Evaluating the Impact of Arginine-to-Lysine Ratios on Growth Performance, Antioxidant Defense, and Immune Modulation in Juvenile Largemouth Bass (Micropterus salmoides)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets, Feeding Procedure, and Sampling
2.2. Sample Analysis
2.3. Biochemical Analysis and Amino Acid Determination
2.4. Determination of Gene Expression in Liver
2.5. Data Analysis
3. Results
3.1. Growth Performance and Morphological Indicators
3.2. Nutritional Components of the Whole Body and Tissues
3.3. Nitrogen, Lipid, and Energy Intake and Utilization
3.4. Plasma Free Amino Acid Profile
3.5. Whole Body Amino Acid Composition
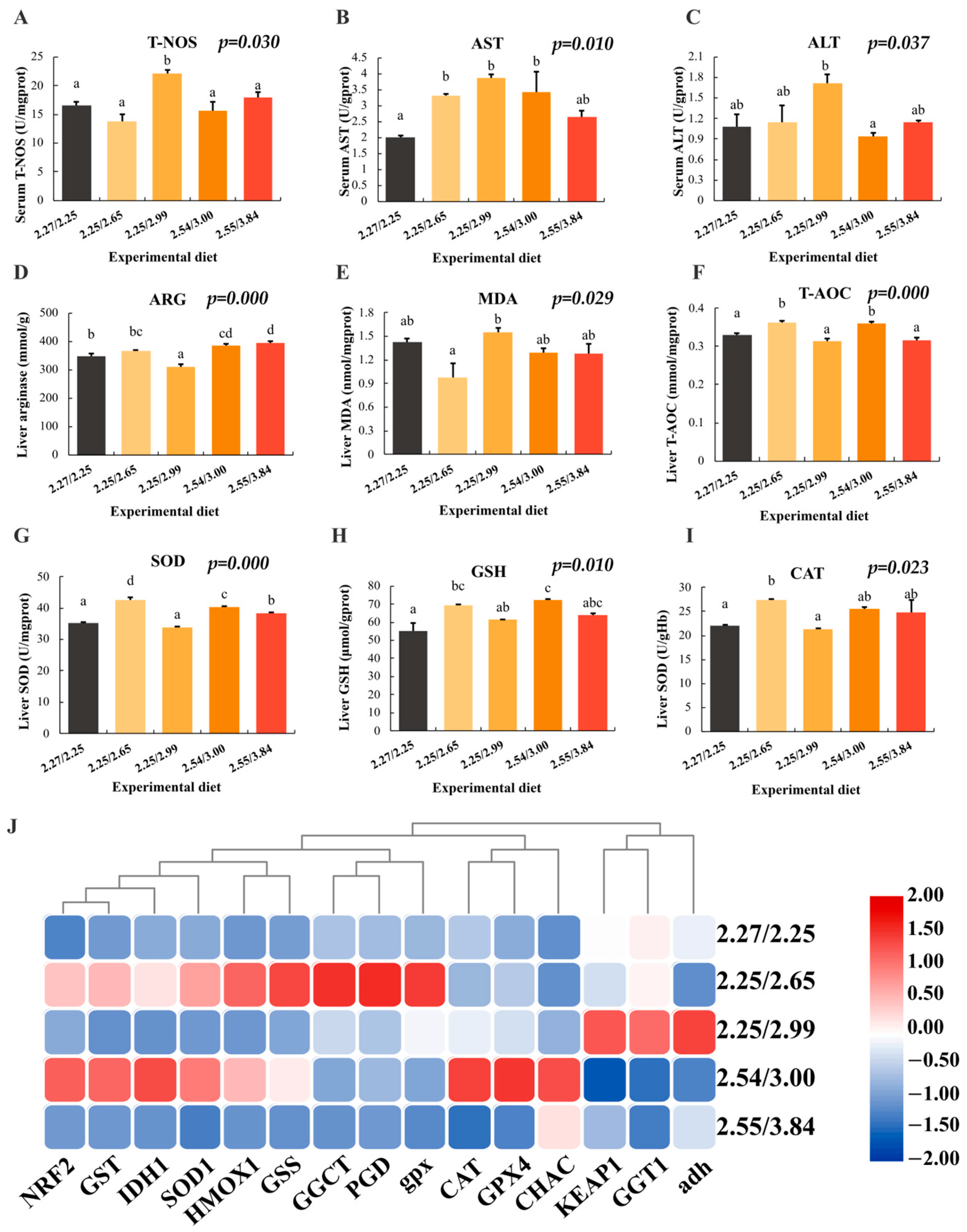
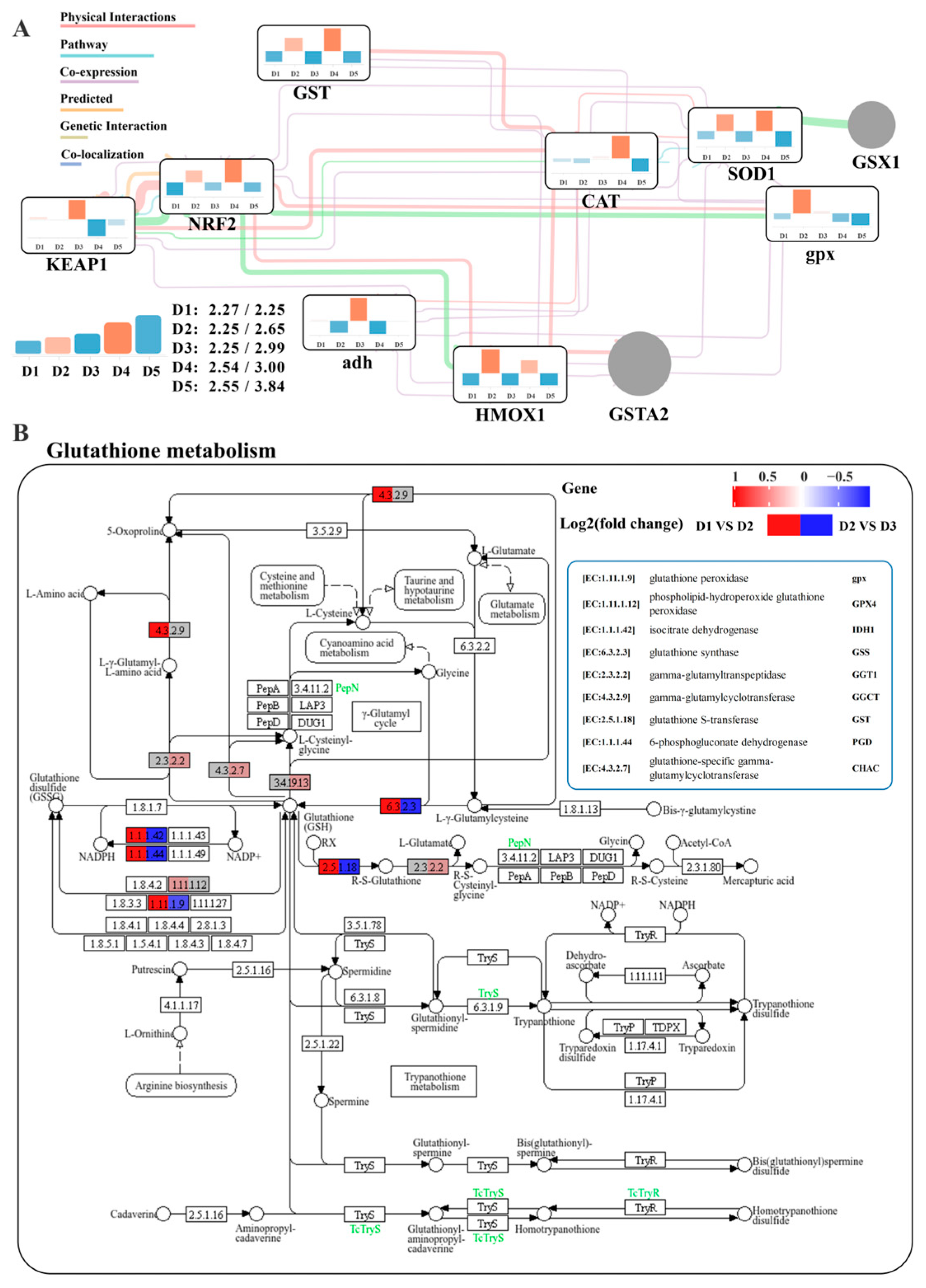
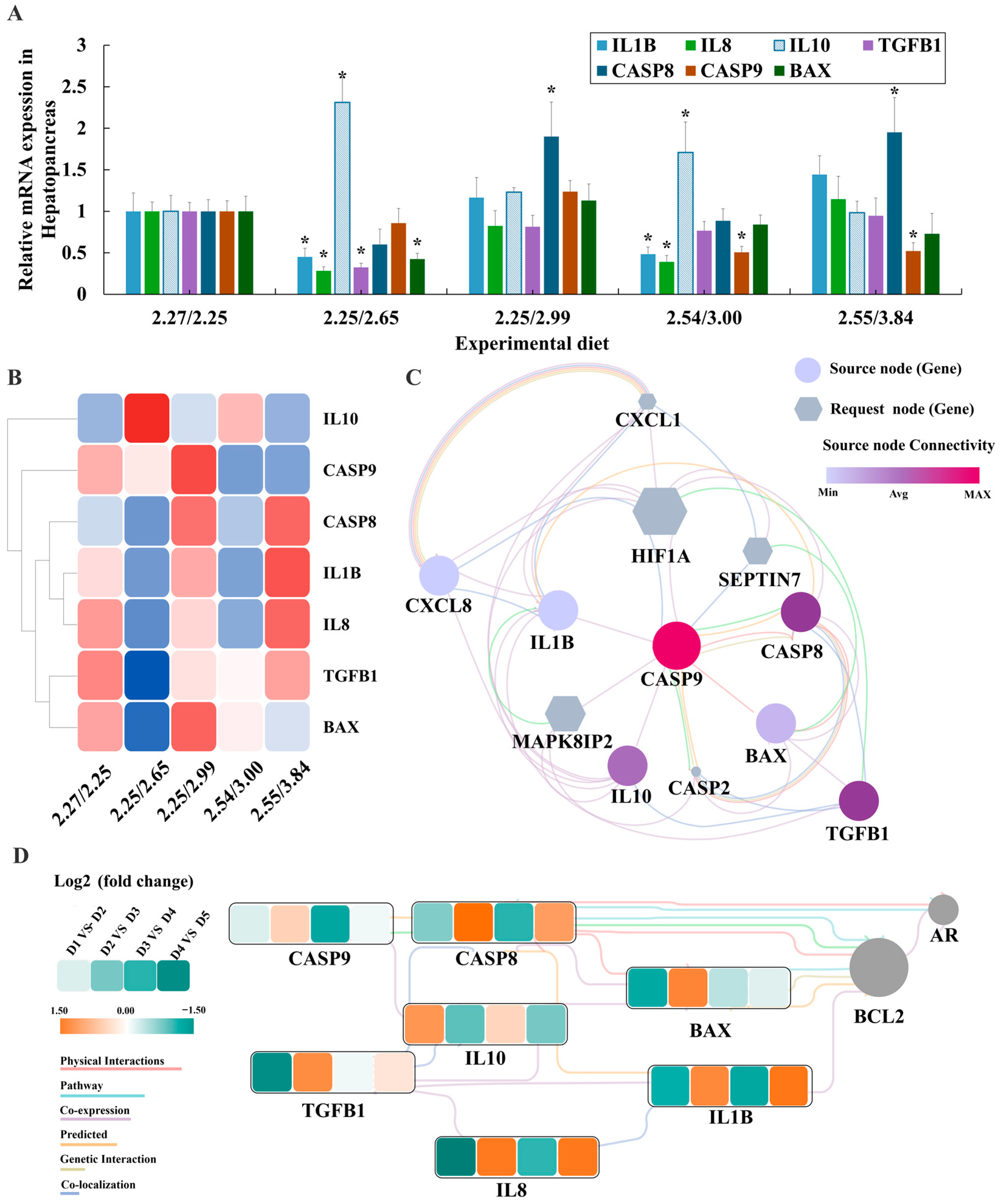
3.6. Serum and Liver Biochemical Indicators and Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-D.; Deng, Y.-P.; Liu, Y.; Qu, B.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Wu, P.; Zhang, Y.-A. Dietary leucine regulates the intestinal immune status, immune-related signalling molecules and tight junction transcript abundance in grass carp (Ctenopharyngodon idella). Aquaculture 2015, 444, 134–142. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Wen, H.-L.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Wu, P.; Zhao, J.; Tang, L.; Tang, W.-N.; Zhang, Y.-A. The tight junction protein transcript abundance changes and oxidative damage by tryptophan deficiency or excess are related to the modulation of the signalling molecules, NF-κB p65, TOR, caspase-(3, 8, 9) and Nrf2 mRNA levels, in the gill of young grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2015, 46, 168–180. [Google Scholar] [PubMed]
- Luo, J.-B.; Feng, L.; Jiang, W.-D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhang, Y.-A.; Zhou, X.-Q. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol. 2014, 40, 197–207. [Google Scholar] [CrossRef]
- Zheng, L.; Feng, L.; Jiang, W.-D.; Wu, P.; Tang, L.; Kuang, S.-Y.; Zeng, Y.-Y.; Zhou, X.-Q.; Liu, Y. Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 77, 53–70. [Google Scholar] [CrossRef]
- Liu, H.-X.; Zhou, X.-Q.; Jiang, W.-D.; Wu, P.; Liu, Y.; Zeng, Y.-Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Feng, L. Optimal α-lipoic acid strengthen immunity of young grass carp (Ctenopharyngodon idella) by enhancing immune function of head kidney, spleen and skin. Fish Shellfish Immunol. 2018, 80, 600–617. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and functions of amino acids in fish. In Amino Acids in Nutrition and Health: Amino Acids in the Nutrition of Companion, Zoo and Farm Animals; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 133–168. [Google Scholar]
- Herring, C.M.; Bazer, F.W.; Wu, G. Amino acid nutrition for optimum growth, development, reproduction, and health of zoo animals. In Amino Acids in Nutrition and Health: Amino Acids in the Nutrition of Companion, Zoo and Farm Animals; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 233–253. [Google Scholar]
- Evoy, D.; Lieberman, M.D.; Fahey, T.J., III; Daly, J.M. Immunonutrition: The role of arginine. Nutrition 1998, 14, 611–617. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Eddy, F.; Tibbs, P. Effects of nitric oxide synthase inhibitors and a substrate, L-arginine, on the cardiac function of juvenile salmonid fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 137–144. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Tanphaichitr, V.; Horne, D.W.; Broquist, H.P. Lysine, a precursor of carnitine in the rat. J. Biol. Chem. 1971, 246, 6364–6366. [Google Scholar] [CrossRef]
