Effects of Different Forms of Organic Selenium on Growth Performance, Antioxidant Capacity, and Intestinal Health in Rice Field Eel (Monopterus albus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Feed Preparation
2.2. Aquaculture Management
2.3. Sample Collection
2.4. Calculation of Growth Performance and Morphological Parameters
2.5. Determination of Nutritional Components
2.6. Determination of Selenium Content
2.7. Liver and Intestinal Biochemical Indices
2.8. Measurement of Related Antioxidant Gene Expression Levels
2.9. Analysis of Intestinal Tissue Structure
2.10. Analysis of Intestinal Microbiota
2.11. Data Processing and Analysis
3. Results
3.1. Growth Performance
3.2. Morphological Parameters
3.3. Whole-Body Composition
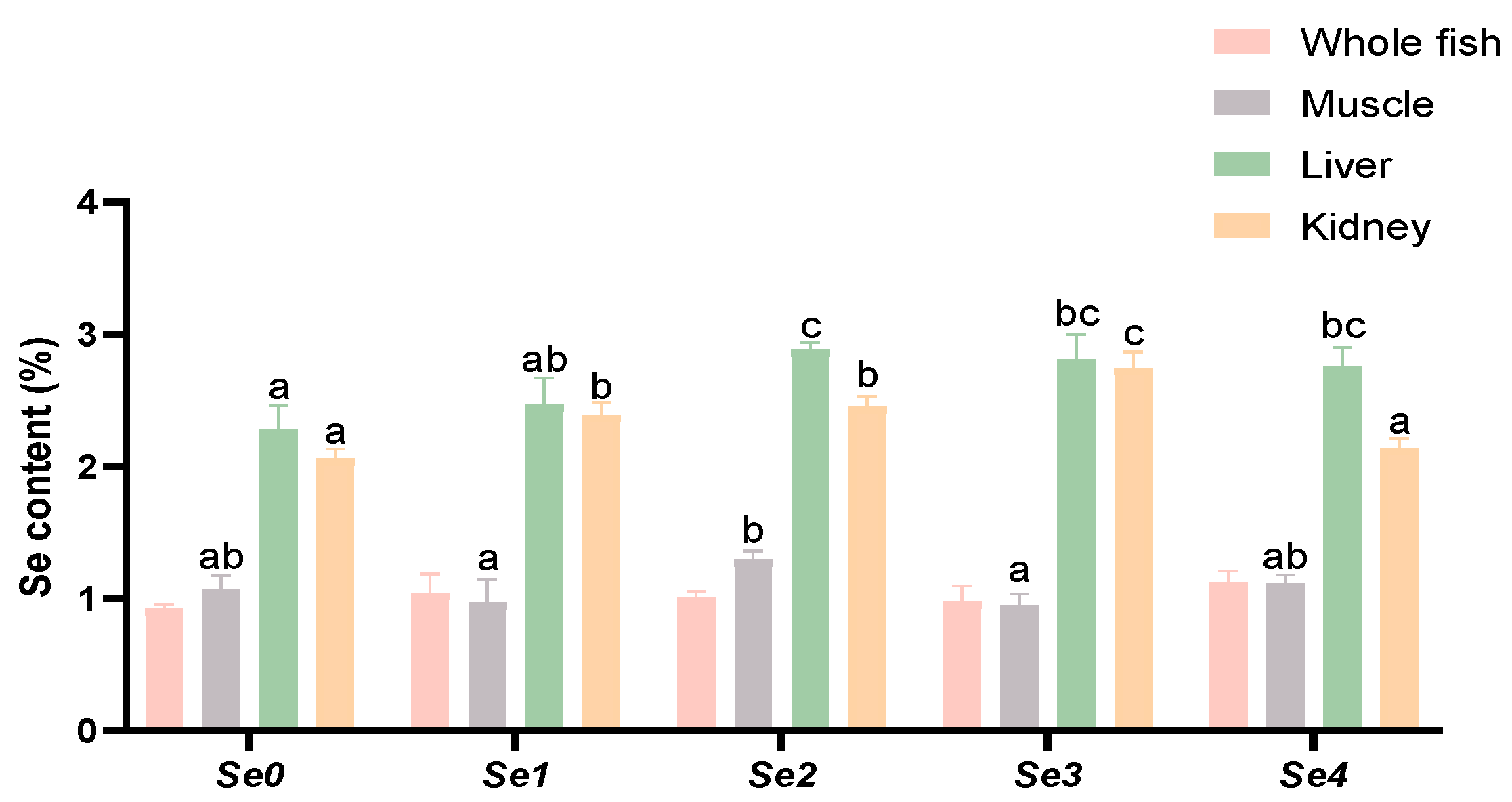
3.4. Se Accumulation
3.5. Serum Biochemical Parameters
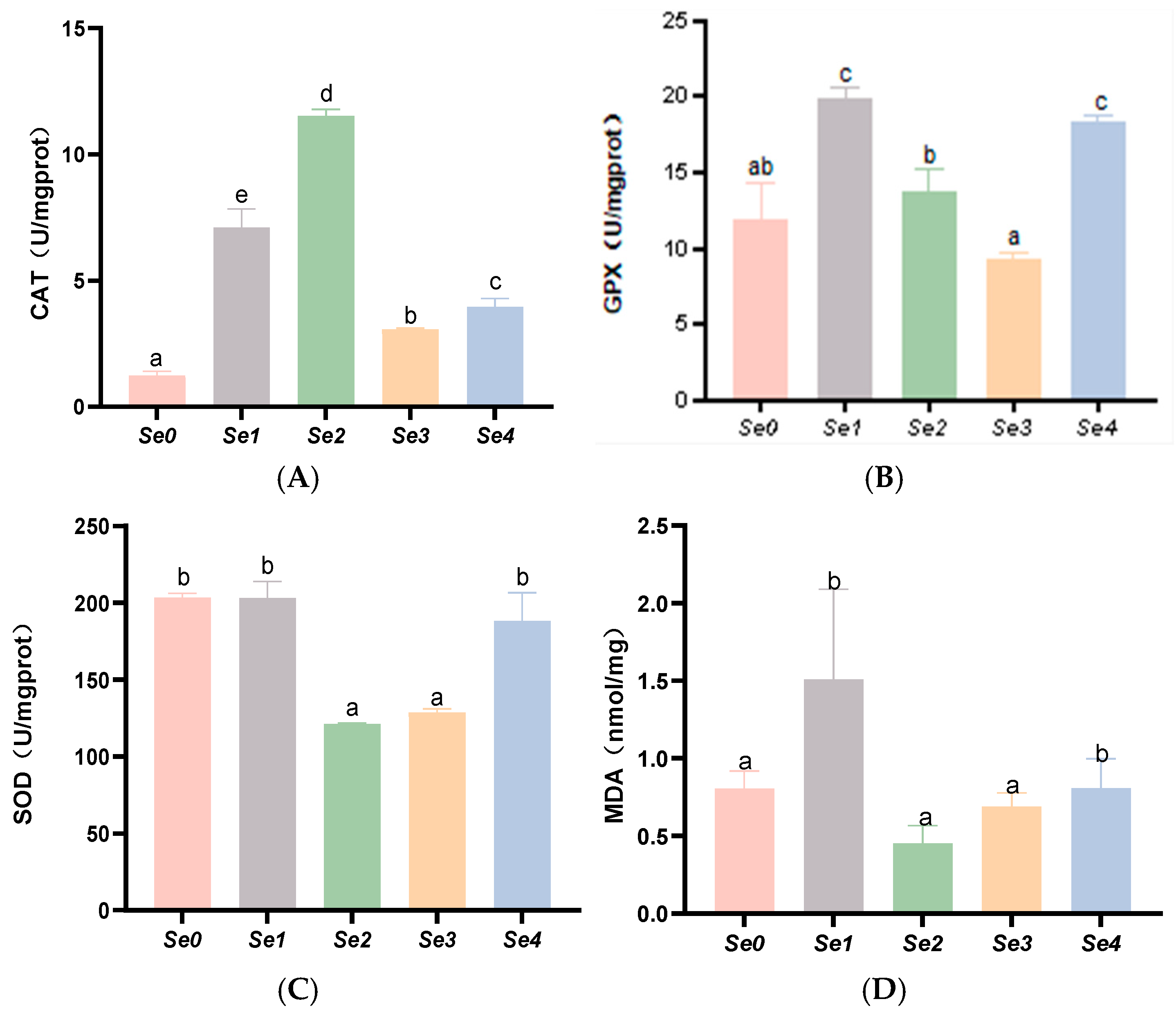
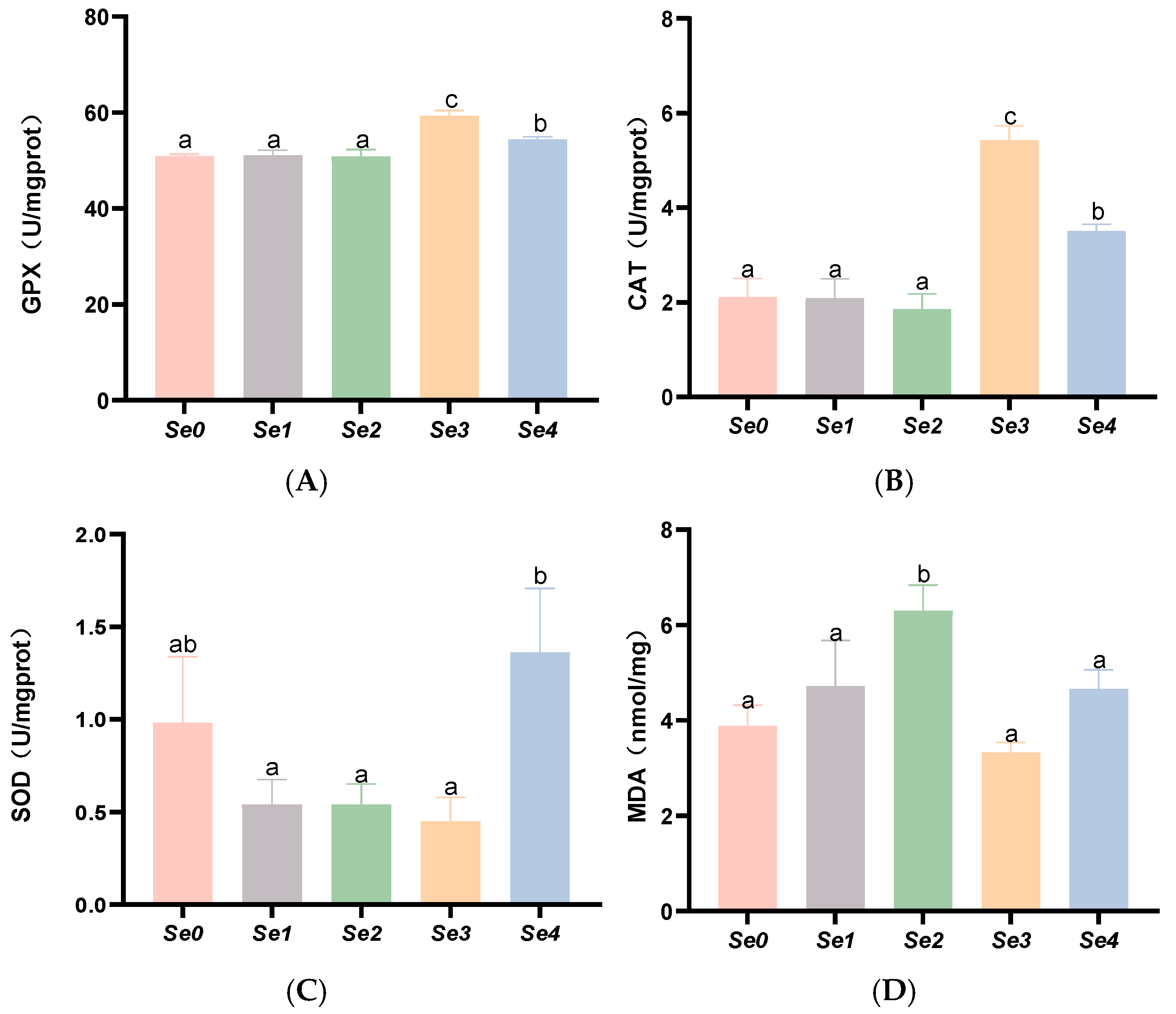
3.6. Hepatic and Intestinal Antioxidant Parameters
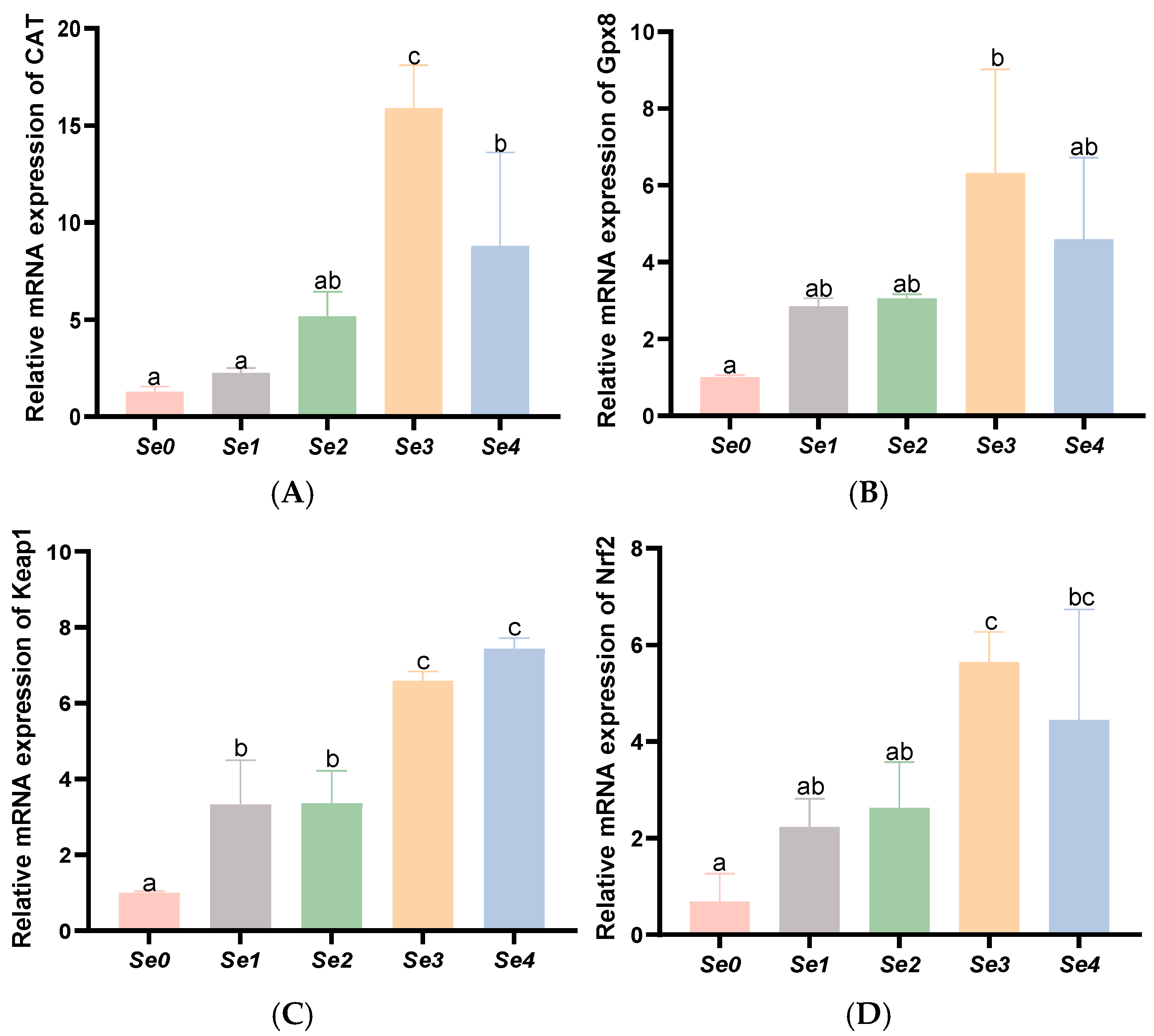
3.7. Expression Levels of Antioxidant-Related Genes in the Liver and Intestine
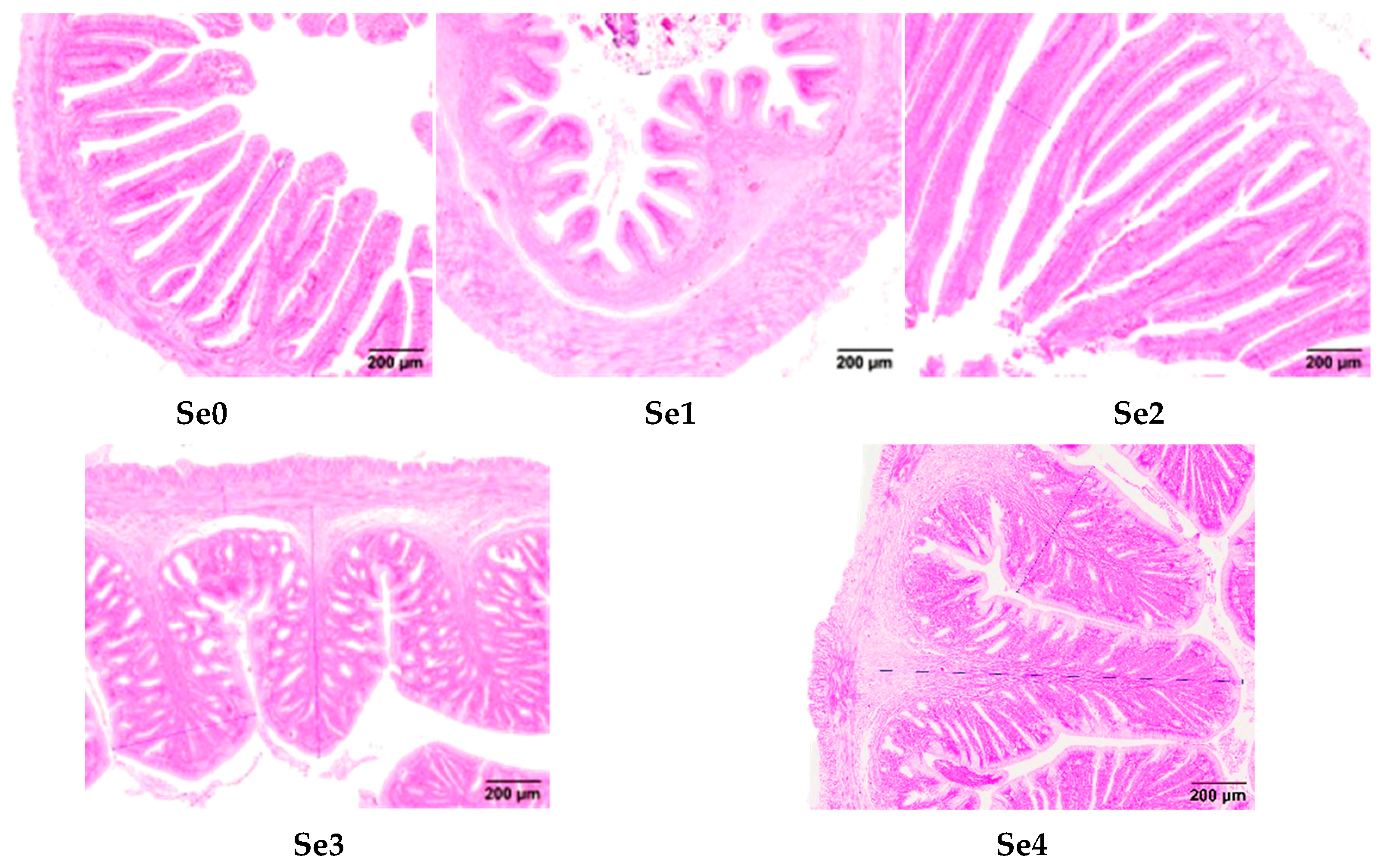
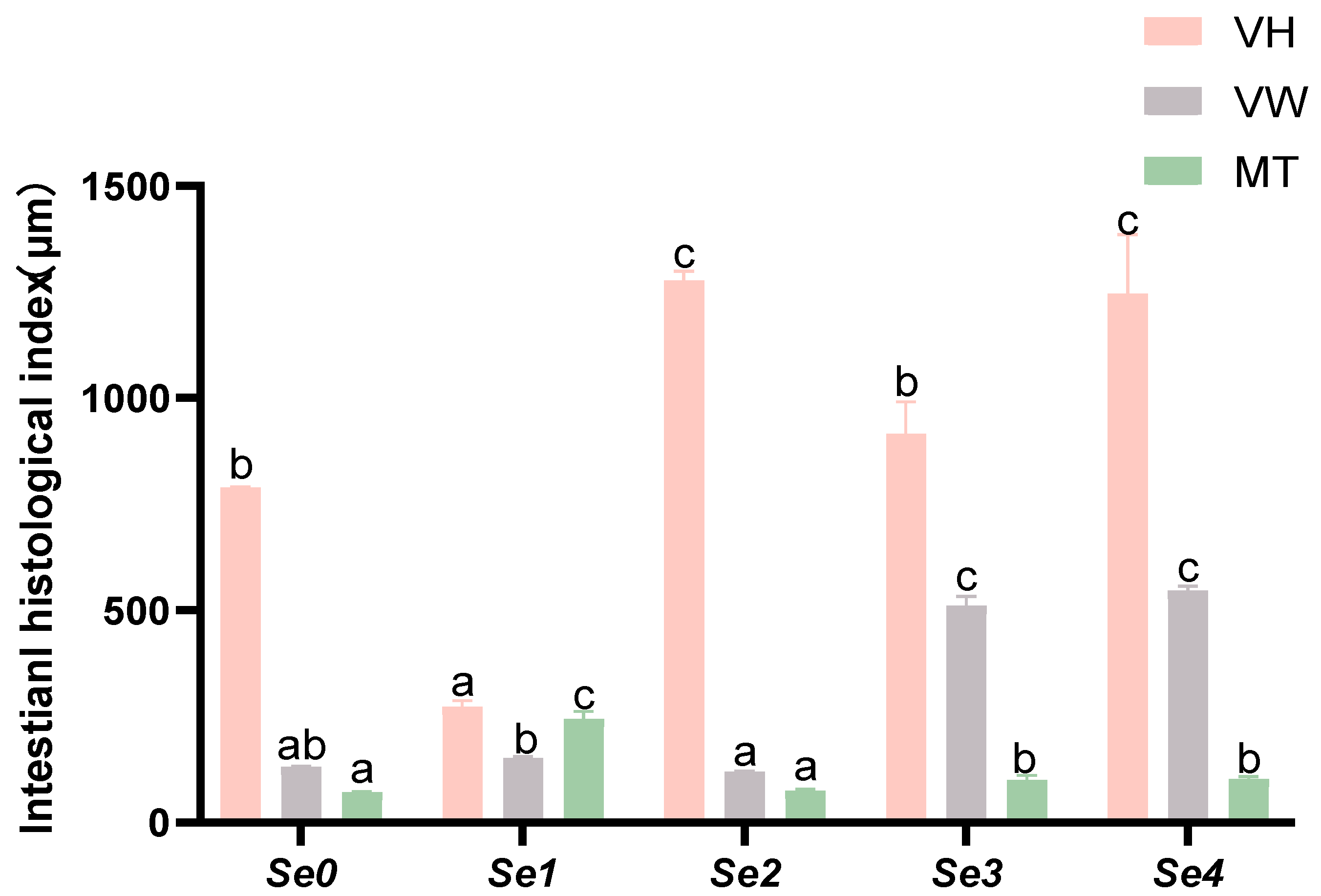
3.8. Intestinal Histomorphology
3.9. Intestinal Digestive Enzyme Activities
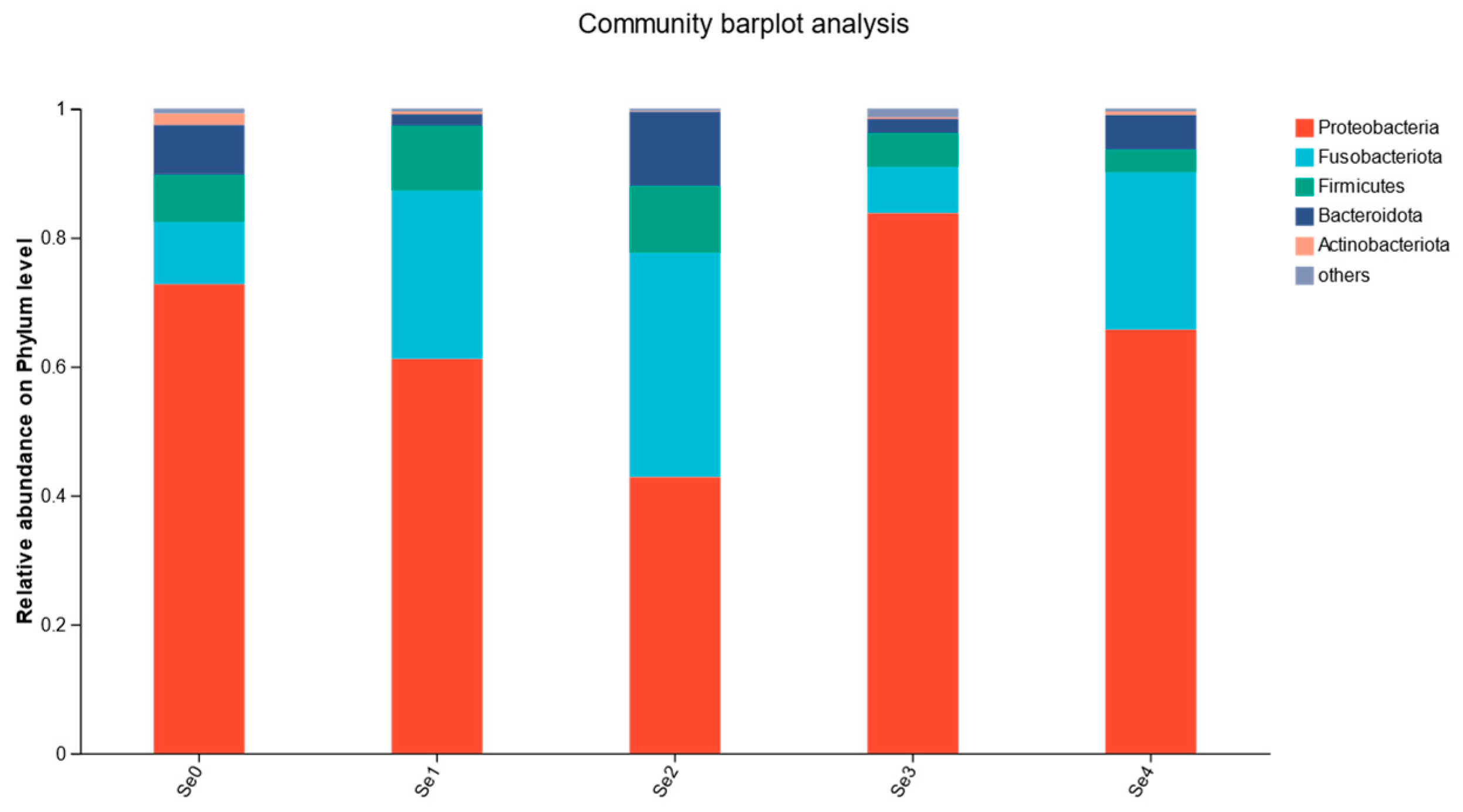
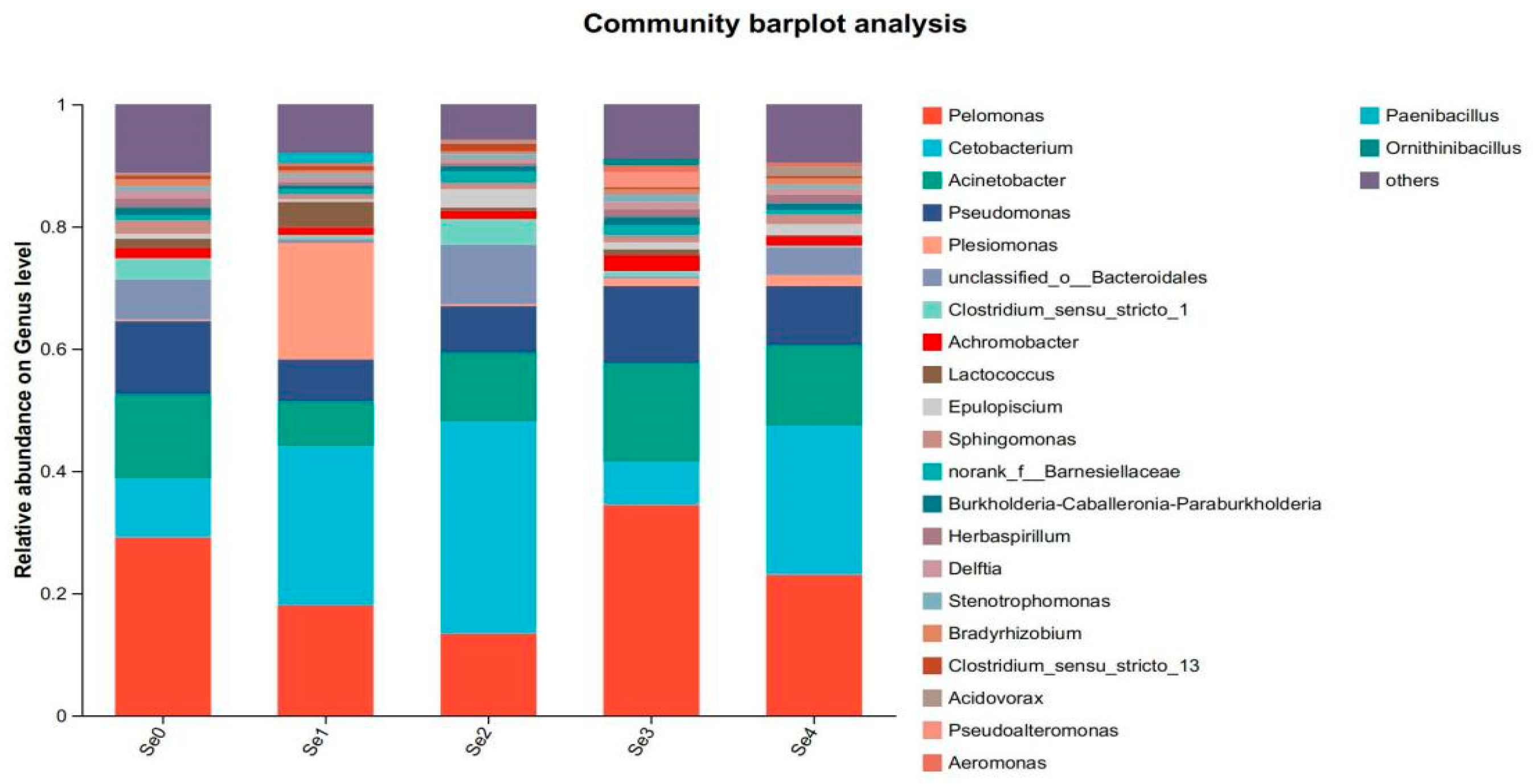
3.10. Intestinal Microbial Flora
4. Discussion
4.1. Growth Performance
4.2. Whole-Body Composition and Morphological Indices
4.3. Se Accumulation
4.4. Serum Biochemical Parameters
4.5. Hepatic and Intestinal Antioxidant Capacity
4.6. Intestinal Histomorphology
4.7. Intestinal Digestive Enzyme Activities
4.8. Intestinal Microbial Flora
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, C.F.; Luo, L.; Li, Q.; Wen, H.; Wang, F.B. Effects of dietary selenium on the anti-oxidation capability and histological structure of grass carpce (Cenopharyngodon idellus). J. Southwest China Norm. Univ. (Nat. Sci. Ed.) 2008, 33, 69–75. (In Chinese) [Google Scholar] [CrossRef]
