Markers of Gut Health in Small Animals: Focus on Fatty Acids and Amino Acids as Indicators of Intestinal Functionality and Microbiome Activity
Simple Summary
Abstract
1. Introduction
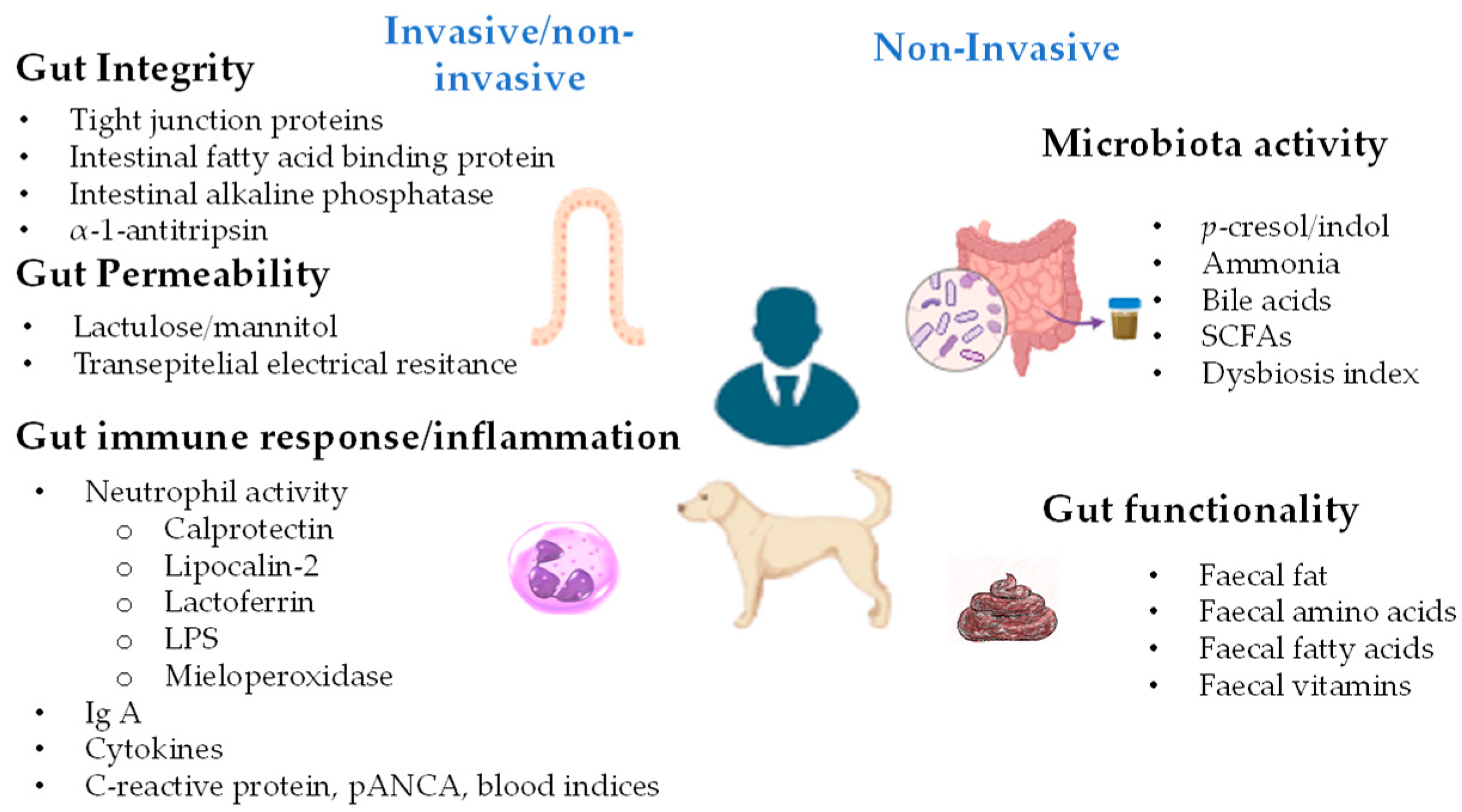
2. Markers for Gut Health Evaluation
2.1. Intestinal Barrier Integrity Indicators
2.2. Indicators of Gut Immune Response
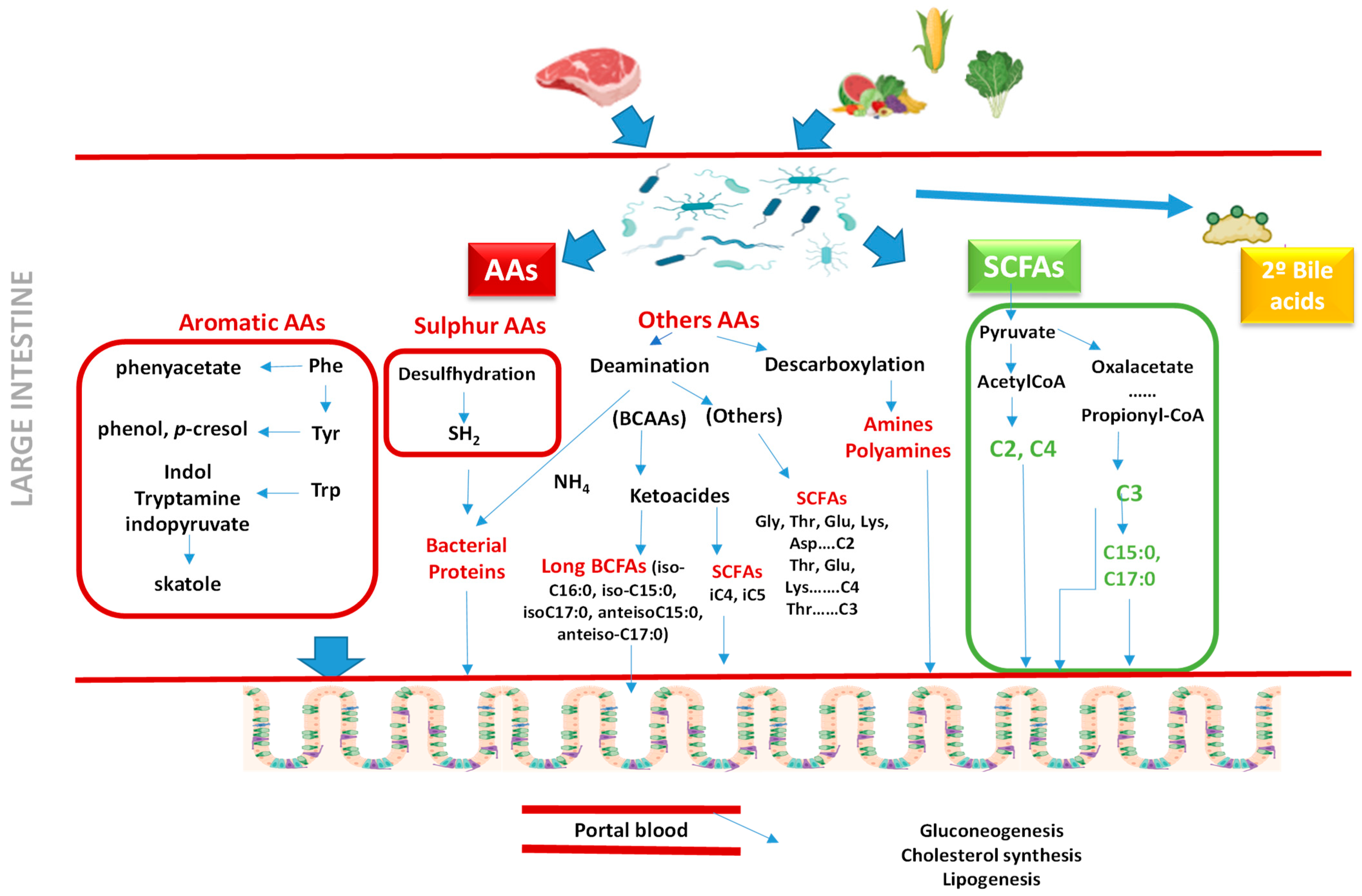
2.3. Indicators of Gut Microbiota Activity
2.4. Indicators of Gut Functionality
2.4.1. Fatty Acids as Markers of Gut Health
| Disease | Species | Sample | Findings | Reference |
|---|---|---|---|---|
| Chronic enteropathy (n = 15) Control (n = 15) | Dogs | Feces | CIEs: SFA: ↑ C16:0, ↑ C18:0, ↑ MUFA n − 9, PUFA: ↑ C22:3n − 3, ↑ AA, ↑ C22:2n − 6 | [125] |
| Food-responsive enteropathy (n = 9) Control (n = 6) | Dogs | Feces | CIEs: ↓ C15:0, ↓ C16:1n − 9, ↓ C16:1n − 7, ↓ C20:5n − 3, ↓ MUFA, ↓ C16:1/C16:0, ↑ C18:0, ↑ elongase C16/C18 | [93] |
| Inflammatory Intestinal Disease (n= 11) T-phenotype small cell lymphoma (n= 11) Control (n= 14) | Cats | Feces | IBD, lymphoma: ↑ long-chain fatty acids: PUFA, AA, DHA, ↑ MUFA n – 9. No differences between control vs. IBD. | [130] |
| Chronic enteropathy (active) (n = 14) Chronic enteropathy (n = 11) Control (n = 26) | Dogs (Yorkshire terrier) | Feces | CIE active: ↑ long fatty acids (↑ MUFA n − 9, ↑ LA, ↑ ALA). Reduction in them after treatment. | [79] |
| Chronic enteropathy (n = 56): Inflammatory Intestinal Disease (n= 22) T-phenotype small cell lymphoma (n = 34) Control (n = 77) | Cats | Feces | CIEs: SFA: ↑ C14:0, ↑ C18:0; MUFA: ↑ C18:1n − 9, ↑ C22:1n − 9, ↑ C20:1n − 9, ↑ C24:1n − 9; PUFA: ↑ ALA, AA | [127] |
| Protein-losing enteropathy (PLE) (n= 38) Control (n= 47) | Dogs | Feces | PLE: ↑ SFA (C16:0, C18:0); ↑ MUFA n − 9 (oleico); PUFA: ↑ LA, ↑ AA; ↑ total fatty acids. After treatment: ↓ MUFA n − 9 y ↓C18:0 | [78] |
| Food-responsive enteropathy (n = 35) Immunosupressant-responsive enteropathy (n = 18) Control (n = 22) | Dogs | Feces | IRE vs. control: ↓ C16:1n − 7, ↓ C18:1n − 9, ↓ C18:2n − 6, ↓ C18:3n − 3, ↓ MUFA, ↓ PUFA, ↓ n − 6, ↓ C16:1n − 7/C16:0, ↓ C18:1n − 9/C18:0, ↑ elongase C18/C16, ↑ SFA:C14:0, C18:0; IRE vs. FRE: ↓ C16:1n − 7, ↓ PUFA, ↓ C16:1n − 7/C16:0, ↑ C14:0, ↑ C18:3n − 3, ↑ C22:5n − 3, ↑ SFA, ↑ n − 3, ↑ elongase C18/C16 | [94] |
| Chronic enteropathy (n = 34): Food-responsive enteropathy (n= 13) Inflammatory Intestinal Disease (n= 15) T-phenotype small cell lymphoma (n = 6) Control (n = 27) | Cats | Feces | CIEs: SFA: ↑ C14:0, ↑ C18:0, ↑ C24:1n − 9 y ↑ C22:1n − 9 (MUFA n − 9), ↑ AA (PUFA); Significant changes in control vs. IBD + lymphoma: C14:0, C18:0, C24:1n − 9, AA. The FRE metabolome was more similar to controls than to IBD or lymphoma | [128] |
2.4.2. Amino Acids as Markers of Gut Health
| Disease | Species | Sample | Findings | Reference |
|---|---|---|---|---|
| Inflammatory Intestinal Disease (n = 12) Control (n = 10) | Dogs | Serum | IBD: alteration in metabolism of amino acids (↓ histidine, ↓ glutamine, ↓ tyrosine, ↓ tryptophan, ↓ cysteine, ↓ proline, ↓ hydroxyproline) | [146] |
| Inflammatory Intestinal Disease (n = 15) Control (n = 10) | Dogs | Plasma | IBD: ↑ valine alanine correlated with CCECAI | [25] |
| Protein-losing enteropathy (n = 30) Control (n = 12) | Dogs | Serum | PLE: ↓ tryptophan correlation tryptophan and albumin | [148] |
| Inflammatory Intestinal Disease (n = 10) Control (n = 12) | Dogs | Plasma | IBD: ↓ tryptophan, ↓ serine, ↓ metionine, ↓ proline. Negative correlation between serine-CCECAI | [147] |
| Inflammatory Intestinal Disease (n = 51) Control (n = 26) | Dogs | Serum | IBD: ↓ tyrosine, ↓ phenylalanine, ↓ tryptophan (AAA); ↑ serine, ↑ glutamic acid, ↑ arginine, ↑ threonine, ↑ proline, ↑ cystine, ↑ lysine, ↑ valine, ↑ isoleucine | [155] |
| Chronic Enteropathy (n = 55) Control (n = 204) | Dogs | Serum | CIEs:↑ phenylalanine, ↓ glycine | [107] |
| Food-responsive enteropathy (n = 9) Control (n = 6) | Dogs | Plasma | Plasma FRE: ↓ histidine, ↓ asparagine, ↓ glycine, ↓ cystine, ↓ leucine, ↓ BCAA/AAA; ↑ phenylalanine | [149] |
| Chronic enteropathy (n = 8) Control (n = 16) | Cats | Plasma, urine | CIEs: Plasma: ↑ alanine, ↑ glutamine, ↑ valine, ↑ isoleucine, ↑ phenylalanine. Plasmatic metabolites (alanine, glutamine, betaine, glycerol) and urine as predictor of the response to diet. | [163] |
| Inflammatory Intestinal Disease (n = 13) T-phenotype small cell lymphoma (n = 13) Control (n = 14) | Cats | Serum | IBD, lymphoma: ↑ alanine, ↑ histidine, ↑ methionine, ↑ lysine, ↑ valine ↓ Metabolites derived from tryptophan; IBD vs. lymphoma: changes in tyrosine and other compounds | [164] |
2.4.3. Other Indicators of Gut Functionality
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIEs | Chronic Inflammatory Enteropathies |
| IBD | Inflammatory Bowel Disease |
| FRE | Food-Responsive Enteropathy |
| IRE | Immunosuppressant-Responsive Enteropathy |
| PLE | Protein-Losing Enteropathy |
| SCFAs | Short-Chain Fatty Acids |
| SAT | Saturated Fatty Acids |
| MUFA | Monounsaturated Fatty Acids |
| PUFA | Polyunsaturated Fatty Acids |
| LA | Linoleic Acid (C18:2n − 6) |
| ALA | Alpha-Linolenic Acid (C18:3n − 3) |
| EPA | Eicosapentaenoic Acid (C20:5n − 3) |
| DHA | Docosahexaenoic Acid (C22:6n − 3) |
| AA | Arachidonic Acid (C20:4n − 6) |
| C2 | Acetic Acid |
| C3 | Propionic Acid |
| C4 | Butyric Acid |
| iC4 | Isobutyric Acid |
| iC5 | Isovaleric Acid |
| CCECAI | Canine Chronic Enteropathy Clinical Activity Index |
| CIBDAI | Canine Inflammatory Bowel Disease Activity Index |
| FABP | Fatty Acid Binding Protein |
| I-FABP | Intestinal Fatty Acid Binding Protein |
| IAP | Intestinal Alkaline Phosphatase |
| L/M | Lactulose/Mannitol Ratio |
| LPS | Lipopolysaccharide |
| MPO | Myeloperoxidase |
| pANCA | Perinuclear Anti-Neutrophilic Cytoplasmic Antibodies |
| CRP | C-Reactive Protein |
| IgA | Immunoglobulin A |
| DI | Dysbiosis Index |
| GPCR | G Protein-Coupled Receptors |
| BCAAs | Branched-Chain Amino Acids |
| AAAs | Aromatic Amino Acids |
| PC | Phosphatidylcholine |
| SII | Systemic Immune-Inflammation Index |
| PLR | Platelet-to-Lymphocyte Ratio |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| OCFA | Odd-Chain Fatty Acids |
References
