears, E.R.; Shanson, M.C.; Waterman-Pearson, A.E. Postoperative pain after ovariohysterectomy in the cat: A comparison of two anaesthetic regimens. Vet. Rec. 1998, 143, 589–590. [Google Scholar] [CrossRef]
- Ansah, O.B.; Raekallio, M.; Vainio, O. Correlation between serum concentrations following continuous intravenous infusion of dexmedetomidine or medetomidine in cats and their sedative and analgesic effects. J. Vet. Pharmacol. Ther. 2000, 23, 1–8. [Google Scholar] [CrossRef]
- Dobbins, S.; Brown, N.O.; Shofer, F.S. Comparison of the Effects of Buprenorphine, Oxymorphone Hydrochloride, and Ketoprofen for Postoperative Analgesia After Onychectomy or Onychectomy and Sterilization in Cats. J. Am. Anim. Hosp. Assoc. 2002, 38, 507–514. [Google Scholar] [CrossRef]
- Porters, N.; Bosmans, T.; Debille, M.; de Rooster, H.; Duchateau, L.; Polis, I. Sedative and antinociceptive effects of dexmedetomidine and buprenorphine after oral transmucosal or intramuscular administration in cats. Vet. Anaesth. Analg. 2014, 41, 90–96. [Google Scholar] [CrossRef]