- Li, X.-Y.; Liu, Y.; Jiang, W.-D.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A. Co-and post-treatment with lysine protects primary fish enterocytes against Cu-induced oxidative damage. PLoS ONE 2016, 11, e0147408. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, L.; Jiang, W.; Wu, P.; Liu, Y.; Kuang, S.; Tang, L.; Zhou, X. Lysine deficiency impaired growth performance and immune response and aggravated inflammatory response of the skin, spleen and head kidney in grown-up grass carp (Ctenopharyngodon idella). Anim. Nutr. 2021, 7, 556–568. [Google Scholar] [CrossRef]
- Li, X.-Y.; Tang, L.; Hu, K.; Liu, Y.; Jiang, W.-D.; Jiang, J.; Wu, P.; Chen, G.-F.; Li, S.-H.; Kuang, S.-Y. Effect of dietary lysine on growth, intestinal enzymes activities and antioxidant status of sub-adult grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2014, 40, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Forster, I.; Ogata, H.Y. Lysine requirement of juvenile Japanese flounder Paralichthys olivaceus and juvenile red sea bream Pagrus major. Aquaculture 1998, 161, 131–142. [Google Scholar] [CrossRef]
- Ketola, H.G. Requirement for dietary lysine and arginine by fry of rainbow trout. J. Anim. Sci. 1983, 56, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Zhao, J.; Limbu, S.M.; Liang, Y.; Deng, J.; Bi, B.; Kong, L.; Yan, H.; Wang, X.; Hu, Q. Arginine supplementation in plant-rich diets affects growth, feed utilization, body composition, blood biochemical indices and gene expressions of the target of rapamycin signaling pathway in juvenile Asian red-tailed catfish (Hemibagrus wyckoiides). J. World Aquac. Soc. 2022, 53, 133–150. [Google Scholar] [CrossRef]
- Chen, G.; Feng, L.; Kuang, S.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.; Li, S.; Tang, L.; Zhou, X. Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Br. J. Nutr. 2012, 108, 195–207. [Google Scholar] [CrossRef]
- Lee, S.; Small, B.C.; Patro, B.; Overturf, K.; Hardy, R.W. The dietary lysine requirement for optimum protein retention differs with rainbow trout (Oncorhynchus mykiss Walbaum) strain. Aquaculture 2020, 514, 734483. [Google Scholar] [CrossRef]
- Zehra, S.; Khan, M.A. Dietary arginine requirement of fingerling Indian major carp, Catla catla (Hamilton). J. World Aquac. Soc. 2013, 44, 363–373. [Google Scholar] [CrossRef]
- Borlongan, I.G.; Coloso, R.M. Requirements of juvenile milkfish (Chanos chanos Forsskal) for essential amino acids. J. Nutr. 1993, 123, 125–132. [Google Scholar] [CrossRef]
- Zhou, Q.-C.; Zeng, W.-P.; Wang, H.-L.; Xie, F.-J.; Zheng, C.-Q. Dietary arginine requirement of juvenile yellow grouper Epinephelus awoara. Aquaculture 2012, 350, 175–182. [Google Scholar] [CrossRef]
- Walton, M. Aspects of amino acid metabolism in teleost fish. In Nutrition and Feeding in Fish; Academic Press: London, UK, 1985; pp. 47–67. [Google Scholar]
- Cynober, L.; Le Boucher, J.; Vasson, M.-P. Arginine metabolism in mammals. J. Nutr. Biochem. 1995, 6, 402–413. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meininger, C.J. Regulation of nitric oxide synthesis by dietary factors. Annu. Rev. Nutr. 2002, 22, 61–86. [Google Scholar] [CrossRef]
- Alam, S.; Teshima, S.-I.; Ishikawa, M.; Koshio, S. Effects of dietary arginine and lysine levels on growth performance and biochemical parameters of juvenile Japanese flounder Paralichthys olivaceus. Fish. Sci. 2002, 68, 509–516. [Google Scholar] [CrossRef]
- Shen, Y.; Qiu, Q.; Sun, L.; Yang, J.; Chen, Y.; Jin, L. Effects of dietary Arg/Lys ratios on digestive enzyme activity and apparent digestibility in all-male yellow catfish. Fish. Sci. 2018, 37, 152–158. [Google Scholar]
- Dong, X.; Wu, J.; Shen, Y.; Chen, J.; Miao, S.; Zhang, X.; Sun, L. Effects of different arginine/lysine level on growth performance, body composition and digestive enzyme activity of Macrobrachium rosenbergii. Aquac. Nutr. 2018, 24, 1101–1111. [Google Scholar] [CrossRef]