- Hao, J.Y. Effects of Dietary Selenium on Growth, Glycolipid Metabolism and Antioxidant Capacity and the Relaxation Effects on Excess Selenium of Bamboo Charcoal in Blunt Snout Bream (Megalobrama amblycephala). Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. (In Chinese) [Google Scholar] [CrossRef]
- Khalil, H.S.; Mansour, A.T.; Goda, A.M.A.; Omar, E.A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre (Argyrosomus regius), fingerlings. Aquaculture 2019, 501, 135–143. [Google Scholar] [CrossRef]
- Foroshani, N.S.; Islami, H.R.; Mousavi, S.A.; Khara, H.; Siahkalroodi, Y.S. Supplementation of juvenile Siberian sturgeon (Acipenser baerii) diet with different levels of selenium: Growth performance, digestive enzyme activities, and intestinal histomorphology. Aquac. Rep. 2024, 38, 102325. [Google Scholar] [CrossRef]
- Liu, L.W.; Liang, X.F.; Li, J.; Yuan, X.H.; Alam, M.S.; Fang, J. Effects of dietary selenium on growth performance and oxidative stress in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Nutr. 2018, 24, 1296–1303. [Google Scholar] [CrossRef]
- Aman, A.J.; Kim, M.; Saunders, L.M.; Parichy, D.M. Thyroid hormone regulates abrupt skin morphogenesis during zebrafish postembryonic development. Dev. Biol. 2021, 477, 205–218. [Google Scholar] [CrossRef]
- Ma, P.; Hu, Z.Y.; Li, L.; Li, D.P.; Tang, R. Dietary selenium promotes the growth performance through growth hormone–insulin-like growth factor and hypothalamic–pituitary–thyroid axes in grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2021, 47, 1313–1327. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, D.F.; Li, S.; Yin, J.J.; Xu, Z.; Zhang, X.Z. Effect of dietary selenium on postprandial protein deposition in the muscle of juvenile rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2021, 125, 721–731. [Google Scholar] [CrossRef]