- van Haaften, K.A.; Forsythe, L.R.E.; Stelow, E.A.; Bain, M.J. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J. Am. Vet. Med Assoc. 2017, 251, 1175–1181. [Google Scholar] [CrossRef]
- Bhalla, R.J.; Trimble, T.A.; Leece, E.A.; Vettorato, E. Comparison of intramuscular butorphanol and buprenorphine combined with dexmedetomidine for sedation in cats. J. Feline Med. Surg. 2018, 20, 325–331. [Google Scholar] [CrossRef]
- Adami, C.; Lardone, E.; Monticelli, P. Inter-rater and inter-device reliability of mechanical thresholds measurement with the Electronic von Frey Anaesthesiometer and the SMALGO in healthy cats. J. Feline Med. Surg. 2019, 21, 979–984. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Vincent, D.; Ezernieks, V.; Rochfort, S.; Spangenberg, G. A Multiple Protease Strategy to Optimise the Shotgun Proteomics of Mature Medicinal Cannabis Buds. Int. J. Mol. Sci. 2019, 20, 5630. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Cottingham, K. Two are not always better than one. J. Proteome Res. 2009, 8, 4172. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pevzner, P.A. False Discovery Rates of Protein Identifications: A Strike against the Two-Peptide Rule. J. Proteome Res. 2009, 8, 4173–4181. