Serum Proteomic Changes in Pet Rabbits with Subclinical and Clinical Encephalitozoonosis in Thailand
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Preparation of Rabbit Sera
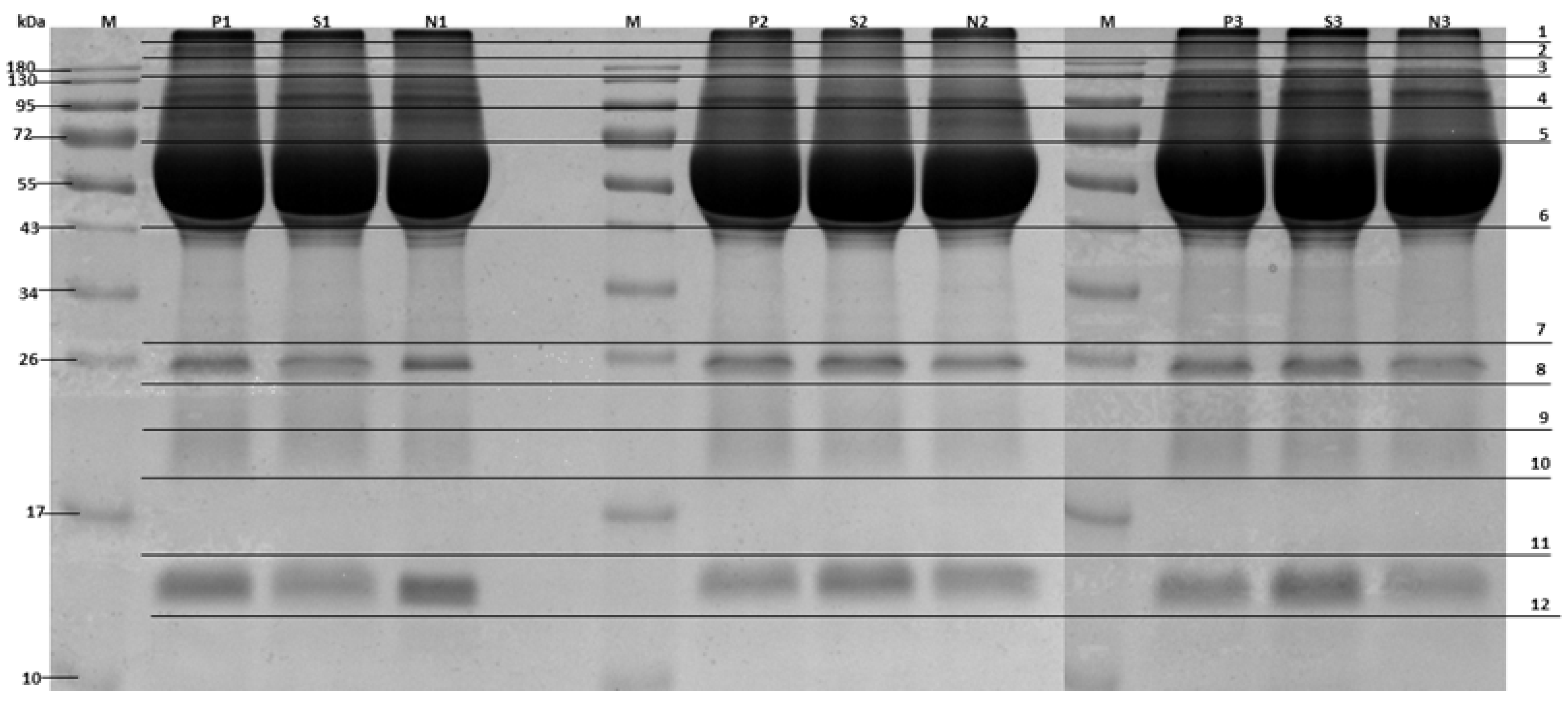
2.3. Serum Protein Separation
2.4. In-Gel Digestion
2.5. Mass Spectrometric Analysis
2.6. Bioinformatics
2.7. Statistical Analysis
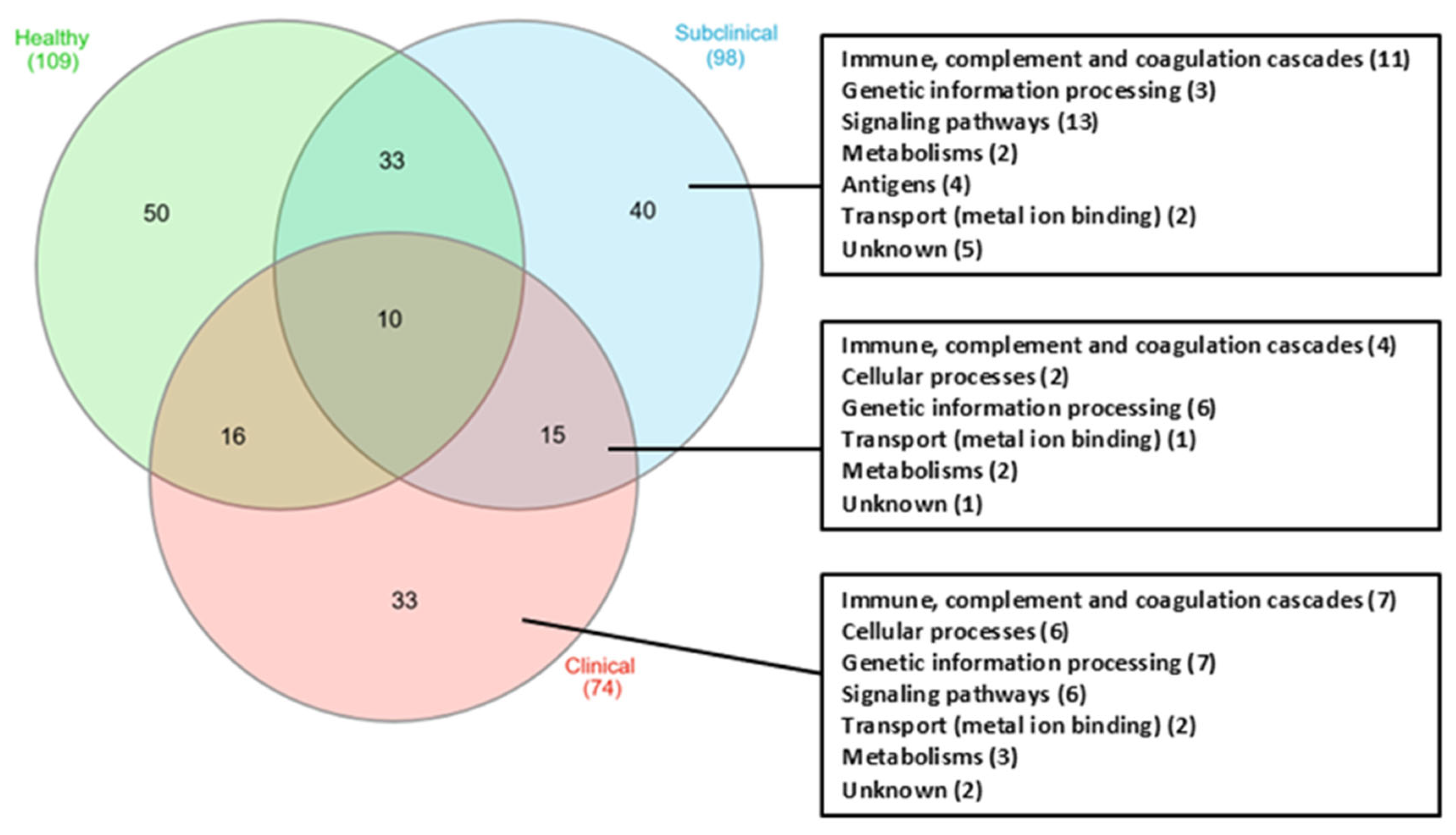
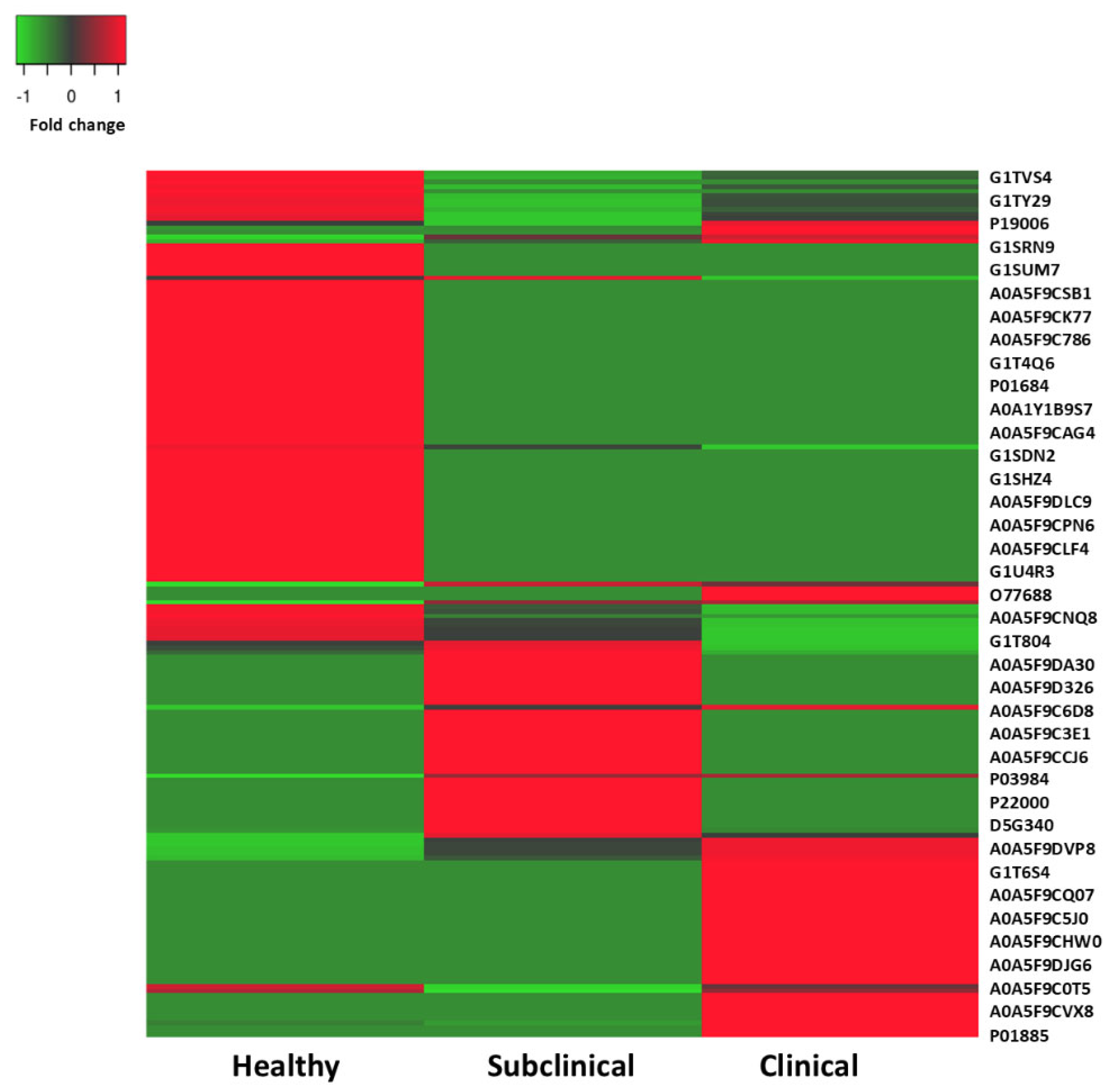
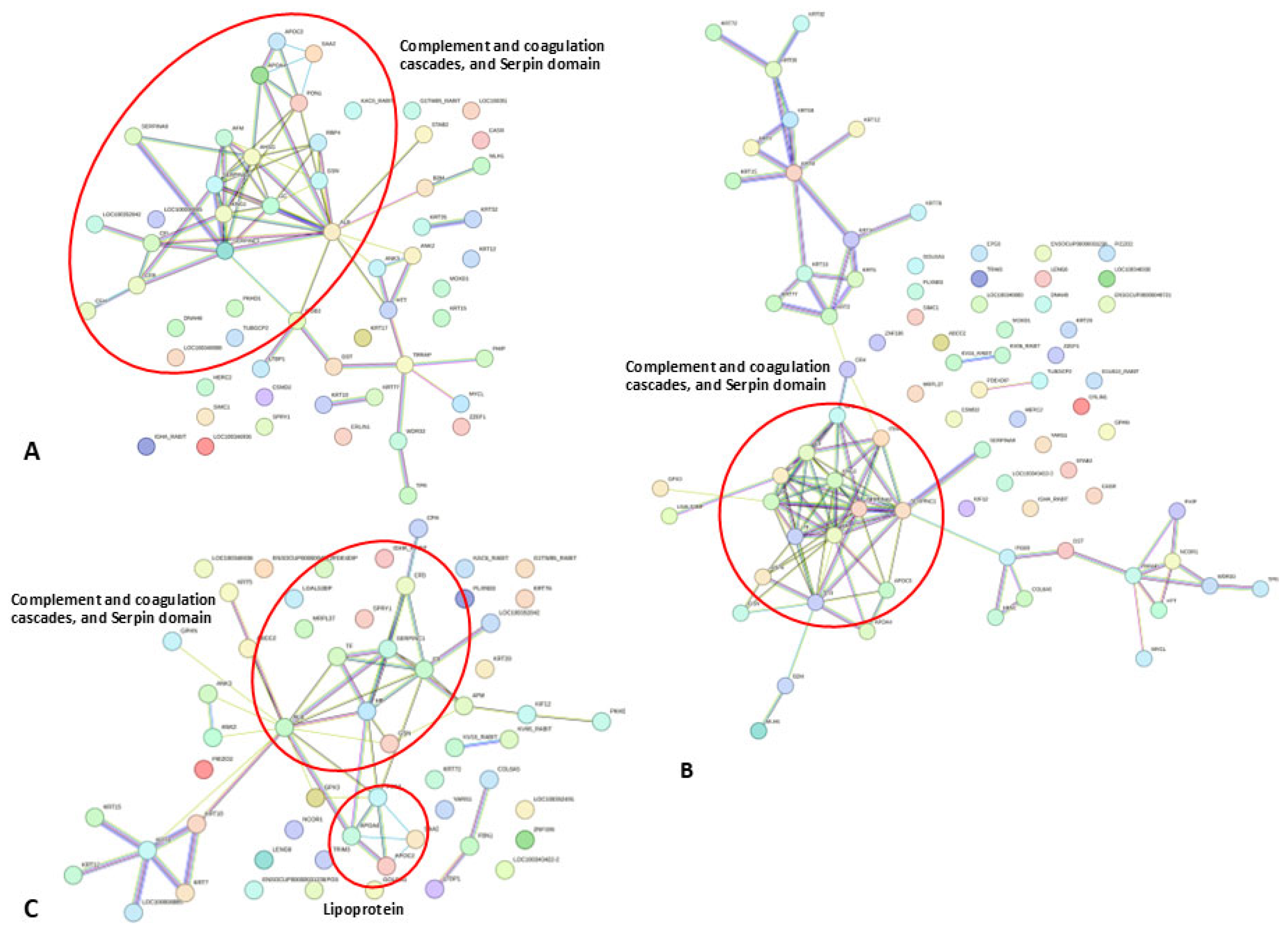
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deplazes, P.; Mathis, A.; Weber, R. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 2000, 6, 236–260. [Google Scholar] [CrossRef]
- Harcourt-Brown, F.M.; Holloway, H.K. Encephalitozoon cuniculi in pet rabbits. Vet. Rec. 2003, 152, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Latney, L.V.; Bradley, C.W.; Wyre, N.R. Encephalitozoon cuniculi in pet rabbits: Diagnosis and optimal management. Vet. Med. 2014, 5, 169–180. [Google Scholar] [CrossRef]
- Doboși, A.-A.; Bel, L.-V.; Paștiu, A.I.; Pusta, D.L. A Review of Encephalitozoon cuniculi in Domestic Rabbits (Oryctolagus cuniculus—Biology, Clinical Signs, Diagnostic Techniques, Treatment, and Prevention. Pathogens 2022, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Duangurai, T.; Khamchomphu, N.; Dusitkul, K.; Tousee, C.; Sukmai, Y.; Rungnirundorn, T.; Areevijittrakul, L.; Jala, S.; Thengchaisri, N. Association of Encephalitozoon cuniculi with Clinical Signs and Abnormal Hematologic/Biochemical Changes in Pet Rabbits in Thailand. Animals 2024, 14, 2766. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.; Cook, C.; Clegg, F. Encephalitozoonosis (nosematosis) causing bilateral cataract in a rabbit. Br. J. Ophthalmol. 1976, 60, 618–631. [Google Scholar] [CrossRef]
