Interaction Between Ruminal Acetate Infusion and Diet Fermentability on Milk Fat Production in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Animal Management and Diets
2.3. Milk Sampling and Analysis
2.4. Rumen and Blood Sampling and Analysis
2.5. Statistical Analysis
3. Results
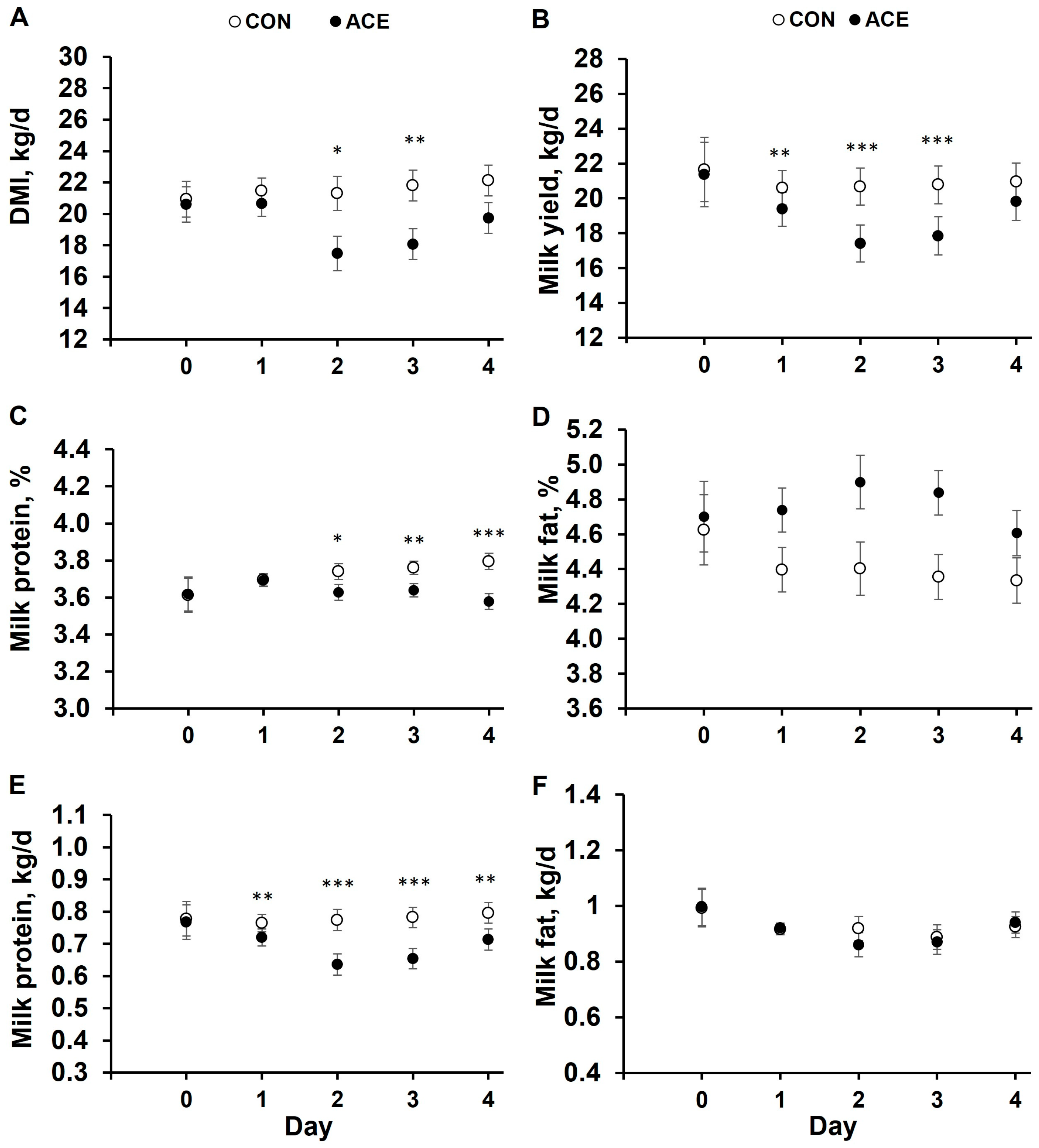
3.1. Dry Matter Intake, Milk Yield, and Composition
3.2. Milk Fatty Acid (FA) Profile
3.3. Plasma Metabolites and Rumen pH
4. Discussion
4.1. Dry Matter Intake, Milk Yield, and Milk Composition
4.2. Milk Fatty Acid Profile and Rumen pH
4.3. Plasma Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HF | High fermentability |
| LF | Low fermentability |
| ACE | Acetate treatment |
| CON | Control treatment |
| DMI | Dry matter intake |
| SCFA | Short-chain fatty acid |
| TMR | Total mixed ration |
| NDF | Neutral detergent fiber |
| DM | Dry matter |
| CP | Crude protein |
| ADF | Acid detergent fiber |
| OM | Organic matter |
| NFC | Non-fiber carbohydrates |
| FA | Fatty acid |
| AM | Before midday |
| PM | After midday |
| NEFA | Non-esterified fatty acid |
| BHB | β-hydroxybutyrate |
References
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J. Volatile fatty acid production. In Quantitative Aspects of Ruminant Digestion and Metabolism, 2nd ed.; CABI: Wallingford, UK, 2005; pp. 157–175. [Google Scholar]
- Bauman, D.E.; Brown, R.E.; Davis, C.L. Pathways of fatty acid synthesis and reducing equivalent generation in mammary gland of rat, sow, and cow. Arch. Biochem. Biophys. 1970, 140, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, N.; Bomberger, R.; Matamoros, C.; Harvatine, K.J. Effect of dietary supplementation of sodium acetate and calcium butyrate on milk fat synthesis in lactating dairy cows. J. Dairy Sci. 2019, 102, 5172–5181. [Google Scholar] [CrossRef]
- Matamoros, C.; Cai, J.; Patterson, A.D.; Harvatine, K.J. Comparison of the effects of short-term feeding of sodium acetate and sodium bicarbonate on milk fat production. J. Dairy Sci. 2021, 104, 7572–7582. [Google Scholar] [CrossRef]
- Maxin, G.; Rulquin, H.; Glasser, F. Response of milk fat concentration and yield to nutrient supply in dairy cows. Animal 2011, 5, 1299–1310. [Google Scholar] [CrossRef]
- IDF (International Dairy Federation). The World Dairy Situation Report 2024; International Dairy Federation: Brussels, Belgium, 2024; p. 236. [Google Scholar]