- Adineh, H.; Naderi, M.; Nazer, A.; Yousefi, M.; Ahmadifar, E. Interactive effects of stocking density and dietary supplementation with Nano selenium and garlic extract on growth, feed utilization, digestive enzymes, stress responses, and antioxidant capacity of grass carp (Ctenopharyngodon idella). J. Word Aquac. Soc. 2021, 52, 789–804. [Google Scholar] [CrossRef]
- Betancor, M.B.; Caballero, M.J.; Terova, G.; Saleh, R.; Atalah, E.; Benítez-Santana, T.; Bell, J.G.; Izquierdo, M. Selenium inclusion decreases oxidative stress indicators and muscle injuries in sea bass larvae fed high DHA microdiets. Br. J. Nutr. 2012, 108, 2115–2128. [Google Scholar] [CrossRef]
- Kong, Y.; Li, S.; Liu, M.; Li, M.; Yang, X.; Yao, C.; Zhao, N. Effect of dietary organic selenium on survival, growth, antioxidation, immunity and gene expressions of selenoproteins in abalone Haliotis discus hannai. Aquac. Res. 2019, 50, 847–855. [Google Scholar] [CrossRef]
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Wang, L.; Sagada, G.; Wang, R.L.; Li, P.W.; Xu, B.Y.; Zhang, C.; Qiao, J.L.; Yan, Y. Different forms of selenium supplementation in fish feed: The bioavailability, nutritional functions, and potential toxicity. Aquaculture 2022, 549, 737819. [Google Scholar] [CrossRef]
- Zheng, L.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Liu, Y.; Tang, L.; Wu, P.; Zeng, Y.Y.; Zhou, X.Q. Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 77, 53–70. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.B.; Gu, Q.; Li, Y.F. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 2009, 291, 78–81. [Google Scholar] [CrossRef]
- Yang, D.Q.; Chen, F.R.; Guo, L. Effects of dietary choline supplementation on growth, tissue fat content, and digestive enzyme activity in rice-field eels. J. Fish. 2006, 5, 676–682. [Google Scholar]