- Donnelly, T. Encephalitozoon cuniculi-associated phacoclastic uveitis in the rabbit: A review. J. Exot. Mammal. Med. Surg. 2003, 1, 1–3. [Google Scholar]
- Csokai, J.; Joachim, A.; Gruber, A.; Tichy, A.; Pakozdy, A.; Künzel, F. Diagnostic markers for encephalitozoonosis in pet rabbits. Vet. Parasitol. 2009, 163, 18–26. [Google Scholar] [CrossRef]
- Santaniello, A.; Dipineto, L.; Rinaldi, L.; Menna, L.F.; Cringoli, G.; Fioretti, A. Serological survey of Encephalitozoon cuniculi in farm rabbits in Italy. Res. Vet. Sci. 2009, 87, 67–69. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Chienwichai, P.; Ampawong, S.; Adisakwattana, P.; Thiangtrongjit, T.; Limpanont, Y.; Chusongsang, P.; Chusongsang, Y.; Reamtong, O. Effect of Praziquantel on Schistosoma mekongi Proteome and Phosphoproteome. Pathogens 2020, 9, 417. [Google Scholar] [CrossRef]
- Han, Y.; Gao, H.; Xu, J.; Luo, J.; Han, B.; Bao, J.; Pan, G.; Li, T.; Zhou, Z. Innate and Adaptive Immune Responses Against Microsporidia Infection in Mammals. Front. Microbiol. 2020, 11, 1468. [Google Scholar] [CrossRef] [PubMed]
- Dalboni, L.C.; Alvares Saraiva, A.M.; Konno, F.T.C.; Perez, E.C.; Codeceira, J.F.; Spadacci-Morena, D.D.; Lallo, M.A. Encephalitozoon cuniculi takes advantage of efferocytosis to evade the immune response. PLoS ONE 2021, 16, e0247658. [Google Scholar] [CrossRef]