- Berge, G.; Sveier, H.; Lied, E. Effects of feeding Atlantic salmon (Salmo salar L.) imbalanced levels of lysine and arginine. Aquac. Nutr. 2002, 8, 239–248. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Y.; Lin, Y.; Chen, J.; Karrow, N.; Ren, X.; Wang, Y. Dietary protein and lipid requirements for juvenile largemouth bass, Micropterus salmoides. J. World Aquac. Soc. 2017, 48, 782–790. [Google Scholar] [CrossRef]
- Coyle, S.D.; Tidwell, J.H.; Webster, C.D. Response of largemouth bass Micropterus salmoides to dietary supplementation of lysine, methionine, and highly unsaturated fatty acids. J. World Aquac. Soc. 2000, 31, 89–95. [Google Scholar] [CrossRef]
- Liang, H.; Xu, P.; Xu, G.; Zhang, L.; Huang, D.; Ren, M.; Zhang, L. Histidine deficiency inhibits intestinal antioxidant capacity and induces intestinal endoplasmic-reticulum stress, inflammatory response, apoptosis, and necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants 2022, 11, 2399. [Google Scholar] [CrossRef]
- Bright, L.A.; Coyle, S.D.; Tidwell, J.H. Effect of dietary lipid level and protein energy ratio on growth and body composition of largemouth bass Micropterus salmoides. J. World Aquac. Soc. 2005, 36, 129–134. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, N.; Qiu, X.; Zhao, M.; Jin, L. Arginine requirement and effect of arginine intake on immunity in largemouth bass, Micropterus salmoides. Aquac. Nutr. 2012, 18, 107–116. [Google Scholar] [CrossRef]
- Dairiki, J.K.; Dias, C.T.d.S.; Cyrino, J.E.P. Lysine requirements of largemouth bass, Micropterus salmoides: A comparison of methods of analysis of dose-response trials data. J. Appl. Aquac. 2007, 19, 1–27. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000; Volume 17. [Google Scholar]
- Meng, J.; Yan, L.; Li, M.; Yue, X. Effect of matrine on inflammatory response and activity of arginase and adenosine nucleotide hydrolase in plateletsin rats with carrageenan-induced arthritis. Acta Chin. Med. 2021, 36, 7. [Google Scholar]
- Matsushita, K. Automatic precolumn derivatization of amino acids and analysis by fast LC using the Agilent 1290 Infinity LC system. Agil. Tech. Note 2010, 5990, 1–4. [Google Scholar]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.-J.; Tian, L.-X.; Xie, M.-Q.; Yang, H.-J.; Wang, Y.; Liang, G.-Y. Quantitative dietary lysine requirement of juvenile grass carp Ctenopharyngodon idella. Aquaculture 2005, 249, 419–429. [Google Scholar] [CrossRef]
- Ebeneezar, S.; Vijayagopal, P.; Srivastava, P.; Gupta, S.; Varghese, T.; Prabu, D.L.; Chandrasekar, S.; Varghese, E.; Sayooj, P.; Tejpal, C. Dietary lysine requirement of juvenile Silver pompano, Trachinotus blochii (Lacepede, 1801). Aquaculture 2019, 511, 734234. [Google Scholar] [CrossRef]
- Feng, F.F.; Ai, Q.; Xu, W.; Mai, K.; Zhang, W. Effects of dietary arginine and lysine on growth and non-specific immune responses of juvenile darkbarbel catfish (Pelteobagrus vachelli). J. Fish. China 2011, 35, 1072–1080. [Google Scholar] [CrossRef]
- Berge, G.E.; Sveier, H.; Lied, E. Nutrition of Atlantic salmon (Salmo salar); the requirement and metabolic effect of lysine. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 477–485. [Google Scholar] [CrossRef]
- Sashaw, J.; Nawata, M.; Thompson, S.; Wood, C.M.; Wright, P.A. Rhesus glycoprotein and urea transporter genes in rainbow trout embryos are upregulated in response to alkaline water (pH 9.7) but not elevated water ammonia. Aquat. Toxicol. 2010, 96, 308–313. [Google Scholar] [CrossRef]
- Barash, H. The influence of the lysine level in the diet on nitrogen excretion and on the concentration of ammonia and free amino acids in the plasma of rainbow trout (Salmo gairdneri). Nutr. Rep. Int. 1984, 29, 283–289. [Google Scholar]