- Bischoff, S.C. “Gut Health”: A New Objective in Medicine. BMC Med. 2011, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the Role of Diet in Maintaining Gut Health to Reduce the Risk of Obesity, Cardiovascular and Other Age-Related Inflammatory Diseases: Recent Challenges and Future Recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, Y.M.; Borcherding, D.; Kanthasamy, A.; Kim, H.J.; Willette, A.A.; Jergens, A.; Allenspach, K.; Mochel, J.P. The Gut-Brain Axis in Neurodegenerative Diseases and Relevance of the Canine Model: A Review. Front. Aging Neurosci. 2019, 11, 130. [Google Scholar] [CrossRef]
- Summers, S.; Quimby, J. Insights into the Gut-Kidney Axis and Implications for Chronic Kidney Disease Management in Cats and Dogs. Vet. J. 2024, 306, 106181. [Google Scholar] [CrossRef] [PubMed]
- Habermaass, V.; Olivero, D.; Gori, E.; Mariti, C.; Longhi, E.; Marchetti, V. Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut–Liver Axis? Animals 2023, 13, 3174. [Google Scholar] [CrossRef]
- Dandrieux, J.R.S. Inflammatory Bowel Disease versus Chronic Enteropathy in Dogs: Are They One and the Same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef]
- Allenspach, K.; Mochel, J.P. Current Diagnostics for Chronic Enteropathies in Dogs. Vet. Clin. Pathol. 2022, 50, 18–28. [Google Scholar] [CrossRef]
- Jergens, A.E.; Heilmann, R.M. Canine chronic enteropathy—Current state-of-the-art and emerging concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.M.; Verlhac, V. Gastrointestinal Functionality in Animal Nutrition and Health: New Opportunities for Sustainable Animal Production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky Gut: Mechanisms, Measurement, and Clinical Implications in Humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Galipeau, H.J.; Verdu, E.F. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol. Motil. 2016, 28, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010, 28, 623–667. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, P.B.; Peeters, R.A.; Groot, J.A.; Dekker, P.R.; Taminiau, J.A.J.M.; van der Meer, R. Differential In Vivo and In Vitro Intestinal Permeability to Lactulose and Mannitol in Animals and Humans: A Hypothesis. Gastroenterology 1995, 108, 687–696. [Google Scholar] [CrossRef]
- Randell, S.C.; Hill, R.C.; Scott, K.C.; Omori, M.; Burrows, C.F. Intestinal Permeability Testing Using Lactulose and Rhamnose: A Comparison Between Clinically Normal Cats and Dogs and Between Dogs of Different Breeds. Res. Vet. Sci. 2001, 71, 45–49. [Google Scholar] [CrossRef]
- Gan, J.; Nazarian, S.; Teare, J.; Darzi, A.; Ashrafian, H.; Thompson, A.J. A case for improved assessment of gut permeability: A meta-analysis quantifying the lactulose: Mannitol ratio in coeliac and Crohn’s disease. BMC Gastroenterol. 2022, 22, 16. [Google Scholar] [CrossRef]
- VanWijck, K.; Verlinden, T.J.M.; van Eijk, H.M.H.; Dekker, J.; Buurman, W.A.; Dejong, C.H.C.; Lenaerts, K. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: A randomized controlled crossover trial. Clin. Nutr. 2013, 32, 245–251. [Google Scholar] [CrossRef]
- Sequeira, I.R.; Lentle, R.G.; Kruger, M.C.; Hurst, R.D. Standardising the Lactulose Mannitol Test of Gut Permeability to Minimise Error and Promote Comparability. PLoS ONE 2014, 9, e99256. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a Newly Discovered Modulator of Intestinal Permeability, and Its Expression in Coeliac Disease. Lancet 2000, 355, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Ural, K.; Erdoğan, H.; Erdoğan, S.; Camkerten, I.; Ahín, N. Circulating Serum Zonulin Levels Before and After Probiotic Enema Treatment in Dogs with Atopic Dermatitis: Randomized Clinical Study. Turk. Klin. J. Vet. Sci. 2021, 12, 70–78. [Google Scholar] [CrossRef]
- Cui, Y.; Li, D.; Zhang, M.; Liu, P.; Wang, H.; Li, Y.; Wu, Y. The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers. Animals 2024, 14, 1713. [Google Scholar] [CrossRef]
- Dinesh, N.; Slovak, J.E.; Kogan, C.; Kopper, J.J. Preliminary Evaluation of Serum Zonulin in Canine Chronic Enteropathies. J. Small Anim. Pract. 2022, 63, 679–685. [Google Scholar] [CrossRef]
- Rossi, G.; Cerquetella, M.; Berardi, S.; Galosi, L.; Mari, S.; Pengo, G.; Gavazza, A. Evaluation of Some Potential New Serological and Faecal Markers in Canine Lymphangiectasia: Correlation with Mucosal Morphology and Histological Score. J. Comp. Pathol. 2020, 174, 173. [Google Scholar] [CrossRef]
- Fragkos, K.C.; Forbes, A. Citrulline as a Marker of Intestinal Function and Absorption in Clinical Settings: A Systematic Review and Meta-Analysis. United Eur. Gastroenterol. J. 2017, 6, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, S.; Pipp, F.C.; Emde, B.; Weigt, S.; Vigna, E.; Hanschke, B.; Kasper, L.; Siddharta, A.; Hellmann, J.; Czasch, S.; et al. L-citrulline: A preclinical safety biomarker for the small intestine in rats and dogs in repeat dose toxicity studies. J. Pharmacol. Tox Met. 2021, 111, 107110. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Verbrugghe, A.; Lourenço, M.; Janssens, G.P.J.; Liu, D.J.X.; Van de Wiele, T.; Eeckhaut, V.; Van Immerseel, F.; Van de Maele, I.; Niu, Y.; et al. Does canine inflammatory bowel disease influence gut microbial profile and host metabolism? BMC Vet. Res. 2016, 12, 114. [Google Scholar] [CrossRef]
- Gerou-Ferriani, M.; Allen, R.; Noble, P.J.M.; German, A.J.; Caldin, M.; Batchelor, D.J. Determining optimal therapy of dogs with chronic enteropathy by measurement of serum citrulline. J. Vet. Intern. Med. 2018, 32, 993–998. [Google Scholar] [CrossRef]
- Niewold, T.A.; Meinen, M.; van der Meulen, J. Plasma Intestinal Fatty Acid Binding Protein (I-FABP) Concentrations Increase Following Intestinal Ischemia in Pigs. Res. Vet. Sci. 2004, 77, 89–91. [Google Scholar] [CrossRef]
- Schurink, M.; Kooi, E.M.W.; Hulzebos, C.V.; Kox, R.G.; Groen, H.; Heineman, E.; Bos, A.F.; Hulscher, J.B.F. Intestinal Fatty Acid-Binding Protein as a Diagnostic Marker for Complicated and Uncomplicated Necrotizing Enterocolitis: A Prospective Cohort Study. PLoS ONE 2015, 10, e0121336. [Google Scholar] [CrossRef]
- Ehlers, M.R. Immune-Modulating Effects of Alpha-1 Antitrypsin. Biol. Chem. 2014, 395, 1187–1193. [Google Scholar] [CrossRef]