- Jeklova, E.; Jekl, V.; Kovarcik, K.; Hauptman, K.; Koudela, B.; Neumayerova, H.; Knotek, Z.; Faldyna, M. Usefulness of detection of specific IgM and IgG antibodies for diagnosis of clinical encephalitozoonosis in pet rabbits. Vet. Parasitol. 2010, 170, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.G.V.; Filho, A.F.S.; Souza, G.O.; Vasconcellos, S.A.; Romero, E.C.; Nascimento, A. Decrease in antithrombin III and prothrombin serum levels contribute to coagulation disorders during leptospirosis. Microbiology 2016, 162, 1407–1421. [Google Scholar] [CrossRef]
- Kim, H.J.; Bar-Sagi, D. Modulation of signalling by Sprouty: A developing story. Nat. Rev. Mol. Cell Biol. 2004, 5, 441–450. [Google Scholar] [CrossRef]
- Collins, S.; Waickman, A.; Basson, A.; Kupfer, A.; Licht, J.D.; Horton, M.R.; Powell, J.D. Regulation of CD4+ and CD8+ effector responses by Sprouty-1. PLoS ONE 2012, 7, e49801. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Keller, T.T.; van Gorp, E.; ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39. [Google Scholar] [CrossRef]
- Brdíčková, K.; Sak, B.; Holubová, N.; Květoňová, D.; Hlásková, L.; Kicia, M.; Kopacz, Ż.; Kváč, M. Encephalitozoon cuniculi Genotype II Concentrates in Inflammation Foci. J. Inflamm. Res. 2020, 13, 583–593. [Google Scholar] [CrossRef]
- Rodríguez-Tovar, L.E.; Nevárez-Garza, A.M.; Trejo-Chávez, A.; Hernández-Martínez, C.A.; Hernández-Vidal, G.; Zarate-Ramos, J.J.; Castillo-Velázquez, U. Encephalitozoon cuniculi: Grading the Histological Lesions in Brain, Kidney, and Liver during Primoinfection Outbreak in Rabbits. J. Pathog. 2016, 2016, 5768428. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Krishnaswamy, S. The transition of prothrombin to thrombin. J. Thromb. Haemost. JTH 2013, 11 (Suppl. 1), 265–276. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Monroe, D.M.; Roberts, H.R.; Hoffman, M. Elevated prothrombin results in clots with an altered fiber structure: A possible mechanism of the increased thrombotic risk. Blood 2003, 101, 3008–3013. [Google Scholar] [CrossRef]
- de Boer, J.P.; Creasey, A.A.; Chang, A.; Abbink, J.J.; Roem, D.; Eerenberg, A.J.; Hack, C.E.; Taylor, F.B., Jr. Alpha-2-macroglobulin functions as an inhibitor of fibrinolytic, clotting, and neutrophilic proteinases in sepsis: Studies using a baboon model. Infect. Immun. 1993, 61, 5035–5043. [Google Scholar] [CrossRef]
- Lagrange, J.; Lecompte, T.; Knopp, T.; Lacolley, P.; Regnault, V. Alpha-2-macroglobulin in hemostasis and thrombosis: An underestimated old double-edged sword. J. Thromb. Haemost. JTH 2022, 20, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Wu, Y.R.; Tseng, M.Y.; Chen, Y.C.; Hsieh, S.Y.; Chen, C.M. Increased prothrombin, apolipoprotein A-IV, and haptoglobin in the cerebrospinal fluid of patients with Huntington’s disease. PLoS ONE 2011, 6, e15809. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef] [PubMed]
- Naryzhny, S.N.; Legina, O.K. Haptoglobin as a biomarker. Biomeditsinskaia Khimiia 2021, 67, 105–118. [Google Scholar] [CrossRef]