- Urrutia, N.L.; Harvatine, K.J. Acetate Dose-Dependently Stimulates Milk Fat Synthesis in Lactating Dairy Cows. J. Nutr. 2017, 147, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.; Dhanoa, M.; Morant, S.; France, J.; Napper, D.; Schuller, E. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and low-roughage diets. J. Dairy Sci. 2003, 86, 3620–3633. [Google Scholar] [CrossRef]
- Fuentes, M.; Calsamiglia, S.; Cardozo, P. Effect of pH and level of concentrate in the diet on the production of biohydrogenation intermediates in a dual-flow continuous culture. J. Dairy Sci. 2009, 92, 4456–4466. [Google Scholar] [CrossRef]
- Larsen, M.; Kristensen, N.B. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal 2013, 7, 1640–1650. [Google Scholar] [CrossRef]
- Matamoros, C.; Hao, F.; Tian, Y.; Patterson, A.D.; Harvatine, K.J. Interaction of sodium acetate supplementation and dietary fiber level on feeding behavior, digestibility, milk synthesis, and plasma metabolites. J. Dairy Sci. 2022, 105, 8824–8838. [Google Scholar] [CrossRef] [PubMed]
- Mertens, D.R. Creating a System for Meeting the Fiber Requirements of Dairy Cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Farmer, E.R.; Tucker, H.A.; Dann, H.M.; Cotanch, K.W.; Mooney, C.S.; Lock, A.L.; Yagi, K.; Grant, R.J. Effect of reducing dietary forage in lower starch diets on performance, ruminal characteristics, and nutrient digestibility in lactating Holstein cows. J. Dairy Sci. 2014, 97, 5742–5753. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Harvatine, K.J. Lipid feeding and milk fat depression. Vet. Clin. Food Anim. Pract. 2014, 30, 623–642. [Google Scholar] [CrossRef]
- Weimer, P.J.; Stevenson, D.M.; Mantovani, H.C.; Man, S.L. Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents. J. Dairy Sci. 2010, 93, 5902–5912. [Google Scholar] [CrossRef] [PubMed]
- Satter, L.D.; Bringe, A.N. Effect of Abrupt Ration Changes on Milk and Blood Components 1. J. Dairy Sci. 1969, 52, 1776–1780. [Google Scholar] [CrossRef]
- Mu, Y.; Qi, W.; Zhang, T.; Zhang, J.; Mei, S.; Mao, S. Changes in rumen fermentation and bacterial community in lactating dairy cows with subacute rumen acidosis following rumen content transplantation. J. Dairy Sci. 2021, 104, 10780–10795. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001; p. 381. [Google Scholar]