- Zhou, F.; Shao, Q.-j.; Xiao, J.-x.; Peng, X.; Ngandzali, B.-O.; Sun, Z.; Ng, W.-K. Effects of dietary arginine and lysine levels on growth performance, nutrient utilization and tissue biochemical profile of black sea bream, Acanthopagrus schlegelii, fingerlings. Aquaculture 2011, 319, 72–80. [Google Scholar] [CrossRef]
- Tantikitti, C.; Chimsung, N. Dietary lysine requirement of freshwater catfish (Mystus nemurus Cuv. & Val.). Aquac. Res. 2001, 32, 135–141. [Google Scholar]
- Zhou, F. Study on Effects of Dietary Lysine and Arginine on Growth Performance, and the Arginine/Lysine Antagonism Mechanism in Juvenile Black Sea bream, Acanthopagrus schlegelii. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2011; pp. 62–68. [Google Scholar]
- Li, X.; Zheng, S.; Jia, S.; Song, F.; Zhou, C.; Wu, G. Oxidation of energy substrates in tissues of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 1017–1032. [Google Scholar] [CrossRef]
- Harper, A.; Benevenga, N.; Wohlhueter, R. Effects of ingestion of disproportionate amounts of amino acids. Physiol. Rev. 1970, 50, 428–558. [Google Scholar] [CrossRef]
- Fico, M.E.; Hassan, A.S.; Milner, J.A. The influence of excess lysine on urea cycle operation and pyrimidine biosynthesis. J. Nutr. 1982, 112, 1854–1861. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kayes, T.B.; Amundson, C.H. Requirements for lysine and arginine by rainbow trout (Oncorhynchus mykiss). Aquaculture 1992, 106, 333–344. [Google Scholar] [CrossRef]
- Iaccarino, D.; Uliano, E.; Agnisola, C. Effects of arginine and/or lysine diet supplementation on nitrogen excretion in zebrafish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, S36. [Google Scholar] [CrossRef]
- Berge, G.E.; Bakke-McKellep, A.M.; Lied, E. In vitro uptake and interaction between arginine and lysine in the intestine of Atlantic salmon (Salmo salar). Aquaculture 1999, 179, 181–193. [Google Scholar] [CrossRef]
- Zhou, Q.; Jin, M.; Elmada, Z.C.; Liang, X.; Mai, K. Growth, immune response and resistance to Aeromonas hydrophila of juvenile yellow catfish, Pelteobagrus fulvidraco, fed diets with different arginine levels. Aquaculture 2015, 437, 84–91. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, B.; Ren, M.; Cui, H.; Xie, J.; Ge, X. Effects of lysine on growth, physiological and biochemical indexes of blood and essential amino acids of serum in juvenile blunt snout bream (Megalobrama amblycephala). J. Fish. China 2013, 37, 1716–1724. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Wen, H.-L.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella): Avoid tryptophan deficiency or excess. Food Chem. 2016, 199, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Kang, B.; Tao, L.; Rong, H.; Zhang, X. Effects of dietary cholesterol on antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in rainbow trout (Oncorhynchus mykiss) fed soybean meal-based diets. Fish Shellfish Immunol. 2013, 34, 324–331. [Google Scholar] [CrossRef]
- Tovar-Ramírez, D.; Mazurais, D.; Gatesoupe, J.; Quazuguel, P.; Cahu, C.; Zambonino-Infante, J. Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture 2010, 300, 142–147. [Google Scholar] [CrossRef]
- Mourente, G.; Dıaz-Salvago, E.; Bell, J.G.; Tocher, D.R. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: Attenuation by dietary vitamin E. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Matthaiou, C.M.; Goutzourelas, N.; Stagos, D.; Sarafoglou, E.; Jamurtas, A.; Koulocheri, S.D.; Haroutounian, S.A.; Tsatsakis, A.M.; Kouretas, D. Pomegranate juice consumption increases GSH levels and reduces lipid and protein oxidation in human blood. Food Chem. Toxicol. 2014, 73, 1–6. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Yang, A.Y.; Guo, Y.; Kong, A.-N.T. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2–ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef]