- Gong, B.; Ju, D.D.; Xu, H.M.; Xiao, M.S. Effects of Selenomethionine on Growth Performance, Selenium Content in Muscle Tissue and Serum Biochemical Indicators of rice field eels. Feed Res. 2023, 46, 61–64. [Google Scholar] [CrossRef]
- GB/T 6435-2014; Meng, F.S.; Zhang, S.; Su, S.L.; Liu, J.M.; Liang, M.; Li, H.; Guo, J.; Gong, L.L. National Standard of the People’s Republic of China: Animal feeding stuff—Determination of crude moisture. China Standards Press: Shanghai, China, 2014.
- GB/T 6432-2018; Xiao, Z.M.; Fan, X.; Ma, D.X.; Li, L.B.; Wang, S.; Jia, Z.; Liu, X.X.; Wang, Z.G.; Liu, J. National Standard of the People’s Republic of China: Determination of crude protein in feeds—Kjeldahl method. China Standards Press: Shanghai, China, 2018.
- GB/T 6433-2006; Zhang, S.; Li, L.P.; Fan, Z.Y.; Yang, S.M.; Su, X.O. National Standard of the People’s Republic of China: Animal feeding stuff—Determination of crude fat. China Standards Press: Shanghai, China, 2016.
- GB/T 6438-2007; Wu, R.L.; Yang, L.; He, Y.F.; Qian, L.; Zhong, M.; Yang, A.H.; Zhang, C.F. National Standard of the People’s Republic of China: Animal feeding stuff—Determination of crude ash. China Standards Press: Shanghai, China, 2007.
- Eggers, D.M. Some recent methods for estimating food-consumption by fish-reply. J. Fish. Res. Board Can. 1979, 36, 1018–1019. [Google Scholar]
- Lee, S.; Nambi, R.W.; Won, S.; Bai, S.C.; Katya, K. Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 464, 153–158. [Google Scholar] [CrossRef]