- Karbach, U.; Ewe, K.; Bodenstein, H. Alpha 1-antitrypsin, a reliable endogenous marker for intestinal protein loss and its application in patients with Crohn’s disease. Gut 1983, 24, 718–723. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Parnell, N.K.; Grützner, N.; Mansell, J.; Berghoff, N.; Schellenberg, S.; Reusch, C.E.; Suchodolski, J.S.; Steiner, J.M. Serum and fecal canine alpha1-proteinase inhibitor concentrations reflect the severity of intestinal crypt abscesses and/or lacteal dilation in dogs. Vet. J. 2016, 207, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Paddock, C.G.; Ruhnke, I.; Berghoff, N.; Suchodolski, J.S.; Steiner, J.M. Development and analytical validation of a radioimmunoassay for the measurement of α1-proteinase inhibitor concentrations in feces from healthy puppies and adult dogs. J. Vet. Diagn. Investig. 2011, 23, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Vaden, S.L.; Vidaurri, A.; Levine, J.F.; Harris, T.; Steiner, J.M.; Williams, D.A. Fecal α1-proteinase inhibitor activity in Soft Coated Wheaten Terriers. J. Vet. Int. Med. 2002, 16, 382. [Google Scholar]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay Between Intestinal Alkaline Phosphatase, Diet, Gut Microbes, and Immunity. World J. Gastroenterol. 2014, 20, 15650–15656. [Google Scholar] [CrossRef]
- Malo, M.S.; Alam, S.N.; Mostafa, G.; Zeller, S.J.; Johnson, P.V.; Mohammad, N.; Chen, K.T.; Moss, A.K.; Rmasamy, S.; Faruqui, A.; et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 2010, 59, 1476–1484. [Google Scholar] [CrossRef]
- Ide, K.; Kato, K.; Sawa, Y.; Hayashi, A.; Takizawa, R.; Nishifuji, K. Comparison of the expression, activity, and fecal concentration of intestinal alkaline phosphatase between healthy dogs and dogs with chronic enteropathy. Am. J. Vet. Res. 2016, 77, 721–729. [Google Scholar] [CrossRef]
- Zollner, A.; Schmiderer, A.; Reider, S.J.; Oberhuber, G.; Pfister, A.; Texler, B.; Watschinger, C.; Koch, R.; Enberger, M.; Raine, T.; et al. Fecal biomarkers in inflammatory bowel diseases: Calprotectin versus lipocalin-2—A comparative study. J. Crohns Colitis 2021, 15, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Grellet, A.; Heilmann, R.M.; Lecoindre, P.; Feugier, A.; Day, M.J.; Peeters, D.; Freiche, V.; Hernandez, J.; Grandjean, D.; Suchodolski, J.D.; et al. Fecal calprotectin concentrations in adult dogs with chronic diarrhea. Am. J. Vet. Res. 2013, 74, 706–711. [Google Scholar] [CrossRef]
- Nestler, J.; Syrjä, P.; Kilpinen, S.; Moniz, C.A.; Spillmann, T.; Hanifeh, M.; Heilmann, R.M. Duodenal and Colonic Mucosal S100A8/A9 (Calprotectin) Expression Is Increased and Correlates with the Severity of Select Histologic Lesions in Dogs with Chronic Inflammatory Enteropathy. BMC Vet. Res. 2024, 20, 393. [Google Scholar] [CrossRef]
- El-Zahar, H.; Abd El-Rahman, Z.; El-Naggar, A. Fecal Calprotectin Concentrations and Other Indicators in Dogs with Idiopathic Inflammatory Bowel Disease. J. Anim. Health Prod. 2022, 10, 88–96. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Berghoff, N.; Mansell, J.; Grützner, N.; Parnell, N.K.; Gurtner, C.; Suchodolski, J.S.; Steiner, J.M. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J. Vet. Intern. Med. 2018, 32, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Hanifeh, M.; Sankari, S.; Rajamäki, M.M.; Syrjä, P.; Kilpinen, S.; Suchodolski, J.S.; Heilmann, R.M.; Guadiano, P.; Lidbury, J.; Steiner, J.M. S100A12 concentrations and myeloperoxidase activities are increased in the intestinal mucosa of dogs with chronic enteropathies. BMC Vet. Res. 2018, 14, 125. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Grellet, A.; Allenspach, K.; Lecoindre, P.; Day, M.J.; Priestnall, S.L.; Toresson, L.; Procoli, F.; Grützner, N.; Suchodolski, J.S.; et al. Association between fecal S100A12 concentration and histologic, endoscopic, and clinical disease severity in dogs with idiopathic inflammatory bowel disease. Vet. Immunol. Immunopathol. 2014, 158, 156–166. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Nestler, J.; Schwarz, J.; Grützner, N.; Ambrus, A.; Seeger, J.; Suchodolski, J.S.; Steiner, J.M.; Gurtner, C. Mucosal expression of S100A12 (calgranulin C) and S100A8/A9 (calprotectin) and correlation with serum and fecal concentrations in dogs with chronic inflammatory enteropathy. Vet. Immunol. Immunopathol. 2019, 211, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef]
- Hellweg, P.; Krammer-Lukas, S.; Strasser, A.; Zentek, J. Effects of bovine lactoferrin on the immune system and the intestinal microflora of adult dogs. Arch. Anim. Nutr. 2008, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Srinivasan, G.; Delgado, M.A.; Young, A.N.; Gewirtz, A.T.; Vijay-Kumar, M. Fecal Lipocalin 2, a Sensitive and Broadly Dynamic Non-Invasive Biomarker for Intestinal Inflammation. PLoS ONE 2012, 7, e44328. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a context of inflammation-induced pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Maden, M.; Gülersoy, E. Serum/fecal S100A12, CRP, and lactoferrin can be used to distinguish infectious and non-infectious canine diarrhea. Vet. Med. Sci. 2023, 9, 2485–2496. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K.; Uchida, K.; Nakashima, K.; Fukushima, K.; Tsukamoto, A.; Nakajima, M.; Fujino, Y.; Tsujimoto, H. Decreased immunoglobulin A concentrations in feces, duodenum, and peripheral blood mononuclear cells of dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2013, 27, 47–55. [Google Scholar] [CrossRef]
- Cunningham-Rundles, C. Physiology of IgA and IgA Deficiency. J. Clin. Immunol. 2001, 21, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Panasevich, M.R.; Daristotle, L.; Quesnell, R.; Reinhart, G.A.; Frantz, N.Z. Altered Fecal Microbiota, IgA, and Fermentative End-Products in Adult Dogs Fed Prebiotics and Nonviable Lactobacillus acidophilus. J. Anim. Sci. 2021, 99, skab347. [Google Scholar] [CrossRef] [PubMed]
- Grellet, A.; Heilmann, R.M.; Polack, B.; Feugier, A.; Boucraut-Baralon, C.; Grandjean, D.; Grützner, N.; Suchodolski, J.S.; Steiner, J.M.; Chastant-Maillard, S. Influence of Breed Size, Age, Fecal Quality, and Enteropathogen Shedding on Fecal Calprotectin and Immunoglobulin A Concentrations in Puppies During the Weaning Period. J. Vet. Intern. Med. 2016, 30, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Kolodziejska-Sawerska, A.; Rychlik, A.; Depta, A.; Wdowiak, M.; Nowicki, M.; Kander, M. Cytokines in canine inflammatory bowel disease. Pol. J. Vet. Sci. 2013, 16, 165–171. [Google Scholar] [CrossRef]