- Urrutia, N.; Harvatine, K.J. Effect of conjugated linoleic acid and acetate on milk fat synthesis and adipose lipogenesis in lactating dairy cows. J. Dairy Sci. 2017, 100, 5792–5804. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 20th ed.; Latimer, G., Ed.; Association of Official Analytical Chemists, International: Rockville, MD, USA, 2016. [Google Scholar]
- MAFF. Carbohydrate, solubles, in herbage. In The Analysis of Agricultural Materials: A Manual of the Analytical Methods Used by the Agricultural Development and Advisory Service, 3rd ed.; HMSO: London, UK, 1986; pp. 43–45. [Google Scholar]
- Sadzawka, M.A.R.; Carrasco, A.M.R.; Demanet, F.R.; Flores, P.H.; Grez, Z.R.; Mora, G.M.L.; Neaman, A. Métodos de Análisis de Tejidos Vegetales; Serie Actas INIA Nº 40; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2007; pp. 91–95. [Google Scholar]
- Van Soest, P.; Wine, R.; Moore, L. Estimation of the true digestibility of forages by the in vitro digestion of cell walls. In Proceedings of the 10th International Grassland Congress, Helsinki, Finland, 7–8 July 1966; pp. 438–441. [Google Scholar]
- ISO. Milk and Liquid Milk Products—Guidelines for the Application of Mid-Infrared Spectrometry (ISO/IDF 9622:2013), 2nd ed.; International Organization for Standardization: Brussels, Switzerland, 2013. [Google Scholar]
- Rico, D.E.; Harvatine, K.J. Induction of and recovery from milk fat depression occurs progressively in dairy cows switched between diets that differ in fiber and oil concentration. J. Dairy Sci. 2013, 96, 6621–6630. [Google Scholar] [CrossRef]
- Raabo, B.E.; Terkildsen, T. On the enzymatic determination of blood glucose. Scand. J. Clin. Lab. Investig. 1960, 12, 402–407. [Google Scholar] [CrossRef]
- Ballou, M.A.; Gomes, R.C.; Juchem, S.O.; DePeters, E.J. Effects of dietary supplemental fish oil during the peripartum period on blood metabolites and hepatic fatty acid compositions and total triacylglycerol concentrations of multiparous Holstein cows. J. Dairy Sci. 2009, 92, 657–669. [Google Scholar] [CrossRef]
- Sheperd, A.; Combs, D. Long-term effects of acetate and propionate on voluntary feed intake by midlactation cows. J. Dairy Sci. 1998, 81, 2240–2250. [Google Scholar] [CrossRef]