- Quaye, I.K. Haptoglobin, inflammation and disease. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 735–742. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Piqueras, M.D.C.; Pantin, A.; Bhattacharya, S.K.; Peschke, R.; Joachim, A.; Cray, C. Application of mass spectrometry to elucidate the pathophysiology of Encephalitozoon cuniculi infection in rabbits. PLoS ONE 2017, 12, e0177961. [Google Scholar] [CrossRef]
- Mehta, S.; Sibley, L.D. Toxoplasma gondii Actin Depolymerizing Factor Acts Primarily to Sequester G-actin. J. Biol. Chem. 2010, 285, 6835–6847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Feng, R.; Liang, G.; Hou, W.; Zhang, Y.; Li, X.; Zhang, L.; Zhang, S. Functional characterization of CpADF, an actin depolymerizing factor protein in Cryptosporidium parvum. Parasitol. Res. 2023, 122, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Stanga, Z.; Nock, S.; Medina-Escobar, P.; Nydegger, U.E.; Risch, M.; Risch, L. Factors other than the glomerular filtration rate that determine the serum beta-2-microglobulin level. PLoS ONE 2013, 8, e72073. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E.C. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 2006, 372, 33–42. [Google Scholar] [CrossRef]
- Haase, S.; Zimmermann, D.; Olshina, M.A.; Wilkinson, M.; Fisher, F.; Tan, Y.H.; Stewart, R.J.; Tonkin, C.J.; Wong, W.; Kovar, D.R.; et al. Disassembly activity of actin-depolymerizing factor (ADF) is associated with distinct cellular processes in apicomplexan parasites. Mol. Biol. Cell 2015, 26, 3001–3012. [Google Scholar] [CrossRef]
- Wang, H.; Sama, A.E. Anti-inflammatory role of fetuin-A in injury and infection. Curr. Mol. Med. 2012, 12, 625–633. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mondal, S.A.; Kumar, M.; Dutta, D. Proinflammatory and antiinflammatory attributes of fetuin-a: A novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr. Pract. 2014, 20, 1345–1351. [Google Scholar] [CrossRef]