- Kaga, C.; Kakiyama, S.; Hokkyo, A.; Ogata, Y.; Shibata, J.; Nagahara, T.; Nakazawa, M.; Nakagawa, T.; Tsujimoto, H.; Chambers, J.; et al. Faecal microbiota and serum inflammatory markers in dogs with chronic enteropathy diagnosed with inflammatory bowel disease and small-cell lymphoma. Res. Square 2023, preprint. [Google Scholar] [CrossRef]
- Jergens, A.E.; Sonea, I.M.; O’Connor, A.M.; Kauffman, L.K.; Grozdanic, S.D.; Ackermann, M.R.; Evans, R.B. Intestinal cytokine mRNA expression in canine inflammatory bowel disease: A meta-analysis with critical appraisal. Comp. Med. 2009, 59, 153–162, Erratum in: Comp. Med. 2009, 59, 220. [Google Scholar]
- Heilmann, R.M.; Steiner, J.M. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J. Vet. Intern. Med. 2018, 32, 1495–1508. [Google Scholar] [CrossRef]
- Nakamura, M.; Takahashi, M.; Ohno, K.; Koshino, A.; Nakashima, K.; Setoguchi, A.; Fujino, Y.; Tsujimoto, H. C-reactive protein concentration in dogs with various diseases. J. Vet. Med. Sci. 2008, 70, 127–131. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.S.; Lee, D.; Yun, T.; Koo, Y.; Chae, Y.; Kang, J.H.; Kang, B.T.; Yang, M.P.; Kim, H. Clinical signs, duodenal histopathological grades, and serum high-mobility group box 1 concentrations in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2021, 35, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Equilino, M.; Théodoloz, V.; Gorgas, D.; Doherr, M.G.; Heilmann, R.M.; Suchodolski, J.S.; Steiner, J.M.; Dvm, I.A.B. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein-losing enteropathy. J. Am. Vet. Med. Assoc. 2015, 246, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gianella, P.; Cagnasso, F.; Giordano, A.; Borrelli, A.; Bottero, E.; Bruno, B.; Ferriani, R.; Borella, F.; Meazzi, S.; Scavone, D.; et al. Comparative evaluation of lipid profile, C-reactive protein and paraoxonase-1 activity in dogs with inflammatory protein-losing enteropathy and healthy dogs. Animals 2024, 14, 3119. [Google Scholar] [CrossRef]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.; Evans, R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, N.; Steiner, J.M. Laboratory tests for the diagnosis and management of chronic canine and feline enteropathies. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 311–328. [Google Scholar] [CrossRef]
- Allenspach, K.; Lomas, B.; Wieland, B.; Harris, T.; Pressler, B.; Mancho, C.; Lees, G.E.; Vaden, S.L. Evaluation of perinuclear anti-neutrophilic cytoplasmic autoantibodies as an early marker of protein-losing enteropathy and protein-losing nephropathy in Soft Coated Wheaten Terriers. Am. J. Vet. Res. 2008, 69, 1301–1304. [Google Scholar] [CrossRef]
- Florey, J.; Viall, A.; Streu, S.; DiMuro, V.; Riddle, A.; Kirk, J.; Perazzotti, L.; Affeldt, K.; Wagner, R.; Vaden, S.; et al. Use of a granulocyte immunofluorescence assay designed for humans for detection of antineutrophil cytoplasmic antibodies in dogs with chronic enteropathies. J. Vet. Intern. Med. 2017, 31, 1062–1066. [Google Scholar] [CrossRef]
- Cristóbal, J.I.; Duque, F.J.; Usón-Casaús, J.; Barrera, R.; López, E.; Pérez-Merino, E.M. Complete blood count-derived inflammatory markers changes in dogs with chronic inflammatory enteropathy treated with adipose-derived mesenchymal stem cells. Animals 2022, 12, 2798. [Google Scholar] [CrossRef]
- Marchesi, M.C.; Maggi, G.; Cremonini, V.; Miglio, A.; Contiero, B.; Guglielmini, C.; Antognoni, M.T. Monocytes count, NLR, MLR and PLR in canine inflammatory bowel disease. Animals 2024, 14, 837. [Google Scholar] [CrossRef]
- Akpinar, M.Y.; Ozin, Y.O.; Kaplan, M.; Ates, I.; Kalkan, I.H.; Kilic, Z.M.Y.; Yuksel, M.; Kayacetin, E. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict mucosal disease severity in ulcerative colitis. J. Med. Biochem. 2018, 37, 155–162. [Google Scholar] [CrossRef]
- Xie, Y.; Zhuang, T.; Ping, Y.; Zhang, Y.; Wang, X.; Yu, P.; Duan, X. Elevated systemic immune inflammation index level is associated with disease activity in ulcerative colitis patients. Clin. Chim. Acta 2021, 517, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.; Suchodolski, J.S.; Steiner, J.M.; Heilmann, R.M. Blood neutrophil-to-lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy. J. Vet. Diagn. Investig. 2021, 33, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, E.; Pierini, A.; Gori, E.; Lucarelli, C.; Lubas, G.; Marchetti, V. Neutrophil-to-lymphocyte ratio (NLR) in canine inflammatory bowel disease (IBD). Vet. Sci. 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Guard, B.C.; Suchodolski, J.S. Canine intestinal microbiology and metagenomics: From phylogeny to function. J. Anim. Sci. 2016, 94, 2247–2261. [Google Scholar] [CrossRef]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal Short-Chain Fatty Acid Concentrations and Dysbiosis in Dogs with Chronic Enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 2016, 1, 16177. [Google Scholar] [CrossRef]
- Cagnasso, F.; Suchodolski, J.S.; Borrelli, A.; Borella, F.; Bottero, E.; Benvenuti, E.; Ferriani, R.; Tolbert, M.K.; Chen, C.C.; Giaretta, P.R.; et al. Dysbiosis index and fecal concentrations of sterols, long-chain fatty acids and unconjugated bile acids in dogs with inflammatory protein-losing enteropathy. Front. Microbiol. 2024, 15, 1433175. [Google Scholar] [CrossRef]
- Galler, A.; Suchodolski, J.S.; Steiner, J.M.; Sung, C.-H.; Hittmair, K.; Richter, B.; Burgener, I. Microbial dysbiosis and fecal metabolomic perturbations in Yorkshire Terriers with chronic enteropathy. Sci. Rep. 2022, 12, 12977. [Google Scholar] [CrossRef]
- Giaretta, P.R.; Rech, R.R.; Guard, B.C.; Blake, A.B.; Blick, A.K.; Steiner, J.M.; Lidbury, J.A.; Cook, A.K.; Hanifeh, M.; Spillmann, T.; et al. Comparison of intestinal expression of the apical sodium-dependent bile acid transporter between dogs with and without chronic inflammatory enteropathy. J. Vet. Int. Med. 2018, 32, 1918–1926. [Google Scholar] [CrossRef]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Int. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Sitkin, S.I.; Tkachenko, E.I.; Vakhitov, T.Y. Metabolic Dysbiosis of the Gut Microbiota and Its Biomarkers. Eksperimental’naia i Klinicheskaia Gastroenterologiia. Exp. Clin. Gastroenterol. 2016, 12, 6–29. [Google Scholar]