- Tabei, Y.; Murotomi, K.; Umeno, A.; Horie, M.; Tsujino, Y.; Masutani, B.; Yoshida, Y.; Nakajima, Y. Antioxidant properties of 5-hydroxy-4-phenyl-butenolide via activation of Nrf2/ARE signaling pathway. Food Chem. Toxicol. 2017, 107, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, Y.-M.; Sun, X.; Guo, N.; Li, J.; Wang, W.; Yao, L.-P.; Fu, Y.-J. Hepatoprotective effect of 2′-O-galloylhyperin against oxidative stress-induced liver damage through induction of Nrf2/ARE-mediated antioxidant pathway. Food Chem. Toxicol. 2017, 102, 129–142. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Frölich, J.C. The l-arginine—Nitric oxide pathway: Role in atherosclerosis and therapeutic implications. Atherosclerosis 1996, 127, 1–11. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.-H.; Zhang, H.; Qiu, W.; Liu, Q.-H.; Peng, X.; Li, Y.-N.; Yang, H.-K. Alkali treatment affects in vitro digestibility and bile acid binding activity of rice protein due to varying its ratio of arginine to lysine. Food Chem. 2012, 132, 925–930. [Google Scholar] [CrossRef]
- Bansal, V.; Ochoa, J.B. Arginine availability, arginase, and the immune response. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 223–228. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Enzymes of arginine metabolism. J. Nutr. 2004, 134, 2743S–2747S. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef]
| Ingredients (g 100 g−1) | Argine/Lysine Ratio | ||||
|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | |
| Soybean meal | 19.00 | 19.00 | 19.00 | 27.00 | 27.00 |
| Fish meal | 26.00 | 26.00 | 26.00 | 26.00 | 26.00 |
| AA mix a | 15.22 | 15.22 | 15.22 | 11.64 | 11.64 |
| Lys | 0.00 | 0.51 | 1.11 | 0.86 | 1.96 |
| Gly | 2.81 | 2.29 | 1.67 | 1.13 | 0.00 |
| Corn starch | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Fish oil | 9.92 | 9.92 | 9.92 | 9.92 | 9.92 |
| Vitamin mix b | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| Mineral mix c | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Calcium dihydrogen phosphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| CMC | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Cellulose | 8.05 | 8.06 | 8.08 | 4.63 | 4.66 |
| 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| Proximate composition (g 100 g−1 dry matter) | |||||
| Moisture | 7.87 | 8.25 | 7.31 | 7.43 | 5.53 |
| Crude protein | 44.41 | 44.05 | 44.02 | 44.84 | 44.07 |
| Crude lipid | 12.26 | 12.32 | 12.32 | 12.05 | 12.61 |
| Ash | 8.74 | 8.96 | 9.08 | 9.06 | 9.50 |
| Energy (MJ/kg) | 20.57 | 20.46 | 20.60 | 21.16 | 21.48 |
| Amino Acid | Argine/Lysine Ratio | ||||
|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | |
| Arg | 2.27 | 2.25 | 2.25 | 2.54 | 2.55 |
| His | 0.84 | 0.85 | 0.84 | 0.96 | 0.97 |
| lle | 1.63 | 1.67 | 1.59 | 1.64 | 1.67 |
| Leu | 2.82 | 2.84 | 2.79 | 2.94 | 2.96 |
| Lys | 2.25 | 2.65 | 2.99 | 3.00 | 3.84 |
| Met | 1.24 | 1.24 | 1.25 | 1.14 | 1.19 |
| Thr | 1.87 | 1.87 | 1.86 | 1.80 | 1.80 |
| Phe | 1.85 | 1.87 | 1.84 | 1.83 | 1.89 |
| Val | 2.40 | 2.37 | 2.33 | 2.28 | 2.27 |
| Ala | 3.15 | 3.15 | 3.12 | 3.07 | 3.07 |
| Asp | 5.22 | 5.23 | 5.21 | 5.16 | 5.19 |
| Glu | 9.10 | 9.10 | 9.05 | 9.00 | 9.02 |
| Gly | 6.60 | 6.14 | 5.53 | 5.00 | 3.94 |
| Pro | 0.55 | 0.56 | 0.54 | 0.54 | 0.57 |
| Ser | 2.01 | 2.00 | 2.03 | 2.13 | 2.15 |
| Tyr | 1.14 | 1.08 | 1.13 | 1.19 | 1.22 |
| Argine/Lysine Ratio | SEM 1 | ANOVA 2 | |||||
|---|---|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | p Value | ||
| Survival 3 | 98.33% | 100.00% | 100.00% | 96.67% | 100.00% | 0.01 | 0.519 |