- Mani, A. PDE4DIP in health and diseases. Cell. Signal. 2022, 94, 110322. [Google Scholar] [CrossRef]

| Access No. | Protein Name | M.W. | No. of Peptide | Fold Change (Clinical vs. Healthy) | Fold Change (Subclinical vs. Healthy) | Fold Change (Clinical vs. Subclinical) |
|---|---|---|---|---|---|---|
| Immune, complement and coagulation cascades | ||||||
| O77688 | Prothrombin (Fragment) | 12,161 | 1 | ON * | ON * | 1.08 |
| A0A5F9CCJ6 | Complement component 4 binding protein alpha | 65,840 | 3 | ON * | ON * | 1.17 |
| A0A5F9CNX4 | Alpha-2-macroglobulin | 156,458 | 11 | ON * | ON * | 1.05 |
| G1SIK0 | Antithrombin-III | 52,603 | 4 | 4.33 | 2 | 2.15 |
| P19006 | Haptoglobin | 36,434 | 2 | 4.25 | 3.25 | 1.3 |
| P01885 | Beta-2-microglobulin | 11,647 | 2 | ON * | — | ON * |
| G1SU82 | Gc-globulin | 53,913 | 2 | ON * | — | ON * |
| G1TW85 | Ig-like domain-containing protein | 11,641 | 12 | 0.73 | OFF ** | ON * |
| G1SS69 | C3/C5 convertase | 85,298 | 3 | OFF ** | 0.5 | OFF ** |
| A0A1Y1B9L0 | IgG light chain (Fragment) | 22,862 | 5 | — | ON ** | OFF ** |
| A0A0C6G056 | IgM light chain | 22,428 | 3 | — | ON * | OFF ** |
| P03988 | Ig mu chain C region secreted form | 49,866 | 9 | — | ON * | OFF ** |
| A0A0C6G052 | IgG heavy chain VDJ region | 23,629 | 3 | — | ON * | OFF ** |
| P03984 | Ig kappa chain b5 variant C region | 11,072 | 2 | — | ON * | OFF ** |
| P22000 | Serum amyloid A-2 protein | 13,443 | 5 | — | ON * | OFF ** |