- Abd El-Kader, M.F.; Fath El-Bab, A.F.; Abd-Elghany, M.F.; Abdel-warith, A.W.A.; Dawood, M.A.O.; Younis, E.M. Selenium nanoparticles act potentially on the growth performance, hemato-biochemical indices, antioxidative, and immune-related genes of European seabass (Dicentrarchus labrax). Biol. Trace Elem. Res. 2021, 199, 3126–3134. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Zaineldin, A.I.; Abdel-daim, M.M.; Elsabagh, M.; Van Doan, H.; Ahmed, H.A. An evaluation of dietary selenium nanoparticles for red sea bream (Pagrus major) aquaculture: Growth, tissue bioaccumulation, and antioxidative responses. Environ. Sci. Pollut. Res. 2019, 26, 30876–30884. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream (Pagrus major). Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Ghrab, W.; Roo, J.; Hamre, K.; Hernández-Cruz, C.M.; Bernardini, G.; Terova, G.; Saleh, R. Organic, inorganic and nanoparticles of Se, Zn and Mn in early weaning diets for gilthead seabream (Sparus aurata). Aquac. Res. 2017, 48, 2852–2867. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Liu, Y.; Yang, H.; Liang, G.; Tian, L. Effect of dietary selenium level on growth performance, body composition and hepatic glutathione peroxidase activities of largemouth bass (Micropterus salmoide). Aquac. Res. 2012, 43, 1660–1668. [Google Scholar] [CrossRef]
- Brandt-Kjelsen, A.; Govasmark, E.; Haug, A.; Salub, B. Turnover of Se in adequately fed chickens using Se-75 as a tracer. J. Anim. Physiol. Anim. Nutr. 2014, 98, 547–558. [Google Scholar] [CrossRef]
- Lin, Y.H. Effects of dietary organic and inorganic selenium on the growth, selenium concentration and meat quality of juvenile grouper Epinephelus malabaricus. Aquaculture 2014, 430, 114–119. [Google Scholar] [CrossRef]
- Davis, K.B. Temperature affects physiological stress responses to acute confinement in sunshine bass (Morone chrysops× Morone saxatilis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 139, 433–440. [Google Scholar] [CrossRef]