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Krimer, P.M. Generating and Interpreting Test Results: Test Validity, Quality Control, Reference Values, and Basic Epidemiology. In Duncan & Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th ed.; Latimer, K.S., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 374–375. [Google Scholar]
- Sirikantaramas, S.; Taura, F.; Morimoto, S.; Shoyama, Y. Recent Advances in Cannabis sativa Research: Biosynthetic Studies and Its Potential in Biotechnology. Curr. Pharm. Biotechnol. 2007, 8, 237–243. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Li, J.-C.; Gu, Y.-F.; Qiu, R.-H.; Huang, J.-Y.; Xue, R.; Li, S.; Zhang, Y.; Zhang, K.; Zhang, Y.-Z. Cannabidiol Exerts Sedative and Hypnotic Effects in Normal and Insomnia Model Mice Through Activation of 5-HT1A Receptor. Neurochem. Res. 2024, 49, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Zuardi, A.W.; Crippa, J.A.S. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Aragona, F.; Tabbì, M.; Gugliandolo, E.; Giannetto, C.; D’aNgelo, F.; Fazio, F.; Interlandi, C. Role of cannabidiolic acid or the combination of cannabigerol/cannabidiol in pain modulation and welfare improvement in horses with chronic osteoarthritis. Front. Vet. Sci. 2024, 11, 1496473. [Google Scholar] [CrossRef]
- Shu, G.; He, Y.; Suo, J.; Wu, C.; Gong, X.; Xiang, Y.; Yang, W.; Cheng, J.; Wang, Y.; Chen, W.; et al. Cannabidiol exhibits anxiolytic-like effects and antipsychotic-like effects in mice models. Neurosci. Lett. 2024, 826, 137723. [Google Scholar] [CrossRef]
- Marliani, G.; Vaccari, L.; Cavallini, D.; Montesano, C.S.; Buonaiuto, G.; Accorsi, P.A. Assessing the effectiveness of cannabidiol additive supplementation on canine behavior and cortisol levels. Heliyon 2024, 10, e31345. [Google Scholar] [CrossRef]
- Jukier, T.; Cruz-Espindola, C.; Martin, D.; Boothe, D.M. Disposition of a single oral dose of a cannabidiol medication in healthy cats. Front. Vet. Sci. 2023, 10, 1181517. [Google Scholar] [CrossRef] [PubMed]
- Crivelaro do Nascimento, G.; Ferrari, D.P.; Guimaraes, F.S.; Del Bel, E.A.; Bortolanza, M.; Ferreira-Junior, N.C. Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson’s disease. Neuropharmacology 2020, 163, 107808. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cardoso, G.K.; Lazarini-Lopes, W.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Garcia-Cairasco, N.; Leite-Panissi, C.R. Cannabidiol effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a neuropathic pain model: Possible role of CB1 and TRPV1 receptors. Neuropharmacology 2021, 197, 108712. [Google Scholar] [CrossRef] [PubMed]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal cannabidiol reduces inflammation and pain-related behaviors in a rat model of arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Brutlag, A.; Hommerding, H. Toxicology of Marijuana, Synthetic Cannabinoids, and Cannabidiol in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 1087–1102. [Google Scholar] [CrossRef]
- Lamanna, M.; Muca, E.; Buonaiuto, G.; Formigoni, A.; Cavallini, D. From posts to practice: Instagram’s role in veterinary dairy cow nutrition education—How does the audience interact and apply knowledge? A survey study. J. Dairy Sci. 2025, 108, 1659–1671. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s Wrong with Bonferroni Adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
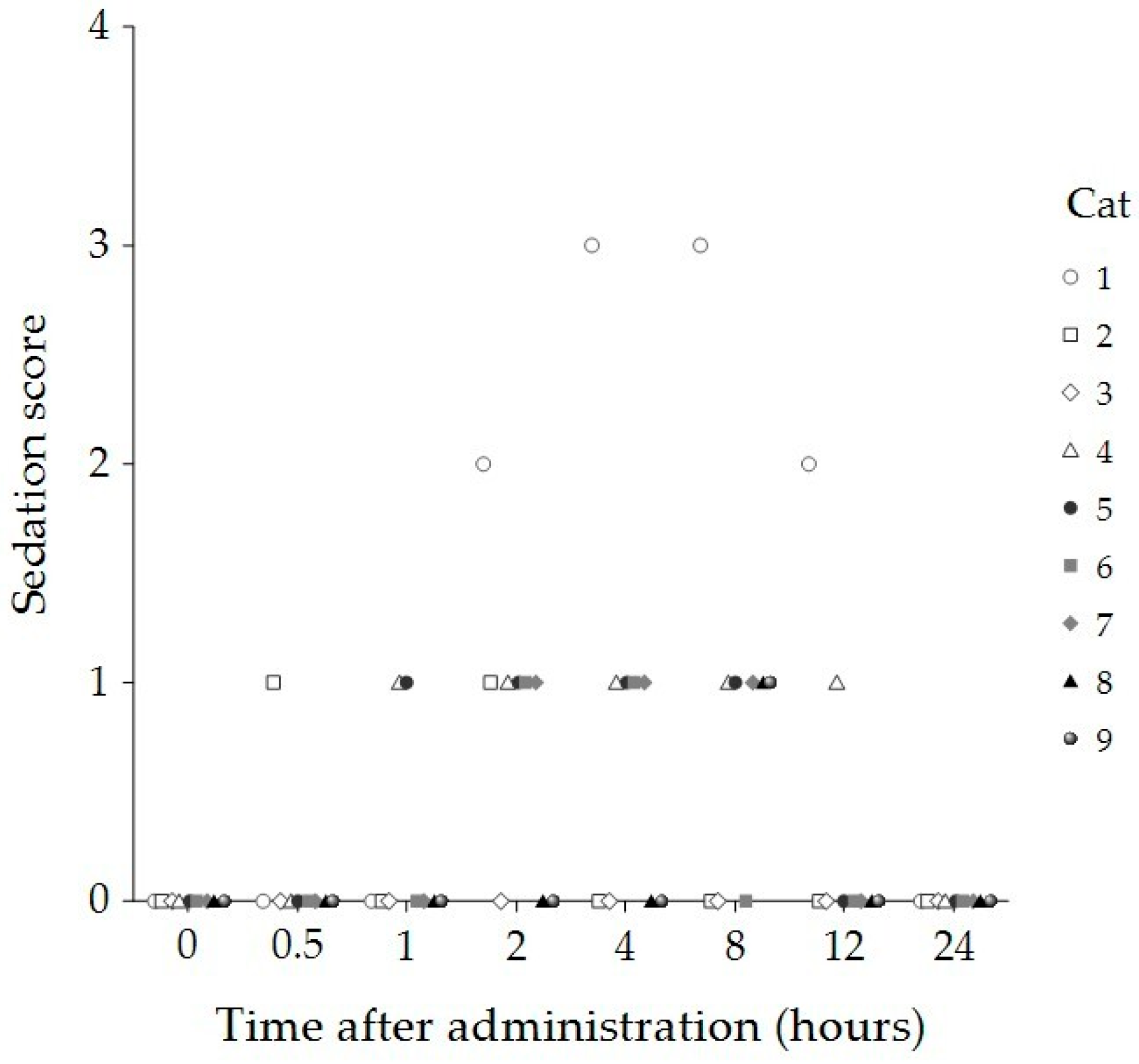
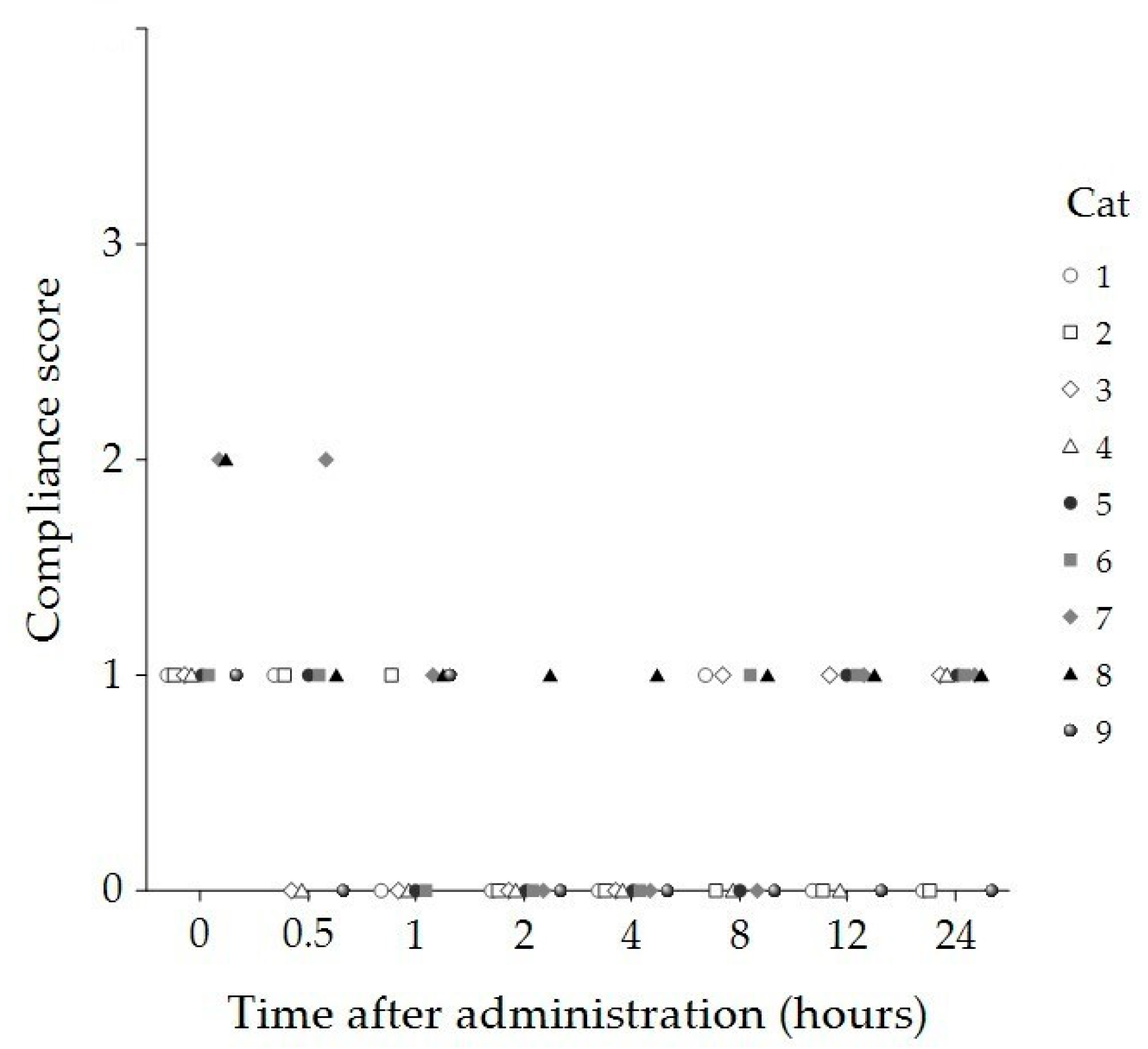
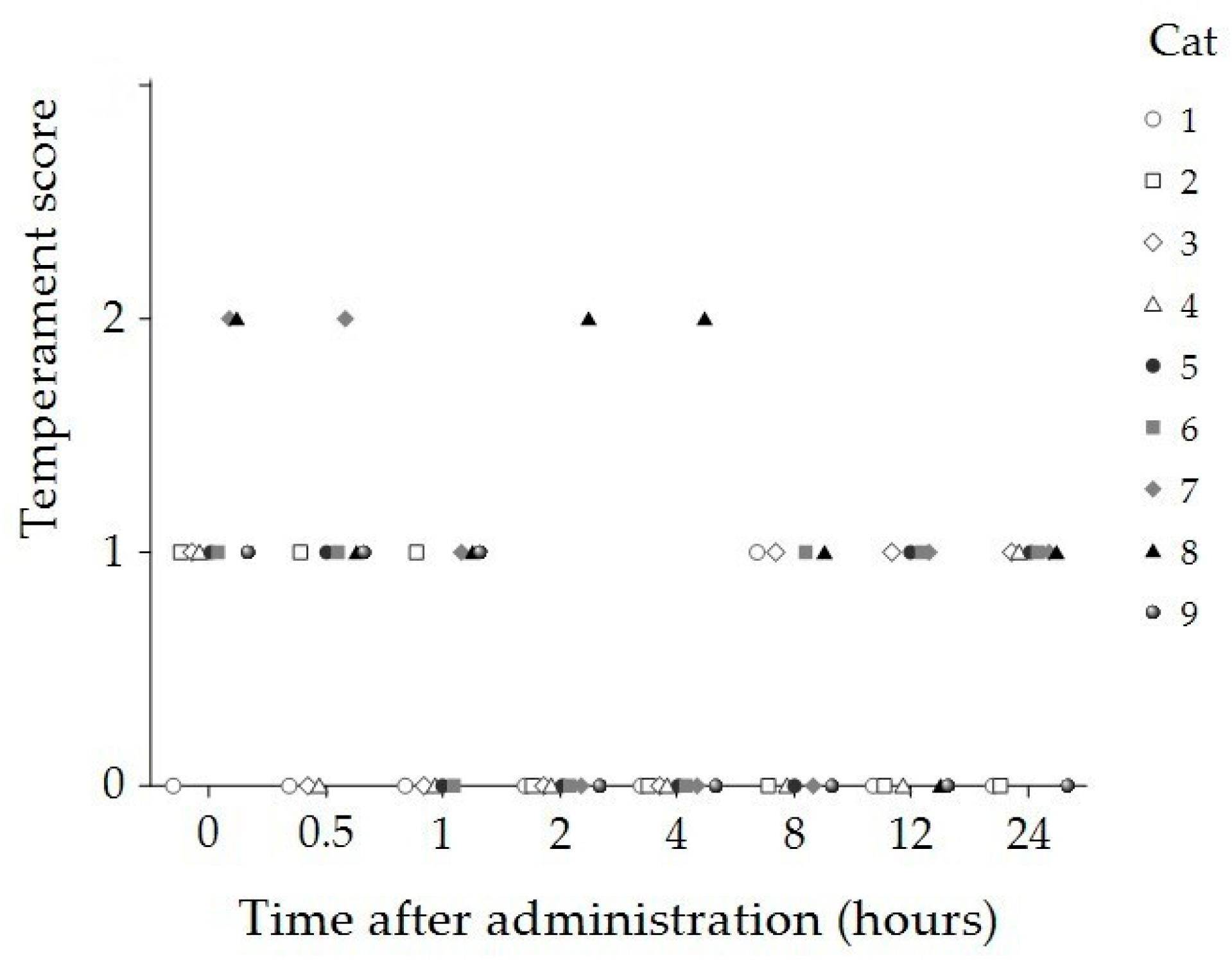
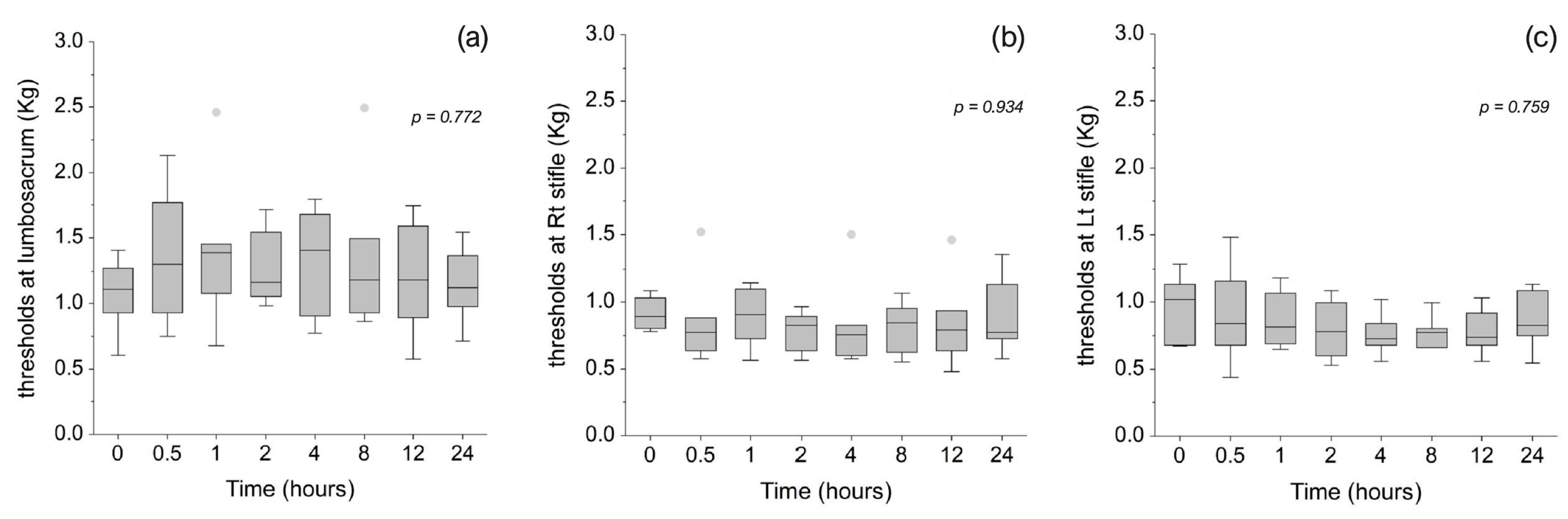
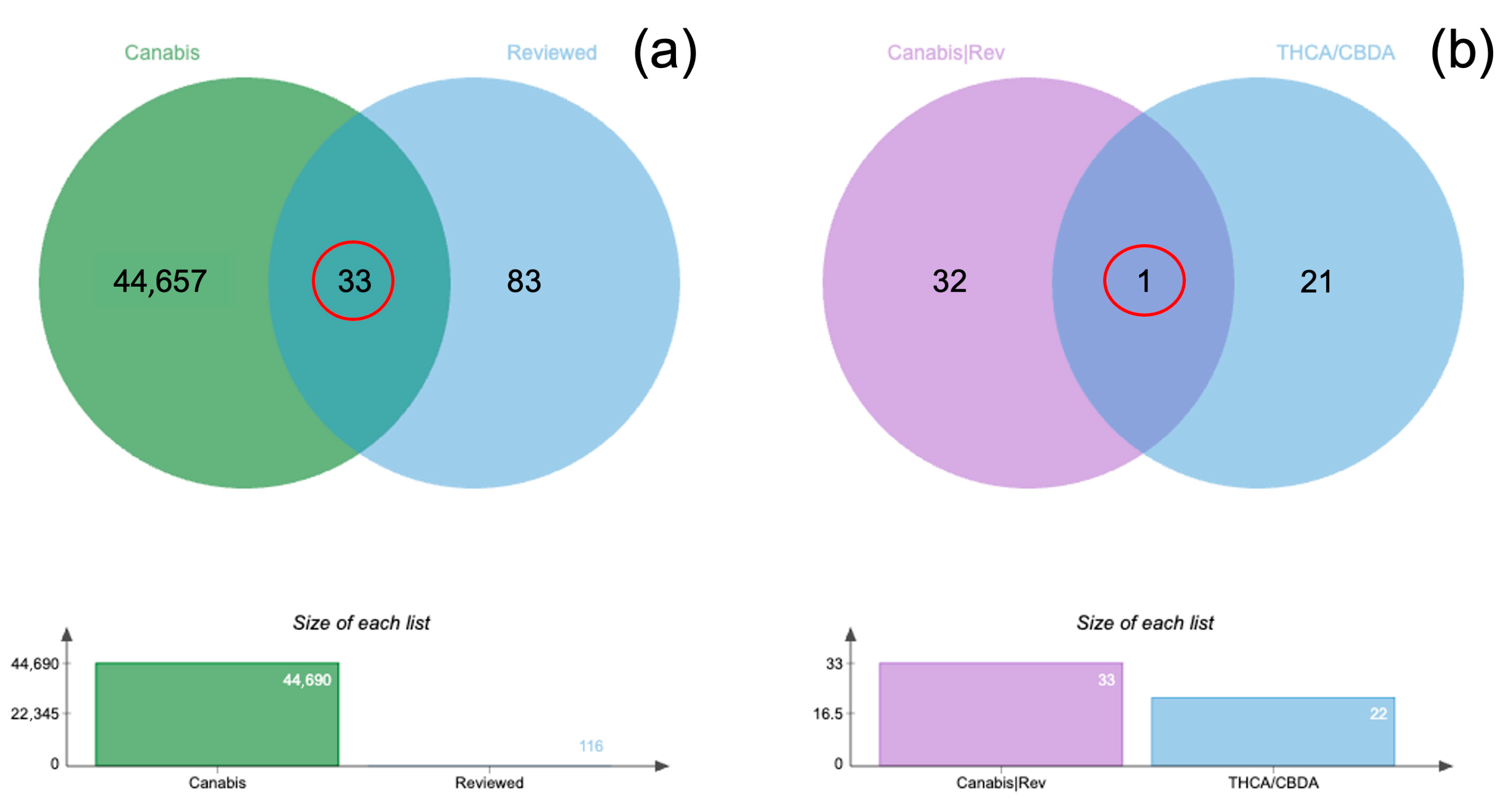
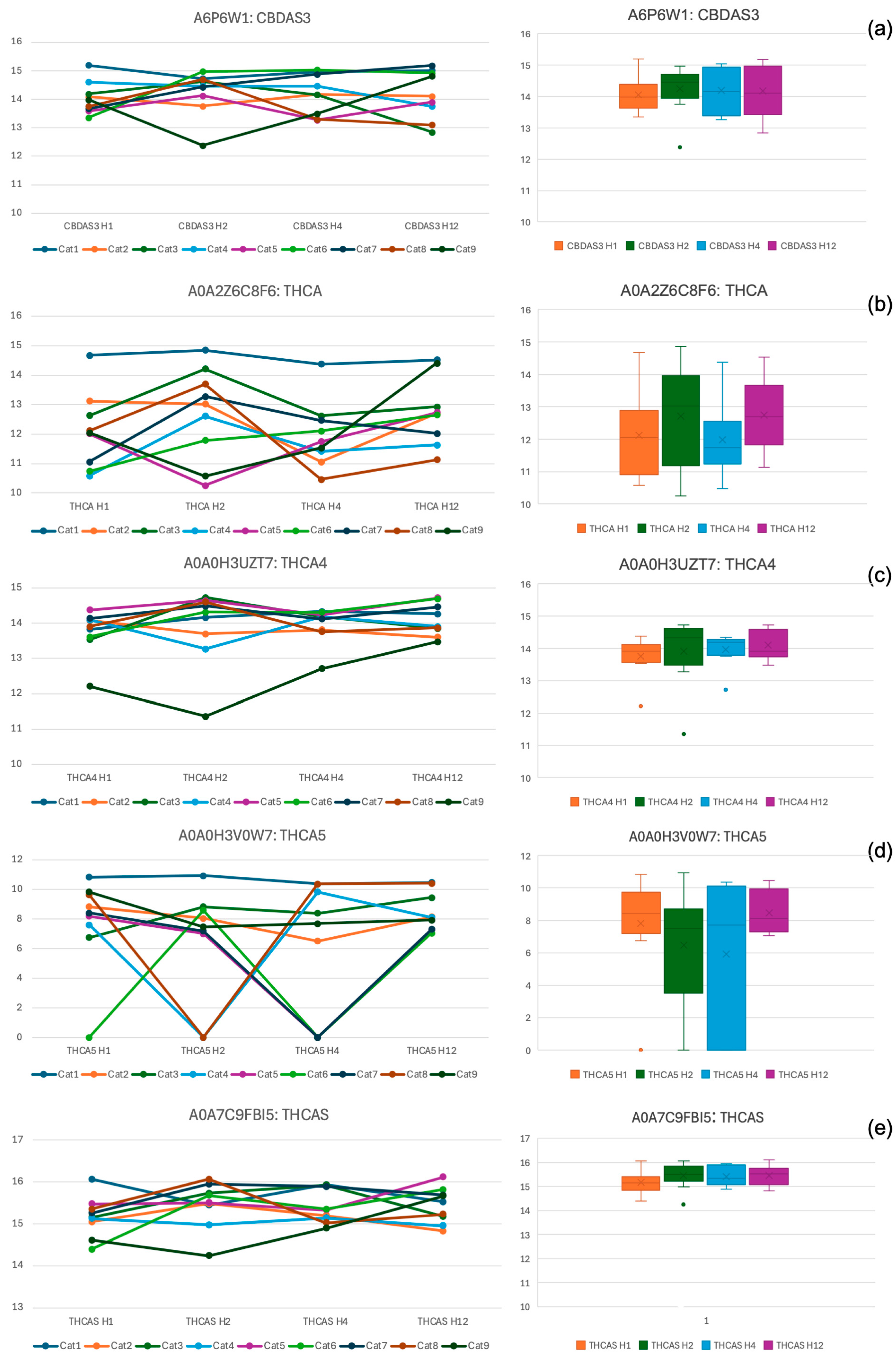
| Time (Hours) | Sedation Score Median (IQR) | Compliance Score Median (IQR) | Temperament Score Median (IQR) |
|---|---|---|---|
| Baseline | 0 (0, 0) | 1 (1, 1.5) | 1 (1, 1.5) |
| 1/2 | 0 (0, 0) | 1 (0, 1) | 1 (0, 1) |
| 1 | 0 (0, 0.5) | 0 (0, 1) | 0 (0, 1) |
| 2 | 1 (0, 1) * | 0 (0, 0) * | 0 (0, 0) * |
| 4 | 1 (0, 1) * | 0 (0, 0) * | 0 (0, 0) * |
| 8 | 1 (0, 1) * | 0 (0, 1) | 0 (0, 1) |
| 12 | 0 (0, 0.5) | 1 (0, 1) | 0 (0, 1) |
| 24 | 0 (0, 0) | 1 (0, 1) | 1 (0, 1) |
| p-value | <0.001 | <0.001 | 0.012 |
| Time (Hours) | HR (Beats/Minute) Median (IQR) | RR (Breaths/Minute) Median (IQR) | Temperature (°C) Median (IQR) |
|---|---|---|---|
| Baseline | 168 (160, 190) | 32 (22, 61) | 38.1 (37.8, 39) |
| 1/2 | 180 (166, 200) | 26 (24, 31) | 38.0 (37.8, 38.4) |
| 1 | 180 (168, 190) | 28 (22, 64) | 37.9 (37.7, 38.6) |
| 2 | 180 (160, 200) | 28 (22, 32) | 37.9 (37.7, 39.1) |