- Zhang, X.; Choi, F.F.K.; Zhou, Y.; Leung, F.P.; Tan, S.; Lin, S.; Xu, H.; Jia, W.; Sung, J.J.; Cai, Z.; et al. Metabolite profiling of plasma and urine from rats with TNBS-induced acute colitis using UPLC-ESI-QTOF-MS-based metabonomics—A pilot study. FEBS J. 2012, 279, 2322–2338. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, L.; Hao, F.; Tang, H.; Wang, Y. Systemic Responses of Mice to Dextran Sulfate Sodium-Induced Acute Ulcerative Colitis Using 1H NMR Spectroscopy. J. Proteome Res. 2013, 12, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered Microbiota, Fecal Lactate, and Fecal Bile Acids in Dogs with Gastrointestinal Disease. PLoS ONE 2019, 14, e0224454. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, C.; Ellis, E.; Abrahamsson, A.; Ericzon, B.G.; Bjorkhem, I.; Axelson, M. Bile Acid Formation in Primary Human Hepatocytes. World J. Gastroenterol. 2000, 6, 522–525. [Google Scholar]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut Microbiota-Derived Bile Acids in Intestinal Immunity, Inflammation, and Tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Honneffer, J.; Guard, B.; Steiner, J.M.; Suchodolski, J.S. Untargeted metabolomics reveals disruption within bile acid, cholesterol, and tryptophan metabolic pathways in dogs with idiopathic inflammatory bowel disease. Gastroenterology 2015, 148, S-715. [Google Scholar] [CrossRef]
- McFarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Zarling, E.J.; Ruchim, M.A. Protein origin of the volatile fatty acids isobutyrate and isovalerate in human stool. J. Lab. Clin. Med. 1987, 109, 566–570. [Google Scholar]
- Langfeld, L.Q.; Stefan, K.D.; Berswill, S.; Heimesaat, M.M. A review of the antimicrobial and immune-modulatory properties of the gut microbiota-derived short-chain fatty acid propionate—What is new? Eur. J. Microbiol. Immunol. 2021, 11, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Higueras, C.; Rey, A.I.; Escudero, R.; Díaz-Regañón, D.; Rodríguez-Franco, F.; García-Sancho, M.; Agulla, B.; Sainz, A. Short-Chain and Total Fatty Acid Profile of Faeces or Plasma as Predictors of Food-Responsive Enteropathy in Dogs: A Preliminary Study. Animals 2022, 12, 89. [Google Scholar] [CrossRef]
- Higueras, C.; Sainz, Á.; García-Sancho, M.; Rodríguez-Franco, F.; Rey, A.I. Faecal Short-Chain, Long-Chain, and Branched-Chain Fatty Acids as Markers of Different Chronic Inflammatory Enteropathies in Dogs. Animals 2024, 14, 1825. [Google Scholar] [CrossRef]
- Miller, J.; Żebrowska-Różańska, P.; Czajkowska, A.; Szponar, B.; Kumala-Ćwikła, A.; Chmielarz, M.; Łaczmański, Ł. Faecal Microbiota and Fatty Acids in Feline Chronic Enteropathy. BMC Vet. Res. 2023, 19, 281. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. Responses of Feeding Prebiotics on Nutrient Digestibility, Faecal Microbiota Composition, and Short-Chain Fatty Acid Concentrations in Dogs: A Meta-Analysis. Animal 2011, 5, 1743–1750. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Holmes, E.; Khan, F.; Kochhar, S.; Scanlan, P.; Shanahan, F.; Wilson, I.D.; Wang, Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 2007, 6, 546–551. [Google Scholar] [CrossRef]
- Stephens, N.S.; Siffledeen, J.; Su, X.; Murdoch, T.B.; Fedorak, R.N.; Slupsky, C.M. Urinary NMR Metabolomic Profiles Discriminate Inflammatory Bowel Disease from Healthy. J. Crohn’s Colitis 2013, 7, e42–e48. [Google Scholar] [CrossRef]
- Bjerrum, J.T.; Wang, Y.; Hao, F.; Coskun, M.; Ludwig, C.; Günther, U.; Nielsen, O.H. Metabonomics of Human Fecal Extracts Characterize Ulcerative Colitis, Crohn’s Disease, and Healthy Individuals. Metabolomics 2015, 11, 122–133. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhang, X.; Xiao, F.; Hu, H.; Li, X.; Dong, F.; Sun, M.; Xiao, Y.; Ge, T.; et al. Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn’s disease. Gut Microbes 2021, 13, 1. [Google Scholar] [CrossRef]
- Kiasat, A.; Rautiainen, S.; Prast-Nielsen, S.; Engstrand, L.; Schuppe-Koistinen, I.; Gustafsson, U.O.; Granström, A.L. Evaluation of plasma short-chain fatty acid levels as markers for inflammatory bowel disease. Scand. J. Gastroenterol. 2023, 58, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, L.V.; Morozova, M.V. Fat of the Gut: Epithelial Phospholipids in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 11682. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.; Catesson, A.; Griffin, J.L.; Holmes, E.; Williams, H.R.T. Metabolomic Analysis in Inflammatory Bowel Disease: A Systematic Review. J. Crohns Colitis 2020, 15, 813–826. [Google Scholar] [CrossRef]
- Kalenyak, K.; Heilmann, R.M.; van de Lest, C.H.A.; Brouwers, J.F.; Burgener, I.A. Comparison of the systemic phospholipid profile in dogs diagnosed with idiopathic inflammatory bowel disease or food-responsive diarrhea before and after treatment. PLoS ONE 2019, 14, e0215435. [Google Scholar] [CrossRef]
- Crisi, P.E.; Luciani, A.; Di Tommaso, M.; Prasinou, P.; De Santis, F.; Chatgilialoglu, C.; Pietra, M.; Procoli, F.; Sansone, A.; Giordano, M.V.; et al. The Fatty Acid-Based Erythrocyte Membrane Lipidome in Dogs with Chronic Enteropathy. Animals 2021, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.K.; Boag, A.M.; Ottka, C.; Lohi, H.; Handel, I.; Gow, A.G.; Mellanby, R.J. Serum metabolomic profiles in dogs with chronic enteropathy. J. Vet. Int. Med. 2022, 36, 1752–1759. [Google Scholar] [CrossRef]
- Crisi, P.E.; Giordano, M.V.; Luciani, A.; Gramenzi, A.; Prasinou, P.; Sansone, A.; Rinaldi, V.; Ferreri, C.; Boari, A. Evaluation of the Fatty Acid-Based Erythrocyte Membrane Lipidome in Cats with Food Responsive Enteropathy, Inflammatory Bowel Disease, and Low-Grade Intestinal T-Cell Lymphoma. PLoS ONE 2024, 19, e0307757. [Google Scholar] [CrossRef]
- Esteve-Comas, M.; Ramirez, M.; Fernandez-Banares, F.; Abad-Lacruz, A.; Gil, A.; Cabre, E.; Gonzalez-Huix, F.; Moreno, J.; Humbert, P.; Guilera, M. Plasma Polyunsaturated Fatty Acid Pattern in Active Inflammatory Bowel Disease. Gut 1992, 33, 1365–1369. [Google Scholar] [CrossRef]
- Figler, M.; Gasztonyi, B.; Cseh, J.; Horváth, G.; Kisbenedek, A.G.; Bokor, S.; Decsi, T. Association of N-3 and N-6 Long-Chain Polyunsaturated Fatty Acids in Plasma Lipid Classes with Inflammatory Bowel Diseases. Br. J. Nutr. 2007, 97, 1154–1161. [Google Scholar] [CrossRef]
- Wiese, D.M.; Horst, S.N.; Brown, C.T.; Allaman, M.M.; Hodges, M.E.; Slaughter, J.C.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T.; et al. Serum Fatty Acids Are Correlated with Inflammatory Cytokines in Ulcerative Colitis. PLoS ONE 2016, 11, e0156387. [Google Scholar] [CrossRef]