- Mansour, A.T.E.; Goda, A.A.; Omar, E.A.; Khalil, H.S.; Esteban, M.A. Dietary supplementation of organic selenium improves growth, survival, antioxidant and immune status of meagre, Argyrosomus regius, juveniles. Fish Shellfish Immunol. 2017, 68, 516–524. [Google Scholar] [CrossRef] [PubMed]
- El-Sharawy, M.E.; Hamouda, M.; Soliman, A.A.; Amer, A.A.; El-Zayat, A.M.; Sewilam, H.; Younis, E.M.; Abdel-Warith, A.W.A.; Dawood, M.A.O. Selenium nanoparticles are required for the optimum growth behavior, antioxidative capacity, and liver wellbeing of Stripped catfish (Pangasianodon hypophthalmus). Saudi J. Biol. Sci. 2021, 28, 7241–7247. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Pourmozaffar, S.; Adeshina, I.; Erfanifar, E.; Mahmoudi, R.; Ajdari, A. Selenium nanoparticle and selenomethionine as feed additives: Effects on growth performance, hepatic enzymes’ activity, mucosal immune parameters, liver histology, and appetite-related gene transcript in goldfish (Carassius auratus). Fish Physiol. Biochem. 2021, 47, 639–652. [Google Scholar] [CrossRef]
- Pour, H.D.; Mousavi, S.M.; Zakeri, M.; Kochanian, P. Synergistic Effects of Selenium and Magnesium Nanoparticles on Growth, Digestive Enzymes, Some Serum Biochemical Parameters and Immunity of Asian Sea Bass (Lates calcarifer). Biol. Trace Elem. Res. 2021, 199, 3102–3111. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Pacini, N.; Abete, M.C.; Dörr, A.J.M.; Elia, A.C.; Natali, M.; Prearo, M. Detoxifying response in juvenile tench fed by selenium diet. Environ. Toxicol. Pharmacol. 2012, 33, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Chen, C.; Shao, Y.X.; ZHAO, T.T. Effects of Selenomethionine on Growth Performance, Tissue Selenium Content, Antioxidant Capacity, and Serum Biochemical Parameters in Striped Bass. Chin. J. Anim. Nutr. 2018, 30, 4746–4756. Available online: https://link.cnki.net/urlid/11.5461.S.20180831.1100.028 (accessed on 31 August 2018). (In Chinese).