| IBW 4 | 5.95 ± 0.10 | 5.92 ± 0.13 | 5.95 ± 0.06 | 5.98 ± 0.09 | 5.98 ± 0.06 | 0.02 | 0.930 |
| FBW5 | 30.64 ± 0.86 | 32.41 ± 1.48 | 29.03 ± 0.59 | 32.21 ± 2.23 | 32.11 ± 1.36 | 0.46 | 0.068 |
| WG 6 | 415.27 ± 5.95 ab | 447.29 ± 21.40 b | 388.34 ± 13.32 a | 438.53 ± 29.83 ab | 436.87 ± 23.23 ab | 7.23 | 0.033 |
| SGR 7 | 3.15 ± 0.03 ab | 3.27 ± 0.08 b | 3.05 ± 0.06 a | 3.24 ± 0.11 ab | 3.23 ± 0.09 ab | 0.03 | 0.033 |
| FCR 8 | 1.06 ± 0.04 | 0.98 ± 0.01 | 1.04 ± 0.03 | 0.97 ± 0.06 | 0.98 ± 0.03 | 0.01 | 0.035 |
| PER 9 | 2.30 ± 0.08 | 2.52 ± 0.03 | 2.36 ± 0.07 | 2.48 ± 0.14 | 2.45 ± 0.06 | 0.03 | 0.053 |
| DFI 10 | 2.76 ± 0.11 | 2.61 ± 0.03 | 2.64 ± 0.04 | 2.57 ± 0.13 | 2.58 ± 0.03 | 0.03 | 0.090 |
| Argine/Lysine Ratio | SEM | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | p Value | ||
| IPF 1 | 7.00 ± 0.33 | 7.37 ± 0.69 | 7.11 ± 0.18 | 6.87 ± 0.03 | 6.69 ± 0.03 | 0.10 | 0.115 |
| HIS 2 | 1.49 ± 0.16 | 1.74 ± 0.32 | 1.53 ± 0.29 | 1.36 ± 0.06 | 1.36 ± 0.20 | 0.06 | 0.303 |
| VSI 3 | 1.21 ± 0.16 | 1.34 ± 0.06 | 1.38 ± 0.03 | 1.39 ± 0.16 | 1.15 ± 0.07 | 0.03 | 0.073 |
| CF 4 | 1.58 ± 0.27 | 1.87 ± 0.05 | 1.55 ± 0.31 | 1.84 ± 0.24 | 1.81 ± 0.03 | 0.06 | 0.109 |
| Argine/Lysine Ratio | SEM | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | p Value | ||
| Whole body% | |||||||
| Moisture | 70.93 ± 1.88 | 71.56 ± 0.09 | 71.13 ± 1.03 | 72.14 ± 1.15 | 72.30 ± 0.11 | 0.28 | 0.212 |
| Crude protein | 17.01 ± 1.51 | 16.89 ± 0.23 | 17.22 ± 0.59 | 16.91 ± 0.41 | 17.17 ± 0.81 | 0.19 | 0.926 |
| Crude lipid | 8.46 ± 1.08 | 7.76 ± 0.38 | 8.18 ± 0.83 | 7.42 ± 0.99 | 6.70 ± 0.40 | 0.24 | 0.134 |
| Ash | 3.70 ± 0.36 | 3.65 ± 0.02 | 3.77 ± 0.07 | 3.41 ± 0.09 | 3.62 ± 0.14 | 0.05 | 0.220 |
| Muscle% | |||||||
| Moisture | 76.55 ± 2.51 | 76.76 ± 0.22 | 73.21 ± 7.44 | 76.48 ± 0.62 | 76.69 ± 0.43 | 0.85 | 0.833 |
| Crude protein | 19.56 ± 1.08 | 19.58 ± 1.15 | 22.57 ± 6.14 | 19.09 ± 0.32 | 19.99 ± 0.46 | 0.70 | 0.600 |
| Crude lipid | 3.21 ± 1.76 | 3.23 ± 0.89 | 3.77 ± 1.75 | 3.34 ± 0.36 | 2.65 ± 0.27 | 0.28 | 0.621 |
| Liver% | |||||||
| Moisture | 68.71 ± 0.77 | 70.06 ± 3.28 | 68.75 ± 3.54 | 69.90 ± 1.24 | 71.56 ± 1.52 | 0.59 | 0.585 |
| Crude protein | 10.70 ± 0.42 | 9.21 ± 1.31 | 10.88 ± 1.45 | 10.90 ± 1.24 | 10.78 ± 1.05 | 0.30 | 0.373 |
| Crude lipid | 3.33 ± 0.21 | 3.24 ± 0.30 | 4.10 ± 0.97 | 3.24 ± 0.19 | 3.17 ± 0.50 | 0.15 | 0.838 |
| Argine/Lysine Ratio | SEM | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | p Value | ||
| Nitrogen | |||||||
| DNI (g kg−1 day−1) 1 | 1.81 ± 0.07 | 1.69 ± 0.02 | 1.72 ± 0.03 | 1.71 ± 0.09 | 1.72 ± 0.02 | 0.02 | 0.132 |
| DNG (g kg −1 day−1) 2 | 0.70 ± 0.08 | 0.71 ± 0.01 | 0.70 ± 0.04 | 0.71 ± 0.01 | 0.72 ± 0.04 | 0.01 | 0.834 |
| NR (%) 3 | 38.74 ± 3.09 | 42.19 ± 0.29 | 40.48 ± 2.80 | 41.58 ± 2.86 | 41.93 ± 3.08 | 0.67 | 0.523 |
| Lipid | |||||||
| DLI (g kg −1 day−1) 4 | 3.12 ± 0.12 a | 2.95 ± 0.03 ab | 3.01 ± 0.05 ab | 2.87 ± 0.15 b | 3.08 ± 0.04 a | 0.03 | 0.044 |
| DLG (g kg −1 day−1) 5 | 2.22 ± 0.36 | 2.04 ± 0.15 | 2.08 ± 0.29 | 1.91 ± 0.34 | 1.68 ± 0.10 | 0.08 | 0.217 |
| LR (%) 6 | 70.90 ± 8.51 | 69.10 ± 4.94 | 69.19 ± 10.56 | 66.54 ± 8.45 | 54.59 ± 3.18 | 2.29 | 0.136 |
| Energy | |||||||
| DEI (102 kJ kg−1 day−1) 7 | 5.20 ± 0.21 | 4.90 ± 0.05 | 5.00 ± 0.08 | 4.87 ± 0.26 | 4.99 ± 0.06 | 0.05 | 0.161 |
| DEG (102 kJ kg −1 day−1) 8 | 2.05 ± 0.18 | 1.88 ± 0.23 | 1.86 ± 0.11 | 1.90 ± 0.20 | 1.94 ± 0.02 | 0.04 | 0.656 |
| ER (%) 9 | 39.35 ± 1.86 | 38.39 ± 4.79 | 37.18 ± 2.77 | 38.94 ± 2.12 | 38.86 ± 0.82 | 0.64 | 0.827 |
| Amino Acid | Argine/Lysine Ratio | SEM | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | p Value | ||
| Arg | 2.96 ± 0.87 a | 5.29 ± 1.16 b | 6.73 ± 0.23 b | 2.97 ± 0.12 a | 2.16 ± 0.95 a | 0.49 | 0.028 |