- Maxin, G.; Glasser, F.; Hurtaud, C.; Peyraud, J.; Rulquin, H. Combined effects of trans-10, cis-12 conjugated linoleic acid, propionate, and acetate on milk fat yield and composition in dairy cows. J. Dairy Sci. 2011, 94, 2051–2059. [Google Scholar] [CrossRef]
- Gualdron-Duarte, L.B.; Allen, M.S. Effects of acetic acid or sodium acetate infused into the rumen or abomasum on feeding behavior and metabolic response of cows in the postpartum period. J. Dairy Sci. 2018, 101, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S. Drives and limits to feed intake in ruminants. Anim. Prod. Sci. 2014, 54, 1513–1524. [Google Scholar] [CrossRef]
- Allen, M.S. Review: Control of feed intake by hepatic oxidation in ruminant animals: Integration of homeostasis and homeorhesis. Animal 2020, 14, s55–s64. [Google Scholar] [CrossRef] [PubMed]
- Storry, J.; Rook, J. Effects of intravenous infusions of acetate, β-hydroxybutyrate, triglyceride and other metabolites on the composition of the milk fat and blood in cows. Biochem. J. 1965, 97, 879. [Google Scholar] [CrossRef]
- Urrutia, N.L. Regulation of Lipogenesis by Spared Nutrients in Bovine Mammary and Adipose Tissue. Ph.D. Thesis, Pennsylvania State University, University Park, PA, USA, 2016. [Google Scholar]
- Matamoros, C.; Klopp, R.N.; Moraes, L.E.; Harvatine, K.J. Meta-analysis of the relationship between milk trans-10 C18:1, milk fatty acids <16 C, and milk fat production. J. Dairy Sci. 2020, 103, 10195–10206. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Harvatine, K.J. Origin of Fatty Acids and Influence of Nutritional Factors on Milk Fat. In Advanced Dairy Chemistry, Volume 2: Lipids; McSweeney, P.L.H., Fox, P.F., O’Mahony, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 33–66. [Google Scholar]
- Jensen, R.G. The Composition of Bovine Milk Lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Salfer, I.; Morelli, M.; Ying, Y.; Allen, M.; Harvatine, K. The effects of source and concentration of dietary fiber, starch, and fatty acids on the daily patterns of feed intake, rumination, and rumen pH in dairy cows. J. Dairy Sci. 2018, 101, 10911–10921. [Google Scholar] [CrossRef]
- Alzahal, O.; Or-Rashid, M.M.; Greenwood, S.L.; Douglas, M.S.; McBride, B.W. The effect of dietary fiber level on milk fat concentration and fatty acid profile of cows fed diets containing low levels of polyunsaturated fatty acids. J. Dairy Sci. 2009, 92, 1108–1116. [Google Scholar] [CrossRef]