- Yu, H.; Zhang, C.; Zhang, X. Dietary nano-selenium enhances antioxidant capacity and hypoxia tolerance of grass carp (Ctenopharyngodon idella) fed with high-fat diet. Aquac. Nutr. 2020, 26, 545–557. [Google Scholar] [CrossRef]
- Tsai, C.F.; Wu, J.Y.; Hsu, Y.W. Protective effects of rosmarinic acid against selenite-induced cataract and oxidative damage in rats. Int. J. Med. Sci. 2019, 16, 729. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Gu, Z.; He, Q.; Ga, L.P.; Du, J.L.; Jeney, G.; Xu, P.; Yin, J.G. Anti-oxidative, anti-inflammatory and hepatoprotective effects of Radix Bupleuri extract against oxidative damage in tilapia (Oreochromis niloticus) via Nrf2 and TLRs signaling pathway. Fish Shellfish Immunol. 2019, 93, 395–405. [Google Scholar] [CrossRef]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, K.B. The Nrf2–Keap1 defence pathway: Role in protection against drug-induced toxicity. Toxicology 2008, 246, 24–33. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Sivieri, K.; Bassan, J.; Peixoto, G.; Monti, R. Gut microbiota and antimicrobial peptides. Curr. Opin. Food Sci. 2017, 13, 56–62. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Carmona, R.; Merino, R.; Domezain, A.; Furne, M.; García-gallego, M.; Sanz, A. Effect of dietary lipid content and stocking density on digestive enzymes profile and intestinal histology of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 497, 10–16. [Google Scholar] [CrossRef]
- Ning, Y.; Wu, X.; Zhou, X.; Zhou, R.; Yang, Z.; Chang, Y.; Ding, J.; Huang, Z. An evaluation on the selenium yeast supplementation in the practical diets of early juvenile sea cucumber (Apostichopus japonicus): Growth performance, digestive enzyme activities, immune and antioxidant capacity, and body composition. Aquac. Nutr. 2021, 27, 2142–2153. [Google Scholar] [CrossRef]
- Li, X.M.; Xu, T.; Sun, G.H.; Yang, J.M.; Li, G.D.; Li, H.Z. Effects of Exogenous Selenium on the Activity of Key Physiological Enzymes and Selenium Levels in Sea Cucumber (Apostichopus japonicus). Prog. Fish. Sci. 2017, 38, 154–163. [Google Scholar]
- Hao, T.T.; Yu, C.L.; Wang, C.Q.; Wang, X.Y.; Li, L. Effects of Selenium Supplementation in High Plant Protein Feed on Liver and Intestinal Health of Juvenile Turbot (Scophthalmus maximus L.). J. Guangdong Ocean Univ. 2024, 44, 1–11. Available online: https://link.cnki.net/urlid/44.1635.n.20241031.0839.002 (accessed on 31 October 2024). (In Chinese).
- Chen, Q.; Li, G.Y.; Luo, K.; Jie, K.; Mo, Z.L. Icrobial Diversity in Water During the Breeding Period of Litopenaeus vannamei Broodstock. Oceanol. Limnol. Sin. 2017, 48, 130–138. [Google Scholar]
- Zhao, X.G.; Luo, H.; Liu, Q.G.; Zhao, L.J.; Cai, L.R.; Dai, L.L.; Zhang, Z. Effects of Rice-Fish Farming with Odontobutis obscura on Microbial Community Structure and Diversity in Paddy Field Water and Sediment. Freshw. Fish. 2017, 47, 8–14. [Google Scholar]
- Ma, Y.; Su, Z.; Chen, F. Terrestrial compound protein replacing dietary fishmeal improved digestive enzymeactivity, immune response, intestinal microflora composition, and protein metabolism of golden pompano (Trachinotus ovatus). Aquac. Nutr. 2023, 2023, 2716724. [Google Scholar] [CrossRef]
- Perry, W.B.; Lindsay, E.; Payne, C.J.; Brodie, C.; Kazlauskaite, R. The role of the gut microbiome in sustainable teleost aquaculture. Proc. Biol. Sci. 2020, 287, 20200184. [Google Scholar] [CrossRef]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef]


| Ingredients | Content (%) |
|---|---|
| Fish meal | 49.0 |
| Soy protein concentrate | 17.0 |
| Corn gluten meal | 11.9 |
| Soy lecithin | 2.0 |
| Wheat meal | 15.0 |
| Fish oil | 2.0 |
| Choline chloride | 0.5 |
| Ca(H2PO4)2 | 1.5 |
| Mineral premix 2 | 0.5 |
| Vitamin premix 1 | 0.5 |
| Cellulose powder | 0.1 |
| Total | 100 |
| Nutrient level | |
| Moisture (%) | 9.82 |
| Crude protein (%) | 47.47 |
| Crude lipid (%) | 17.67 |
| Crude ash (%) | 13.46 |
| Gene Name | Primer Sequence (5′–3′) | Product Length (bp) |
|---|---|---|
| RPL17 | F: GTTGTAGCGACGGAAAGGGAC R: GACTAAATCATGCAAGTCGAGGG | 160 |
| CAT | F: GTCCAAGTCTAAGGCATCTCC R: CTCCTCTTCGTTCAGCACC | 106 |
| GPX8 | F: GTCCACTTACGGTGTTACCT R: ATGGGCTCGTCAGTTCTC | 180 |
| Keap1 | F: AGCCTGGGTGCGATACGA R: CAAGAAATGACTTTGGTGGG | 198 |