| His | 70.22 ± 12.35 | 73.82 ± 11.65 | 101.46 ± 11.99 | 79.93 ± 17.46 | 104.28 ± 16.71 | 4.91 | 0.042 |
| Ile | 132.12 ± 30.69 | 139.91 ± 35.77 | 170.51 ± 14.73 | 146.28 ± 13.55 | 169.21 ± 14.65 | 6.66 | 0.248 |
| Leu | 227.08 ± 53.64 | 245.53 ± 58.98 | 299.12 ± 16.45 | 257.89 ± 28.41 | 297.29 ± 24.92 | 11.61 | 0.188 |
| Lys | 80.75 ± 35.35 a | 139.80 ± 31.71 ab | 147.26 ± 5.36 ab | 90.35 ± 18.10 a | 209.56 ± 46.78 b | 14.07 | 0.003 |
| Met | 77.33 ± 10.50 a | 82.35 ± 13.66 a | 120.80 ± 1.74 b | 95.59 ± 17.56 ab | 95.45 ± 21.61 ab | 5.14 | 0.035 |
| Thr | 206.75 ± 31.46 | 186.33 ± 40.69 | 252.06 ± 2.98 | 175.32 ± 41.32 | 225.96 ± 33.78 | 10.33 | 0.102 |
| Phe | 94.73 ± 23.25 | 96.60 ± 29.15 | 104.73 ± 17.76 | 103.79 ± 5.72 | 105.19 ± 18.93 | 4.62 | 0.946 |
| Val | 219.30 ± 48.31 | 230.31 ± 44.91 | 300.16 ± 21.31 | 246.26 ± 22.67 | 274.02 ± 24.26 | 10.89 | 0.087 |
| EAA | 1111.2467 ± 234.24 | 1199.94 ± 220.36 | 1502.83 ± 75.43 | 1198.39 ± 124 | 1483.13 ± 166.92 | 57.60 | 0.061 |
| Ala | 726.65 ± 178.62 | 693.38 ± 165.24 | 901.69 ± 37.56 | 667.97 ± 46.07 | 888.09 ± 95.40 | 37.26 | 0.103 |
| Asp | 23.90 ± 11.70 | 25.44 ± 1.49 | 26.71 ± 0.70 | 26.27 ± 2.07 | 28.01 ± 3.27 | 1.27 | 0.693 |
| Asn | 43.05 ± 16.60 a | 55.29 ± 5.92 ab | 87.41 ± 9.22 b | 61.20 ± 17.14 ab | 85.68 ± 9.66 b | 5.39 | 0.005 |
| Glu | 39.59 ± 13.75 | 46.58 ± 13.79 | 39.11 ± 4.53 | 35.48 ± 5.60 | 53.06 ± 8.82 | 2.77 | 0.287 |
| Gln | 213.62 ± 77.16 | 256.20 ± 80.53 | 365.19 ± 59.59 | 275.75 ± 51.72 | 248.46 ± 11.33 | 20.27 | 0.060 |
| Gly | 1880.44 ± 483.90 b | 1451.10 ± 245.11 ab | 1547.77 ± 110.18 ab | 1104.66 ± 253.94 a | 1116.80 ± 45.86 a | 97.78 | 0.036 |
| Pro | 83.42 ± 26.63 | 85.02 ± 25.21 | 100.46 ± 16.43 | 89.03 ± 2.56 | 115.26 ± 11.58 | 5.18 | 0.270 |
| Ser | 258.02 ± 58.75 ab | 282.89 ± 96.55 b | 304.09 ± 14.10 b | 134.23 ± 29.65 a | 136.82 ± 37.29 a | 22.95 | 0.032 |
| Tyr | 123.61 ± 19.47 ab | 108.69 ± 21.66 a | 175.84 ± 23.84 b | 140.32 ± 22.20 ab | 149.74 ± 30.02 ab | 8.02 | 0.049 |
| Cit | 44.59 ± 12.59 a | 42.96 ± 6.00 a | 74.76 ± 9.80 b | 49.65 ± 8.17 a | 45.54 ± 1.72 a | 3.67 | 0.005 |
| Orn | 78.52 ± 29.42 | 92.00 ± 23.10 | 120.25 ± 20.30 | 96.89 ± 1.56 | 109.71 ± 12.87 | 5.80 | 0.176 |
| NEAA | 3515.41 ± 861.72 | 3139.54 ± 653.80 | 3743.26 ± 215.71 | 2681.45 ± 392.72 | 3077.17 ± 144.54 | 151.43 | 0.201 |
| Amino Acid | Argine/Lysine Ratio | SEM | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | p Value | ||
| Arg | 7.62 ± 0.13 | 7.58 ± 0.05 | 7.48 ± 0.02 | 7.52 ± 0.12 | 7.55 ± 0.16 | 0.03 | 0.573 |
| His | 2.72 ± 0.05 a | 2.73 ± 0.03 ab | 2.77 ± 0.02 abc | 2.82 ± 0.04 c | 2.82 ± 0.04 bc | 0.01 | 0.012 |
| Ile | 4.84 ± 0.11 | 4.90 ± 0.01 | 4.86 ± 0.06 | 4.79 ± 0.08 | 4.66 ± 0.15 | 0.03 | 0.064 |
| Leu | 8.17 ± 0.12 | 8.19 ± 0.09 | 8.30 ± 0.04 | 8.31 ± 0.13 | 8.27 ± 0.21 | 0.03 | 0.576 |
| Lys | 9.23 ± 0.19 | 9.37 ± 0.10 | 9.45 ± 0.07 | 9.51 ± 0.16 | 9.49 ± 0.22 | 0.04 | 0.269 |
| Met | 3.21 ± 0.07 | 3.27 ± 0.03 | 3.20 ± 0.09 | 3.22 ± 0.09 | 3.21 ± 0.06 | 0.02 | 0.792 |
| Thr | 4.94 ± 0.09 ab | 4.91 ± 0.04 a | 5.00 ± 0.04 ab | 5.03 ± 0.04 ab | 5.06 ± 0.04 b | 0.02 | 0.027 |
| Phe | 4.80 ± 0.05 | 4.81 ± 0.03 | 4.86 ± 0.01 | 4.85 ± 0.06 | 4.83 ± 0.07 | 0.01 | 0.466 |
| Val | 6.00 ± 0.05 b | 6.03 ± 0.02 b | 6.00 ± 0.02 b | 5.90 ± 0.09 ab | 5.76 ± 0.09 a | 0.03 | 0.003 |
| EAA | 51.52 ± 0.54 | 51.79 ± 0.27 | 51.92 ± 0.23 | 51.96 ± 0.42 | 51.64 ± 0.67 | 0.11 | 0.745 |
| Ala | 1.23 ± 0.02 | 1.21 ± 0.06 | 1.47 ± 0.04 | 1.32 ± 0.17 | 1.29 ± 0.05 | 0.03 | 0.071 |
| Asp | 10.84 ± 0.07 | 10.88 ± 0.05 | 10.92 ± 0.04 | 10.98 ± 0.13 | 10.99 ± 0.15 | 0.03 | 0.334 |
| Glu | 15.87 ± 0.12 | 15.86 ± 0.08 | 15.93 ± 0.06 | 15.98 ± 0.06 | 16.01 ± 0.10 | 0.02 | 0.205 |
| Gly | 10.81 ± 0.63 | 10.49 ± 0.34 | 10.06 ± 0.38 | 9.96 ± 0.70 | 9.97 ± 0.97 | 0.17 | 0.445 |