- Park, Y.; Pariza, M.W. Mechanisms of body fat modulation by conjugated linoleic acid (CLA). Food Res. Int. 2007, 40, 311–323. [Google Scholar] [CrossRef]
- Allen, M.S.; Bradford, B.J.; Harvatine, K.J. The cow as a model to study food intake regulation. Annu. Rev. Nutr. 2005, 25, 523–547. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Ying, Y.; Bartell, P.A.; Harvatine, K.J. The effects of feeding time on milk production, total-tract digestibility, and daily rhythms of feeding behavior and plasma metabolites and hormones in dairy cows. J. Dairy Sci. 2014, 97, 7764–7776. [Google Scholar] [CrossRef]
- Radloff, H.D.; Schultz, L.H.; Hoekstra, W.G. Relationship of Plasma Free Fatty Acids to Other Blood Components in Ruminants under Various Physiological Conditions 1, 2, 3, 4. J. Dairy Sci. 1966, 49, 179–182. [Google Scholar] [CrossRef]
- Urrutia, N.; Ying, Y.; Harvatine, K.J. The effect of conjugated linoleic acid, acetate, and their interaction on adipose tissue lipid metabolism in nonlactating cows. J. Dairy Sci. 2017, 100, 5058–5067. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Jiang, L.; Mao, S. Effect of high-concentrate diets on microbial composition, function, and the VFAs formation process in the rumen of dairy cows. Anim. Feed. Sci. Technol. 2020, 269, 114619. [Google Scholar] [CrossRef]
- Palmquist, D.; Davis, C.; Brown, R.; Sachan, D. Availability and metabolism of various substrates in ruminants. V. Entry rate into the body and incorporation into milk fat of d(-) β-hydroxybutyrate. J. Dairy Sci. 1969, 52, 633–638. [Google Scholar] [CrossRef]
- Miller, P.S.; Reis, B.; Calvert, C.; DePeters, E.; Baldwin, R. Patterns of nutrient uptake by the mammary glands of lactating dairy cows. J. Dairy Sci. 1991, 74, 3791–3799. [Google Scholar] [CrossRef]
- Izumi, K.; Fukumori, R.; Oikawa, S.; Oba, M. Short communication: Effects of butyrate supplementation on the productivity of lactating dairy cows fed diets differing in starch content. J. Dairy Sci. 2019, 102, 11051–11056. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tunaru, S.; Offermanns, S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol. Sci. 2009, 30, 557–562. [Google Scholar] [CrossRef] [PubMed]