| Cellular processes | ||||||
| G1U9R8 | Actin-depolymerizing factor | 81,346 | 7 | ON * | ON * | 2.82 |
| U3KMR2 | Alpha-2-HS-glycoprotein | 36,005 | 6 | ON * | ON * | 2.15 |
| G1TIT1 | ER lipid raft associated 1 | 39,027 | 4 | ON * | — | ON* |
| Genetic information processing | ||||||
| A0A5F9DVP8 | Retinol-binding protein | 24,043 | 10 | ON * | ON * | 2.29 |
| P04916 | Retinol-binding protein | 23,205 | 5 | ON * | ON * | 2.29 |
| P06912 | Retinol-binding protein 4 | 23,087 | 11 | 2.7 | 1 | 2.7 |
| G1SZH0 | Plasma retinol-binding protein | 49,814 | 13 | 2.38 | 1.06 | 2.26 |
| A0A5F9C9K8 | Phosphodiesterase 4D interacting protein | 278,239 | 10 | ON * | ON * | 1.0 |
| A0A5F9C619 | Remodeling and spacing factor 1 | 158,986 | 5 | ON * | ON * | 1.0 |
| G1T8D0 | Sprouty homolog 1 protein | 34,904 | 4 | 1 | 2 | 0.5 |
| Transport (metal ion binding) | ||||||
| G1STF7 | Beta-1 metal-binding globulin | 76,635 | 39 | ON * | ON * | 0.77 |
| A0A5F9DMU6 | Inter-alpha-trypsin inhibitor heavy chain H3 | 98,412 | 5 | 0.28 | 0.5 | 0.5 |
| G1SV85 | Monooxygenase DBH like 1 | 71,508 | 5 | ON * | — | ON * |
| P49065 | Albumin | 68,865 | 44 | — | ON * | OFF * |
| Signaling pathways | ||||||
| A0A5F9CQ07 | Fibrillin 2 | 311,658 | 21 | ON * | — | ON * |
| P27170 | Serum paraoxonase/aryl- esterase 1 | 39,984 | 5 | — | ON * | OFF * |
| Metabolism | ||||||
| A0A5F9CSE7 | Protein AMBP | 49,014 | 3 | ON * | ON * | 1.5 |
| B7NZM1 | Apolipoprotein A-I (Predicted) | 30,585 | 26 | — | ON * | OFF ** |
| G1U2V8 | Apolipoprotein C-III | 17,367 | 1 | ON * | — | ON * |