| 4 | 180 (160, 190) | 24 (20, 26) | 37.9 (37.7, 38.2) |
| 8 | 180 (164, 194) | 26 (20, 36) | 37.7 (37.2, 38.3) |
| 12 | 184 (176, 200) | 24 (22, 27) | 37.8 (37.8, 38.2) |
| 24 | 180 (160, 180) | 24 (20, 24) | 37.8 (37.8, 38.1) |
| p-value | 0.792 | 0.182 | 0.232 |
| Blood Chemistry | Baseline (Pre-CBD) | 1 Day Post-CBD | 2 Weeks Post-CBD | p-Value | Reference Interval ** |
|---|---|---|---|---|---|
| BUN (mg/dL) | 27 ± 5.37 | 24 ± 5.64 | 26 ± 4.52 | 0.052 | 19–34 |
| Creatinine (mg/dL) | 1.45 ± 0.27 | 1.37 ± 0.34 | 1.35 ± 0.30 | 0.376 | 0.9–2.2 |
| ALT (U/L) | 40.27 ± 16.40 | 49.22 ± 13.57 | 48.33 ± 13.80 | 0.134 | 25–97 |
| ALP (U/L) | 35.89 ± 17.74 | 42.33 ± 21.18 | 38.78 ± 17.03 | 0.179 | 0–45 |
| AST (U/L) | 28.22 ± 7.36 | 48.89 ± 27.22 | 26.22 ± 6.94 | 0.018 * | 7–38 |
| Total protein (g/dL) | 7.30 ± 0.52 | 7.00 ± 0.44 | 6.92 ± 0.45 | 0.201 | 6–7.9 |
| Albumin (g/dL) | 3.52 ± 0.21 | 3.41 ± 0.22 | 3.40 ± 0.28 | 0.651 | 2.8–3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanapinit, K.; Niyom, S.; Suriyawongpongsa, P.; Khathatip, S.; Tancharoen, K.; Roytrakul, S.; Ploypetch, S. Evaluation of Cannabidiol Oil’s Effects on Sedation, Behavioral Responses to Handling, and Nociceptive Thresholds in Healthy Cats. Animals 2025, 15, 1987. https://doi.org/10.3390/ani15131987