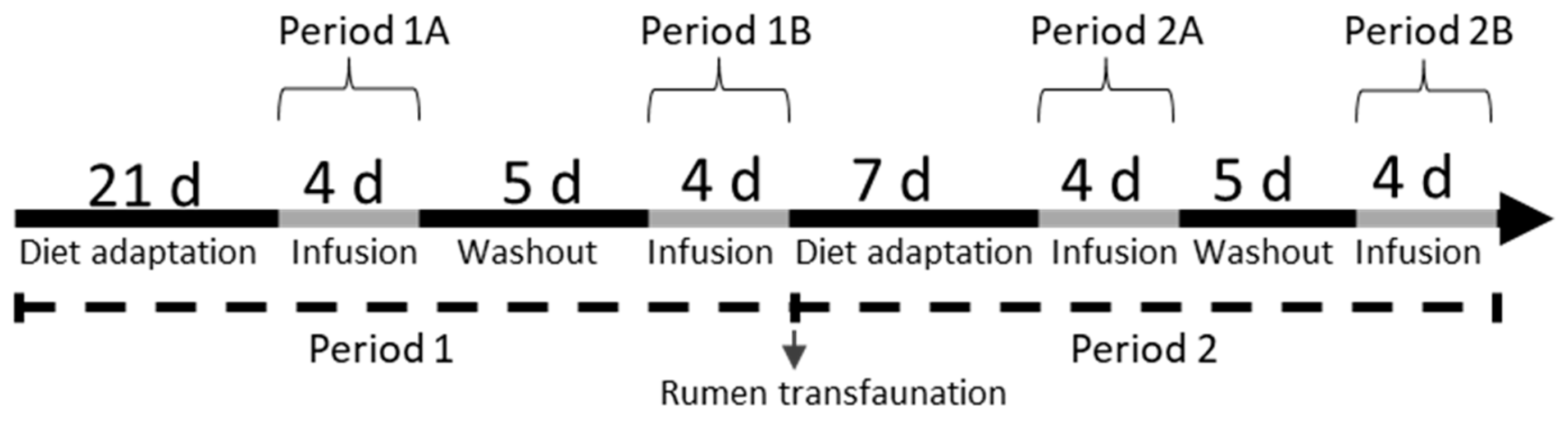

| Item | HF | LF |
|---|---|---|
| Ingredients, g/100 g DM | ||
| Corn silage | 65 | 65 |
| Ground corn | 17.4 | 5.4 |
| Soybean meal | 15.5 | 16.5 |
| Oat hulls | - | 11 |
| Mineral–vitamin mix 3 | 1.25 | 1.25 |
| NPN 4 | 0.85 | 0.85 |
| Nutrients, g/100 g DM | ||
| NDF | 34.6 ± 1.1 | 38.6 ± 1.1 |
| ADF | 21.6 ± 1.1 | 23.8 ± 0.8 |
| CP | 16.1 ± 0.2 | 16.8 ± 0.4 |
| Starch | 21.2 ± 1.1 | 16.3 ± 1.3 |
| Ether extract | 2.14 ± 0.21 | 1.98 ± 0.2 |
| Ash | 6.26 ± 0.24 | 6.60 ± 0.24 |
| NFC | 40.9 ± 1 | 36.0 ± 1.1 |
| Digestibility 5 | 77.9 ± 1.9 | 73.8 ± 1.3 |
| NEL 6, Mcal/kg | 1.58 ± 1.9 | 1.51 ± 0.02 |
| Variable | Treatments 1 | SE | p-Value 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HF | LF | |||||||||
| CON | ACE | CON | ACE | F | I | T | F × I | I × T | ||
| DMI, kg/d | 21.6 | 19.4 | 21.7 | 18.6 | 1.1 | 0.74 | 0.01 | 0.001 | 0.67 | 0.003 |
| Milk yield, kg/d | 20.6 | 18.7 | 20.9 | 18.5 | 1.0 | 0.93 | <0.001 | 0.015 | 0.40 | 0.03 |
| Milk fat % | 4.38 | 4.91 | 4.37 | 4.63 | 0.12 | 0.08 | <0.001 | 0.29 | 0.12 | 0.58 |
| Milk fat yield, kg/d | 0.90 | 0.91 | 0.92 | 0.89 | 0.03 | 0.99 | 0.66 | 0.40 | 0.29 | 0.71 |
| Milk protein % | 3.76 | 3.62 | 3.74 | 3.65 | 0.04 | 0.99 | 0.001 | 0.87 | 0.27 | <0.001 |
| Milk protein, kg/d | 0.78 | 0.68 | 0.78 | 0.68 | 0.03 | 0.85 | 0.001 | 0.02 | 0.78 | 0.03 |
| Apparent NEL Balance 3, Mcal/d | 32.7 | 29.3 | 31.1 | 27.0 | 2.59 | 0.21 | 0.03 | 0.003 | 0.81 | 0.12 |
| Milk FA | Treatments 1 | SE | p-Value 2 | |||||
|---|---|---|---|---|---|---|---|---|
| HF | LF | |||||||
| CON | ACE | CON | ACE | F | I | F × I | ||
| FA content by source 3, % | ||||||||
| De novo | 31.6 | 30.0 | 31.7 | 29.4 | 1.1 | 0.68 | 0.01 | 0.60 |
| Mixed | 37.0 | 40.9 | 37.5 | 38.9 | 1.2 | 0.55 | 0.03 | 0.31 |
| Preformed | 22.8 | 23.7 | 24.9 | 26.4 | 1.1 | 0.04 | 0.28 | 0.77 |
| OBCFA 4 | 3.50 | 3.08 | 3.56 | 3.40 | 0.15 | 0.19 | 0.05 | 0.36 |
| FA yield by source, g/d | ||||||||
| De novo | 258 | 251 | 254 | 231 | 20 | 0.50 | 0.36 | 0.64 |
| Mixed | 301 | 341 | 306 | 307 | 28 | 0.57 | 0.42 | 0.44 |
| Preformed | 211 | 213 | 220 | 216 | 16 | 0.52 | 0.95 | 0.75 |
| OBCFA | 28.9 | 25.4 | 29.4 | 26.2 | 2.3 | 0.76 | 0.13 | 0.94 |
| Specific FA of interest, % of FA | ||||||||
| C16:0 | 35.5 | 39.4 | 36.0 | 37.5 | 1.1 | 0.54 | 0.02 | 0.26 |