| Pro | 1.85 ± 0.10 | 1.91 ± 0.14 | 1.79 ± 0.05 | 1.77 ± 0.06 | 2.00 ± 0.15 | 0.03 | 0.135 |
| Ser | 4.85 ± 0.08 ab | 4.74 ± 0.07 a | 4.88 ± 0.06 ab | 4.92 ± 0.03 b | 4.99 ± 0.03 b | 0.03 | 0.004 |
| Tyr | 3.05 ± 0.12 | 3.12 ± 0.07 | 3.04 ± 0.20 | 3.12 ± 0.23 | 3.10 ± 0.20 | 0.04 | 0.963 |
| NEAA | 48.49 ± 0.54 | 48.21 ± 0.27 | 48.09 ± 0.22 | 48.05 ± 0.41 | 48.35 ± 0.67 | 0.11 | 0.741 |
| Amino Acid | Argine/Lysine Ratio | SEM | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| D1: 1.01 (2.27/2.25) | D2: 0.85 (2.25/2.65) | D3: 0.75 (2.25/2.99) | D4: 0.85 (2.54/3.00) | D5: 0.66 (2.55/3.84) | p Value | ||
| Arg | 58.81 ± 3.44 ab | 64.25 ± 0.27 b | 59.99 ± 3.62 ab | 55.01 ± 3.62 a | 55.22 ± 3.46 a | 1.14 | 0.027 |
| His | 56.50 ± 3.31 | 61.46 ± 0.25 | 59.60 ± 3.59 | 54.72 ± 3.60 | 54.25 ± 3.40 | 1.01 | 0.070 |
| Ile | 52.21 ± 3.06 | 55.90 ± 0.23 | 55.05 ± 3.32 | 53.97 ± 3.55 | 52.07 ± 3.26 | 0.76 | 0.457 |
| Leu | 50.81 ± 2.97 | 55.14 ± 0.23 | 53.66 ± 3.23 | 52.38 ± 3.45 | 52.15 ± 3.27 | 0.74 | 0.468 |
| Lys | 72.09 ± 4.22 c | 67.46 ± 0.28 c | 57.10 ± 3.44 b | 58.79 ± 3.87 b | 46.16 ± 2.89 a | 2.50 | 0.000 |
| Met | 45.58 ± 2.67 | 50.41 ± 0.21 | 46.14 ± 2.78 | 52.31 ± 3.45 | 50.46 ± 3.16 | 0.92 | 0.047 |
| Thr | 46.26 ± 2.71 | 50.10 ± 0.21 | 48.41 ± 2.92 | 51.80 ± 3.41 | 52.50 ± 3.28 | 0.86 | 0.106 |
| Phe | 45.61 ± 2.67 | 49.08 ± 0.20 | 47.62 ± 2.87 | 49.23 ± 3.24 | 47.76 ± 2.99 | 0.67 | 0.492 |
| Val | 43.88 ± 2.57 | 48.60 ± 0.20 | 46.56 ± 2.81 | 48.02 ± 3.16 | 47.30 ± 2.97 | 0.71 | 0.268 |
| EAA | 52.67 ± 3.08 | 56.14 ± 0.23 | 52.81 ± 3.18 | 53.15 ± 3.50 | 50.38 ± 3.16 | 0.80 | 0.270 |
| Ala | 6.86 ± 0.41 a | 7.33 ± 0.03 a | 8.48 ± 0.51 b | 7.98 ± 0.53 ab | 7.84 ± 0.49 ab | 0.18 | 0.009 |
| Asp | 36.43 ± 2.13 | 39.74 ± 0.17 | 37.82 ± 2.28 | 39.44 ± 2.60 | 39.53 ± 2.48 | 0.58 | 0.316 |
| Glu | 30.62 ± 1.79 | 33.28 ± 0.13 | 31.75 ± 1.91 | 32.90 ± 2.17 | 33.13 ± 2.07 | 0.47 | 0.367 |
| Gly | 28.74 ± 1.69 a | 32.63 ± 0.13 ab | 32.80 ± 1.98 ab | 36.92 ± 2.43 b | 47.19 ± 2.95 c | 1.75 | 0.000 |
| Pro | 58.74 ± 3.44 | 65.54 ± 0.27 | 59.18 ± 3.57 | 60.32 ± 3.97 | 64.81 ± 4.06 | 1.06 | 0.090 |
| Ser | 42.37 ± 2.48 | 45.22 ± 0.19 | 43.33 ± 2.61 | 42.79 ± 2.82 | 43.34 ± 2.71 | 0.58 | 0.651 |
| Tyr | 47.08 ± 2.75 a | 55.09 ± 0.23 b | 48.48 ± 2.92 a | 48.62 ± 3.20 a | 47.49 ± 2.98 a | 0.97 | 0.023 |
| NEAA | 30.66 ± 1.80 a | 33.77 ± 0.14 ab | 32.58 ± 1.96 ab | 34.13 ± 2.25 ab | 35.86 ± 2.24 b | 0.61 | 0.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, S.; Luan, X.; Liu, T.; He, J.; Wang, J.; Han, T. Evaluating the Impact of Arginine-to-Lysine Ratios on Growth Performance, Antioxidant Defense, and Immune Modulation in Juvenile Largemouth Bass (Micropterus salmoides). Animals 2025, 15, 1947. https://doi.org/10.3390/ani15131947
Sun Y, Zhang S, Luan X, Liu T, He J, Wang J, Han T. Evaluating the Impact of Arginine-to-Lysine Ratios on Growth Performance, Antioxidant Defense, and Immune Modulation in Juvenile Largemouth Bass (Micropterus salmoides). Animals. 2025; 15(13):1947. https://doi.org/10.3390/ani15131947
Chicago/Turabian StyleSun, Yulong, Shuailiang Zhang, Xueyao Luan, Tao Liu, Jiale He, Jiteng Wang, and Tao Han. 2025. "Evaluating the Impact of Arginine-to-Lysine Ratios on Growth Performance, Antioxidant Defense, and Immune Modulation in Juvenile Largemouth Bass (Micropterus salmoides)" Animals 15, no. 13: 1947. https://doi.org/10.3390/ani15131947
APA StyleSun, Y., Zhang, S., Luan, X., Liu, T., He, J., Wang, J., & Han, T. (2025). Evaluating the Impact of Arginine-to-Lysine Ratios on Growth Performance, Antioxidant Defense, and Immune Modulation in Juvenile Largemouth Bass (Micropterus salmoides). Animals, 15(13), 1947. https://doi.org/10.3390/ani15131947