Wanapinit K, Niyom S, Suriyawongpongsa P, Khathatip S, Tancharoen K, Roytrakul S, Ploypetch S. Evaluation of Cannabidiol Oil’s Effects on Sedation, Behavioral Responses to Handling, and Nociceptive Thresholds in Healthy Cats. Animals. 2025; 15(13):1987. https://doi.org/10.3390/ani15131987
Chicago/Turabian StyleWanapinit, Kannika, Sirirat Niyom, Panisara Suriyawongpongsa, Sakunrat Khathatip, Kaittisak Tancharoen, Sittiruk Roytrakul, and Sekkarin Ploypetch. 2025. "Evaluation of Cannabidiol Oil’s Effects on Sedation, Behavioral Responses to Handling, and Nociceptive Thresholds in Healthy Cats" Animals 15, no. 13: 1987. https://doi.org/10.3390/ani15131987
APA StyleWanapinit, K., Niyom, S., Suriyawongpongsa, P., Khathatip, S., Tancharoen, K., Roytrakul, S., & Ploypetch, S. (2025). Evaluation of Cannabidiol Oil’s Effects on Sedation, Behavioral Responses to Handling, and Nociceptive Thresholds in Healthy Cats. Animals, 15(13), 1987. https://doi.org/10.3390/ani15131987