| Nrf2 | F: CTTCAGACAGCGGTGACAGG R: GCCTCATTCAGTTGGTGCTT | 260 |
| Experiment | Time |
|---|---|
| Aquaculture Experiment | 20 July 2024–20 September 2024 |
| Sample Collection | 22 September 2024–24 September 2024 |
| Nutritional Components | 29 September 2024–30 September 2024 |
| Liver and Intestinal Biochemical Indices | 8 October 2024–5 November 2024 |
| Related Antioxidant Gene Expression | 10 November 2024–5 December 2024 |
| Parameters | Se0 | Se1 | Se2 | Se3 | Se4 |
|---|---|---|---|---|---|
| IW (g) | 18.53 ± 0.18 | 18.33 ± 0.00 | 18.40 ± 0.18 | 18.40 ± 0.12 | 18.49 ± 0.08 |
| FCR | 0.88 ± 0.18 | 0.99 ± 0.02 | 1.07 ± 0.04 | 1.05 ± 0.15 | 1.04 ± 0.02 |
| FW (g) | 61.93 ± 5.63 a | 67.99 ± 5.84 b | 62.74 ± 4.41 a | 61.94 ± 1.55 a | 57.63 ± 6.25 a |
| WGR (%) | 234.02 ± 27.60 a | 270.84 ± 31.87 b | 240.85 ± 20.65 a | 236.65 ± 9.50 a | 211.80 ± 35.06 a |
| SGR (%·d−1) | 2.01 ± 0.13 a | 2.18 ± 0.15 b | 2.04 ± 0.10 a | 2.02 ± 0.05 a | 1.89 ± 0.19 a |
| Parameters | Se0 | Se1 | Se2 | Se3 | Se4 |
|---|---|---|---|---|---|
| CF (g/cm3) | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 |
| HSI (%) | 4.64 ± 0.64 | 4.23 ± 0.17 | 5.24 ± 0.77 | 4.38 ± 0.70 | 4.72 ± 0.22 |
| VSI (%) | 7.78 ± 0.65 | 8.10 ± 0.50 | 8.99 ± 0.81 | 8.03 ± 0.33 | 8.72 ± 0.44 |
| Parameters | Se0 | Se1 | Se2 | Se3 | Se4 |
|---|---|---|---|---|---|
| Moisture (%) | 68.04 ± 0.23 | 66.42 ± 0.90 | 66.13 ± 0.91 | 69.99 ± 0.20 | 68.48 ± 0.02 |
| Crude protein (%) | 52.46 ± 1.13 b | 50.21 ± 0.88 a | 49.74 ± 0.46 a | 57.79 ± 0.09 c | 51.78±0.96 ab |
| Crude lipid (%) | 34.36 ± 0.12 d | 31.38 ± 0.50 c | 34.50 ± 0.74 d | 26.59 ± 0.22 b | 24.62 ± 0.08 a |
| Ash (%) | 7.18 ± 0.69 a | 6.35 ± 0.08 a | 6.25 ± 0.78 a | 8.74 ± 0.56 b | 8.88 ± 0.64 b |
| Parameters | Se0 | Se1 | Se2 | Se3 | Se4 |
|---|---|---|---|---|---|
| TP (g/L) | 39.62 ± 3.42 | 43.22 ± 4.24 | 46.22 ± 2.69 | 41.42 ± 2.05 | 43.65 ± 0.34 |
| ALB (g/L) | 13.06 ± 0.47 | 13.33 ± 1.01 | 14.20 ± 0.57 | 12.97 ± 0.36 | 13.67 ± 0.33 |
| AST (U/L) | 63.50 ± 0.50 b | 57.00 ± 1.00 a | 53.33 ± 1.15 a | 93.00 ± 4.36 c | 52.50 ± 0.50 a |
| ALT (U/L) | 5.00 ± 1.00 bc | 5.33 ± 1.53 bc | 4.67 ± 1.53 bc | 7.00 ± 1.00 c | 2.00 ± 0.00 a |
| TC (mmol/L) | 3.46 ± 0.35 a | 3.50 ± 0.45 a | 4.39 ± 0.23 b | 3.65 ± 0.28 a | 4.19 ± 0.20 ab |
| TG (mmol/L) | 1.27 ± 0.28 | 1.28 ± 0.36 | 1.55 ± 0.17 | 1.19 ± 0.27 | 1.26 ± 0.02 |
| GLU (mmol/L) | 4.03 ± 0.49 ab | 5.27 ± 0.50 b | 4.87 ± 0.95 b | 3.60 ± 0.00 a | 3.50 ± 0.30 a |
| HDL (mmol/L) | 1.64 ± 0.14 | 1.65 ± 0.25 | 2.05 ± 0.06 | 1.69 ± 0.17 | 1.57 ± 0.69 |
| LDL (mmol/L) | 0.54 ± 0.08 | 0.49 ± 0.09 | 0.72 ± 0.07 | 0.51 ± 0.06 | 0.51 ± 0.24 |
| Parameters | Se0 | Se1 | Se2 | Se3 | Se4 |
|---|---|---|---|---|---|
| ACE | 199.26 ± 18.02 | 227.04 ± 82.67 | 168.79 ± 43.76 | 191.27 ± 14.60 | 178.84 ± 31.90 |
| Chao | 198.87 ± 17.73 | 229.49 ± 79.70 | 167.19 ± 46.35 | 191.14 ± 14.79 | 177.43 ± 32.02 |
| Shannon | 2.98 ± 0.35 | 2.61 ± 0.91 | 2.67 ± 0.62 | 2.96 ± 0.35 | 2.70 ± 0.46 |
| Simpson | 0.16 ± 0.10 | 0.21 ± 0.13 | 0.18 ± 0.10 | 0.16 ± 0.07 | 0.20 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Liu, Y.; Wan, J.; Chen, J.; Qiu, Y.; Zhang, C. Effects of Different Forms of Organic Selenium on Growth Performance, Antioxidant Capacity, and Intestinal Health in Rice Field Eel (Monopterus albus). Animals 2025, 15, 1949. https://doi.org/10.3390/ani15131949
Yu D, Liu Y, Wan J, Chen J, Qiu Y, Zhang C. Effects of Different Forms of Organic Selenium on Growth Performance, Antioxidant Capacity, and Intestinal Health in Rice Field Eel (Monopterus albus). Animals. 2025; 15(13):1949. https://doi.org/10.3390/ani15131949
Chicago/Turabian StyleYu, Denghang, Yujia Liu, Jincheng Wan, Jiaxiang Chen, Yangjie Qiu, and Chi Zhang. 2025. "Effects of Different Forms of Organic Selenium on Growth Performance, Antioxidant Capacity, and Intestinal Health in Rice Field Eel (Monopterus albus)" Animals 15, no. 13: 1949. https://doi.org/10.3390/ani15131949
APA StyleYu, D., Liu, Y., Wan, J., Chen, J., Qiu, Y., & Zhang, C. (2025). Effects of Different Forms of Organic Selenium on Growth Performance, Antioxidant Capacity, and Intestinal Health in Rice Field Eel (Monopterus albus). Animals, 15(13), 1949. https://doi.org/10.3390/ani15131949