- Schwarz, J.; Vecka, M.; Stožický, F.; Pomahačová, R.; Staňková, B.; Tvrzická, E.; Kreslová, M.; Zahálková, R.; Sýkora, J. The Assessment of Plasma Fatty Acid Profiles in Newly Diagnosed Treatment-Naïve Paediatric Crohn’s Disease. Physiol. Res. 2021, 70, 799–808. [Google Scholar] [CrossRef]
- Murgia, A.; Hinz, C.; Liggi, S.; Dénes, J.; Hall, Z.; West, J.; Santoru, M.L.; Piras, C.; Manis, C.; Usai, P.; et al. Italian Cohort of Patients Affected by Inflammatory Bowel Disease Is Characterised by Variation in Glycerophospholipid, Free Fatty Acids, and Amino Acid Levels. Metabolomics 2018, 14, 140. [Google Scholar] [CrossRef]
- Galler, A.I.; Klavins, K.; Burgener, I.A. A Preliminary Metabolomic Study of Yorkshire Terrier Enteropathy. Metabolites 2022, 12, 264. [Google Scholar] [CrossRef]
- Yilmaz, M.; Claiborn, K.C.; Hotamisligil, G.S. De novo lipogenesis products and endogenous lipokines. Diabetes 2016, 65, 1800–1807. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Sig. Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Park, W.J.; Kothapalli, K.S.D.; Lawrence, P.; Tyburczy, C.; Brenna, J.T. An Alternate Pathway to Long-Chain Polyunsaturates: The FADS2 Gene Product D8-Desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009, 50, 1195–1202. [Google Scholar] [CrossRef]
- Kuroki, F.; Iida, M.; Matsumoto, T.; Aoyagi, K.; Kanamoto, K.; Fujishima, M. Serum n-3 polyunsaturated fatty acids are depleted in Crohn’s disease. Dig. Dis. Sci. 1997, 42, 1137–1141. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, L.; Sun, R.; Yu, T.; Jiang, S.; Chen, H. Characterization of serum polyunsaturated fatty acid profile in patients with inflammatory bowel disease. Ther. Adv. Chronic Dis. 2023, 14, 20406223231156826. [Google Scholar] [CrossRef]
- Geerling, B.J.; Houwelingen, A.C.V.; Badart-Smook, A.; Stockbrügger, R.W.; Brummer, R.-J.M. Fat Intake and Fatty Acid Profile in Plasma Phospholipids and Adipose Tissue in Patients With Crohn’s Disease, Compared With Controls. Am. J. Gastroenterol. 1999, 94, 410–417. [Google Scholar] [CrossRef]
- Lai, Y.; Xue, J.; Liu, C.-W.; Gao, B.; Chi, L.; Tu, P.; Lu, K.; Ru, H. Serum metabolomics identifies altered bioenergetics, signaling cascades in parallel with exposome markers in Crohn’s disease. Molecules 2019, 24, 449. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, U.; Daniluk, J.; Kucharski, R.; Kowalczyk, T.; Pietrowska, K.; Samczuk, P.; Filimoniuk, A.; Kretowski, A.; Lebensztejn, D.; Ciborowski, M. Untargeted Metabolomics and Inflammatory Markers Profiling in Children with Crohn’s Disease and Ulcerative Colitis—A Preliminary Study. Inflamm. Bowel Dis. 2019, 25, 1120–1128. [Google Scholar] [CrossRef]
- James, M.J.; Gibson, R.A. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343s–348s. [Google Scholar] [CrossRef] [PubMed]
- Hengstermann, S.; Valentini, L.; Schaper, L.; Buning, C.; Koernicke, T.; Maritschnegg, M.; Buhner, S.; Tillinger, W.; Regano, N.; Guglielmi, F.; et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin. Nutr. 2008, 27, 571–578. [Google Scholar] [CrossRef]
- Honneffer, J.B. Microbiota and Metabolomic Changes Across Various Canine Gastrointestinal Diseases. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2017; pp. 1–239. [Google Scholar]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.-H.; Pilla, R.; Marsilio, S.; Chow, B.; Zornow, K.A.; Slovak, J.E.; Lidbury, J.A.; Steiner, J.M.; Hill, S.L.; Suchodolski, J.S. Fecal Concentrations of Long-Chain Fatty Acids, Sterols, and Unconjugated Bile Acids in Cats with Chronic Enteropathy. Animals 2023, 13, 2753. [Google Scholar] [CrossRef]
- Giordano, M.V.; Crisi, P.E.; Gramenzi, A.; Cattaneo, D.; Corna, L.; Sung, C.-H.; Tolbert, K.M.; Steiner, J.M.; Suchodolski, J.S.; Boari, A. Fecal microbiota and concentrations of long-chain fatty acids, sterols, and unconjugated bile acids in cats with chronic enteropathy. Front. Vet. Sci. 2024, 11, 1401592. [Google Scholar] [CrossRef]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS ONE 2009, 4, e6386. [Google Scholar] [CrossRef]
- Marsilio, S.; Chow, B.; Hill, S.L.; Ackermann, M.R.; Estep, J.S.; Sarawichitr, B.; Pilla, R.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Untargeted metabolomic analysis in cats with naturally occurring inflammatory bowel disease and alimentary small cell lymphoma. Sci. Rep. 2021, 11, 9198. [Google Scholar] [CrossRef]
- Bueno-Hernández, N.; Dominguez-López, A. Quantification of Low Expressed SCD1 Gene in Colonic Mucosa from Patients with Active Ulcerative Colitis. Inflamm. Bowel Dis. 2011, 17, E155. [Google Scholar] [CrossRef]
- Chen, C.; Shah, Y.M. Metabolomics Reveals that Hepatic Stearoyl-CoA Desaturase 1 Downregulation Exacerbates Inflammation and Acute Colitis. Cell Metab. 2008, 7, 135–147. [Google Scholar] [CrossRef]
- Garg, M.L.; Keelan, M. Fatty acid desaturation in the intestinal mucosa. Biochim. Biophys. Acta 1988, 958, 139–141. [Google Scholar] [CrossRef]
- Weng, Y.J.; Gan, H.Y.; Li, X.; Huang, Y.; Li, Z.C.; Deng, H.M.; Chen, S.Z.; Zhou, Y.; Wang, L.S.; Han, Y.P.; et al. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J. Dig. Dis. 2019, 20, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.A.; Pereira, L. Roles of Palmitoleic Acid and Its Positional Isomers, Hypogeic and Sapienic Acids, in Inflammation, Metabolic Diseases, and Cancer. Cells 2022, 11, 2146. [Google Scholar] [CrossRef]
- Chen, Y.; Mai, Q. Dietary Palmitoleic Acid Reprograms Gut Microbiota and Improves Biological Therapy against Colitis. Gut Microbes 2023, 15, 2211501. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Odahara, S.; Nakamura, M.; Koido, S.; Katahira, K.; Shiraishi, H.; Ohkusa, T.; Fujise, K.; Tajiri, H. The Fatty Acid Profile of the Erythrocyte Membrane in Initial-Onset Inflammatory Bowel Disease Patients. Dig. Dis. Sci. 2013, 58, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Gerasimidis, K.; Blackburn, G.; Akinci, D.; Edwards, C.; Russell, R.; Watson, D. Untargeted Metabolomics of Extracts from Faecal Samples Demonstrates Distinct Differences between Paediatric Crohn’s Disease Patients and Healthy Controls but No Significant Changes Resulting from Exclusive Enteral Nutrition Treatment. Metabolites 2018, 8, 82. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2018, 4, 293–305. [Google Scholar] [CrossRef]