| Q07298 | Alpha-1-antiproteinase S-1 | 45,721 | 4 | ON * | — | ON * |
| Unknown | ||||||
| G1U6P5 | Uncharacterized protein | 38,221 | 4 | 0.44 | 0.21 | 2.07 |
| A0A5F9C4L3 | Uncharacterized protein | 70,533 | 7 | ON * | — | ON * |
| A0A5F9D345 | Uncharacterized protein | 54,050 | 7 | OFF ** | 2 | OFF ** |
| G1T804 | Uncharacterized protein | 34,065 | 2 | OFF ** | 0.21 | OFF ** |
| G1TBS8 | Uncharacterized protein | 139,280 | 31 | OFF ** | 3.71 | OFF ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duangurai, T.; Reamtong, O.; Thiangtrongjit, T.; Jala, S.; Chienwichai, P.; Thengchaisri, N. Serum Proteomic Changes in Pet Rabbits with Subclinical and Clinical Encephalitozoonosis in Thailand. Animals 2025, 15, 1962. https://doi.org/10.3390/ani15131962
Duangurai T, Reamtong O, Thiangtrongjit T, Jala S, Chienwichai P, Thengchaisri N. Serum Proteomic Changes in Pet Rabbits with Subclinical and Clinical Encephalitozoonosis in Thailand. Animals. 2025; 15(13):1962. https://doi.org/10.3390/ani15131962
Chicago/Turabian StyleDuangurai, Taksaon, Onrapak Reamtong, Tipparat Thiangtrongjit, Siriluk Jala, Peerut Chienwichai, and Naris Thengchaisri. 2025. "Serum Proteomic Changes in Pet Rabbits with Subclinical and Clinical Encephalitozoonosis in Thailand" Animals 15, no. 13: 1962. https://doi.org/10.3390/ani15131962
APA StyleDuangurai, T., Reamtong, O., Thiangtrongjit, T., Jala, S., Chienwichai, P., & Thengchaisri, N. (2025). Serum Proteomic Changes in Pet Rabbits with Subclinical and Clinical Encephalitozoonosis in Thailand. Animals, 15(13), 1962. https://doi.org/10.3390/ani15131962