| C18:0 | 7.21 | 7.96 | 7.94 | 8.82 | 0.37 | 0.02 | 0.02 | 0.83 |
| C18:1 trans-10 | 0.15 | 0.14 | 0.15 | 0.15 | 0.01 | 0.23 | 0.49 | 0.28 |
| C18:1 trans-11 | 0.37 | 0.46 | 0.44 | 0.54 | 0.03 | 0.002 | <0.001 | 0.80 |
| Total trans C18:1 | 0.99 | 1.10 | 1.10 | 1.20 | 0.07 | 0.04 | 0.03 | 0.98 |
| Variable | Treatments 1 | p-Value 2,3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HF | LF | SE | |||||||||
| CON | ACE | CON | ACE | F | I | T | F × I 4 | F × T | I × T | ||
| Glucose, mg/dL | 52.9 | 52.2 | 51.7 | 52.0 | 1.3 | 0.53 | 0.83 | 0.001 | 0.63 | 0.74 | 0.41 |
| AM | 55.5 | 53.4 | 53.4 | 53.5 | 1.7 | ||||||
| PM | 50.2 | 50.9 | 50.1 | 50.5 | 1.5 | ||||||
| NEFA, μEq/L | 82.5 | 101.3 | 81.3 | 123.9 | 12 | 0.37 | 0.02 | <0.001 | 0.32 | 0.29 | 0.13 |
| AM | 98.9 | 119.5 | 94.2 | 169.0 | 21 | ||||||
| PM | 66.1 | 83.2 | 68.5 | 78.8 | 9.5 | ||||||
| BHB 4, μM | 0.65 b | 1.08 a | 0.62 b | 0.77 b | 0.1 | 0.05 | 0.002 | <0.001 | 0.08 | 0.08 | 0.12 |
| AM | 0.42 | 0.70 | 0.47 | 0.56 | 0.09 | ||||||
| PM | 0.87 | 1.46 | 0.77 | 0.98 | 0.14 | ||||||
| Rumen pH | 6.31 | 6.63 | 6.51 | 6.77 | 0.07 | 0.01 | <0.001 | <0.001 | 0.65 | 0.66 | 0.26 |
| AM | 6.77 | 6.9 | 6.83 | 7.1 | 0.12 | ||||||
| PM | 5.86 | 6.36 | 6.19 | 6.44 | 0.08 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urrutia, N.L.; Muñoz, C.; Ungerfeld, E.M.; Cisterna, C.; Harvatine, K.J. Interaction Between Ruminal Acetate Infusion and Diet Fermentability on Milk Fat Production in Dairy Cows. Animals 2025, 15, 1931. https://doi.org/10.3390/ani15131931
Urrutia NL, Muñoz C, Ungerfeld EM, Cisterna C, Harvatine KJ. Interaction Between Ruminal Acetate Infusion and Diet Fermentability on Milk Fat Production in Dairy Cows. Animals. 2025; 15(13):1931. https://doi.org/10.3390/ani15131931
Chicago/Turabian StyleUrrutia, Natalie L., Camila Muñoz, Emilio M. Ungerfeld, Claudia Cisterna, and Kevin J. Harvatine. 2025. "Interaction Between Ruminal Acetate Infusion and Diet Fermentability on Milk Fat Production in Dairy Cows" Animals 15, no. 13: 1931. https://doi.org/10.3390/ani15131931
APA StyleUrrutia, N. L., Muñoz, C., Ungerfeld, E. M., Cisterna, C., & Harvatine, K. J. (2025). Interaction Between Ruminal Acetate Infusion and Diet Fermentability on Milk Fat Production in Dairy Cows. Animals, 15(13), 1931. https://doi.org/10.3390/ani15131931