- Vich, A.V.; Hu, S.; Andreu-Sánchez, S.; Collij, V.; Jansen, B.H.; Augustijn, H.E.; Bolte, L.A.; Abu-Ali, R.G.; Giallourakis, C.; Schneider, J.; et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut 2023, 78, 1472–1485. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Hernández-Ledesma, B.; Chaparro, M.; Indiano-Romacho, P.; Bernardo, D.; Gisbert, J.P. Role of Food Proteins and Bioactive Peptides in Inflammatory Bowel Disease. Trends Food Sci. Technol. 2019, 88, 194–206. [Google Scholar] [CrossRef]
- Beaumont, M.; Blachier, F. Amino Acids in Intestinal Physiology and Health; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the Fecal Microbiota and Serum Metabolite Profiles in Dogs with Idiopathic Inflammatory Bowel Disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Ohta, H.; Kagawa, Y.; Osuga, T.; Morishita, K.; Sasaki, N.; Takiguchi, M. Plasma amino acid profiles in dogs with inflammatory bowel disease. J. Vet. Int. Med. 2019, 33, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Kathrani, A.; Allenspach, K.; Fascetti, A.J.; Larsen, J.A.; Hall, E.J. Alterations in serum amino acid concentrations in dogs with protein-losing enteropathy. J. Vet. Intern. Med. 2018, 32, 1026–1032. [Google Scholar] [CrossRef]
- Higueras, C.; Escudero, R.; Rebolé, A.; García-Sancho, M.; Rodríguez-Franco, F.; Sainz, Á.; Rey, A.I. Changes in faecal and plasma amino acid profile in dogs with food-responsive enteropathy as indicators of gut homeostasis disruption: A pilot study. Vet. Sci. 2023, 10, 112. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Okamoto, S.; Hashimoto, M.; Muramatsu, T.; Andou, A.; Uo, M.; Kitazume, M.T.; Matsuoka, K.; Yajima, T.; Inoue, N.; et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS ONE 2012, 7, e31131. [Google Scholar] [CrossRef]
- Williams, H.R.T.; Willsmore, J.D.; Cox, I.J.; Walker, D.G.; Cobbold, J.F.L.; Taylor-Robinson, S.D.; Orchard, T.R. Serum Metabolic Profiling in Inflammatory Bowel Disease. Dig. Dis. Sci. 2012, 57, 2157–2165. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Ono, N.; Imaizumi, A.; Mori, M.; Suzuki, H.; Uo, M.; Hashimoto, M.; Naganuma, M.; Matsuoka, K.; Mizuno, S.; et al. Decreased plasma histidine level predicts risk of relapse in patients with ulcerative colitis in remission. PLoS ONE 2015, 10, e0140716. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Brown, C.T.; Motley, A.K.; Horst, S.N.; Williams, C.S.; Koyama, T.; Zhao, Z.; Adams, D.W.; Beaulieu, D.B.; et al. Alterations in Lipid, Amino Acid, and Energy Metabolism Distinguish Crohn’s Disease from Ulcerative Colitis and Control Subjects by Serum Metabolomic Profiling. Metabolomics 2018, 14, 17. [Google Scholar] [CrossRef]
- Tews, H.C.; Schmelter, F.; Kandulski, A.; Büchler, C.; Schmid, S.; Schlosser, S.; Elger, T.; Loibl, J.; Sommersberger, S.; Fererberger, T.; et al. Unique Metabolomic and Lipidomic Profile in Serum From Patients With Crohn’s Disease and Ulcerative Colitis Compared With Healthy Control Individuals. Inflamm. Bowel Dis. 2023, 30, 2405–2417. [Google Scholar] [CrossRef]
- Benvenuti, E.; Pierini, A.; Gori, E.; Bartoli, F.; Erba, P.; Ruggiero, P.; Marchetti, V. Serum Amino Acid Profile in 51 Dogs with Immunosuppressant-Responsive Enteropathy (IRE): A Pilot Study on Clinical Aspects and Outcomes. BMC Vet. Res. 2020, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Schicho, R.; Shaykhutdinov, R.; Ngo, J.; Nazyrova, A.; Schneider, C.; Panaccione, R.; Kaplan, G.G.; Vogel, H.J.; Storr, M. Quantitative Metabolomic Profiling of Serum, Plasma, and Urine by 1H NMR Spectroscopy Discriminates Between Patients with Inflammatory Bowel Disease and Healthy Individuals. J. Proteome Res. 2012, 11, 3344–3357. [Google Scholar] [CrossRef]
- Dawiskiba, T.; Deja, S.; Mulak, A.; Zabek, A.; Jawien, E.; Pawelka, D.; Banasik, M.; Mastalerz-Migas, A.; Balcerzak, W.; Kaliszewski, K.; et al. Serum and Urine Metabolomic Fingerprinting in Diagnostics of Inflammatory Bowel Diseases. World J. Gastroenterol. 2014, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, I.; Vincenzo, O.D.; Imperatore, N.; Fisco, M.; Testa, A.; Filippo Scialò; Castiglione, F.; Ruoppolo, M.; Fabrizio Pasanisi; Santarpia, L. Amino acid profiles, disease activity, and protein intake in adult patients with Crohn’s disease. Front. Nutr. 2023, 10, 1245574. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Holeček, M. Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition 2002, 18, 130–133. [Google Scholar] [CrossRef]
- Crown, S.B.; Marze, N.; Antoniewicz, M.R. Catabolism of Branched-Chain Amino Acids Contributes Significantly to Synthesis of Odd-Chain and Even-Chain Fatty Acids in 3T3-L1 Adipocytes. PLoS ONE 2015, 10, e0145850. [Google Scholar] [CrossRef]
- Higueras, C.; Ruiz-Capillas, C.; Herrero, A.; Sainz, A.; García-Sancho, M.; Rodríguez-Franco, F.; Larrosa, M.; Rey, A.I. Differentiating Canine Chronic Inflammatory Enteropathies Using Faecal Amino Acid Profiles: Potential and Limitations. Animals 2025, 15, 1185. [Google Scholar] [CrossRef]
- Kathrani, A.; Yen, S.; Hall, E.J.; Swann, J.R. The effects of a hydrolyzed protein diet on the plasma, fecal and urine metabolome in cats with chronic enteropathy. Sci. Rep. 2023, 13, 19979. [Google Scholar] [CrossRef]
- Matthews, D.E. An overview of phenylalanine and tyrosine kinetics in humans. J. Nutr. 2007, 137, 1549S–1555S. [Google Scholar] [CrossRef] [PubMed]
- Questa, M.; Weimer, B.C.; Fiehn, O.; Chow, B.; Hill, S.L.; Ackermann, M.R.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S.; Marsilio, S. Unbiased Serum Metabolomic Analysis in Cats with Naturally Occurring Chronic Enteropathies Before and After Medical Intervention. Sci. Rep. 2024, 14, 6939. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Guard, B.C.; Blake, A.B.; Ackermann, M.; Webb, C.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Long-Term Recovery of the Fecal Microbiome and Metabolome of Dogs with Steroid-Responsive Enteropathy. Animals 2021, 11, 2498. [Google Scholar] [CrossRef] [PubMed]
- Passmore, I.J.; Letertre, M.P.M.; Preston, M.D.; Bianconi, I.; Harrison, M.A.; Nasher, F.; Kaur, H.; Hong, H.A.; Baines, S.D.; Cutting, S.M.; et al. Para-cresol production by Clostridium difficile affects microbial diversity and membrane integrity of Gram-negative bacteria. PLoS Pathog. 2018, 14, e1007191. [Google Scholar] [CrossRef]
- Rémond, D.; Buffière, C.; Godin, J.-P.; Mirand, P.P.; Obled, C.; Papet, I.; Dardevet, D.; Williamson, G.; Breuillé, D.; Faure, M. Intestinal Inflammation Increases Gastrointestinal Threonine Uptake and Mucin Synthesis in Enterally Fed Minipigs. J. Nutr. 2009, 139, 720–726. [Google Scholar] [CrossRef]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross-Sectional Evaluation of the Gut-Microbiome Metabolome Axis in an Italian Cohort of IBD Patients. Sci. Rep. 2017, 7, 9523. [Google Scholar] [CrossRef]
- Bosch, S.; Struys, E.A.; van Gaal, N.; Bakkali, A.; Jansen, E.W.; Diederen, K.; Benninga, M.A.; Mulder, C.J.; de Boer, N.K.H.; de Meij, T.G.J. Fecal Amino Acid Analysis Can Discriminate De Novo Treatment-Naïve Pediatric Inflammatory Bowel Disease from Controls. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 773–778. [Google Scholar] [CrossRef]
- Diederen, K.; Li, J.V.; Donachie, G.E.; Meij, T.G.; Waart, D.R.; Hakvoort, T.B.M.; Kindermann, A.; Wagner, J.; Auyeung, V.; Velde, A.A.; et al. Exclusive Enteral Nutrition Mediates Gut Microbial and Metabolic Changes Associated with Remission in Children with Crohn’s Disease. Sci. Rep. 2020, 10, 10758. [Google Scholar] [CrossRef]
- Ruaux, C.G. Cobalamin in companion animals: Diagnostic marker, deficiency states and therapeutic implications. Vet. J. 2013, 196, 145–152. [Google Scholar] [CrossRef]
- Kather, S.; Grützner, N.; Kook, P.H.; Dengler, F.; Heilmann, R.M. Review of cobalamin status and disorders of cobalamin metabolism in dogs. J. Vet. Intern. Med. 2020, 34, 13–28. [Google Scholar] [CrossRef]
- Toresson, L.; Suchodolski, J.S.; Spillmann, T.; Lopes, B.C.; Shih, J.; Steiner, J.M.; Pilla, R. The intestinal microbiome in dogs with chronic enteropathies and cobalamin deficiency or normocobalaminemia—A comparative study. Animals 2023, 13, 1378. [Google Scholar] [CrossRef]
- Berghoff, N.; Parnell, N.K.; Hill, S.L.; Suchodolski, J.S.; Steiner, J.M. Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease. Am. J. Vet. Res. 2013, 74, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, M.; Steiner, J.M.; Fosgate, G.T.; Zentek, J.; Hartmann, S.; Kohn, B. Chronic diarrhea in dogs—Retrospective study in 136 cases. J. Vet. Intern. Med. 2017, 31, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Cristóbal-Verdejo, I.; Duque-Carrasco, F.J.; Espadas-González, L.; Pastor-Sirvent, N.; Usón-Casaús, J.M. Relationship between serum cobalamin concentration and endoscopic ileal appearance and histology in dogs with chronic inflammatory enteropathy. J. Vet. Intern. Med. 2022, 36, 957–965. [Google Scholar] [CrossRef]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Lucena, R.; Olmedilla, A.B.; Blanco, B.; Novales, M.; Ginel, P.J. Effect of Enterococcus faecium SF68 on serum cobalamin and folate concentrations in healthy dogs. J. Small Anim. Pract. 2018, 59, 438–443. [Google Scholar] [CrossRef]
- Toresson, L.; Steiner, J.M.; Suchodolski, J.S.; Spillmann, T. Oral cobalamin supplementation in dogs with chronic enteropathies and hypocobalaminemia. J. Vet. Intern. Med. 2016, 30, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kook, P.; Bigler, B.; Reusch, C.; Lutz, S.; Sewell, A. Evaluation of serum cobalamin concentration in cats with clinical signs of gastrointestinal disease. Schweiz. Arch. Tierheilkd. 2012, 154, 479–486. [Google Scholar] [CrossRef]
- Kunath, T.; Kather, S.; Dengler, F.; Nexo, E.; Pfannkuche, H.; Heilmann, R.M. Serum Transcobalamin Concentration in Cats-Method Validation and Evaluation in Chronic Enteropathies and Other Conditions. Vet. Sci. 2024, 11, 552. [Google Scholar] [CrossRef]
- Simpson, K.; Lamb, S.; Fyfe, J.; Cornetta, A.; Strauss-Ayali, D.; Sachs, A.; Reimers, T. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J. Vet. Intern. Med. 2001, 15, 26–32. [Google Scholar] [CrossRef]
- Steiner, J.; Suchodolski, J.; Toresson, L.; Larsen, M.; Olmedal, G.; Spillmann, T. Oral cobalamin supplementation in cats with hypocobalaminaemia: A retrospective study. J. Feline Med. Surg. 2017, 19, 1302–1306. [Google Scholar] [CrossRef]
- Ullal, T.V.; Marks, S.L.; Huebner, S.N.; Taylor, S.L.; Shelley, C.D. Association of folate concentrations with clinical signs and laboratory markers of chronic enteropathy in dogs. J. Vet. Intern. Med. 2023, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Batt, R.M.; Needham, J.R.; Carter, M.W. Bacterial overgrowth associated with a naturally occurring enteropathy in the German shepherd dog. Res. Vet. Sci. 1983, 35, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Dossin, O. Laboratory tests for diagnosis of gastrointestinal and pancreatic diseases. Top. Companion Anim. Med. 2011, 26, 86–97. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Day, M.J.; Ruaux, C.G.; Steiner, J.M.; Williams, D.A.; Hall, E.J. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J. Vet. Intern. Med. 2003, 17, 33–43. [Google Scholar] [CrossRef]
- Sacoor, C.; Barros, L.M.; Montezinho, L. What are the potential biomarkers that should be considered in diagnosing and managing canine chronic inflammatory enteropathies? Open Vet. J. 2020, 10, 412–430. [Google Scholar] [CrossRef]
| Disease | Species | Sample | Findings | Reference |
|---|---|---|---|---|
| Inflammatory Intestinal Disease (n = 15) Control (n = 10) | Dogs | Feces | No differences in SCFAs between groups | [25] |
| Chronic enteropathy (n = 73) Control (n = 49) | Dogs | Feces | CIEs: ↓ C2, C3, ∑SCFAs | [76] |
| Food-responsive enteropathy (n = 9) Control (n = 6) | Dogs | Feces | FRE: ↓ C2, C3, iC4, iC5, ∑SCFAs | [93] |
| Inflammatory Intestinal Disease (n = 6) Control (n= 16) | Dogs | Feces | IBD: ↓ C2, C3 | [57] |
| CIEs: Inflammatory Intestinal Disease (n= 6) T-phenotype small cell lymphoma (n = 6) Unidentified enteropathy (n = 3) Control (n = 13) | Cats | Feces | CIEs: ↓ C3, iC4, ↑ C4 and ∑SCFAs | [95] |
| Food-responsive enteropathy (n = 35) Immunosuppressant-enteropathy (n = 18) Control (n = 22) | Dogs | Feces | IRE vs. control: ↓ C2, C3, ∑SCFAs | [94] |
| Disease | Species | Sample | Findings | Reference |
|---|---|---|---|---|
| Inflammatory Intestinal Disease (n = 12) Control (n = 10) | Dogs | Serum | IBD: no differences in lipid metabolism with controls. | [96] |
| Food-responsive enteropathy (n = 16) Immunosuppressants-responsive enteropathy (n= 16) | Dogs | Blood and plasma phospholipids | Differences in phospholipids between both groups. Phosphatidylcholine changed from PC38:4 before treatment to lysophosphatidylcholine 18:0 after treatment. | [105] |
| Chronic enteropathy (n = 48): Food-responsive enteropathy (n = 28) Antibiotic-responsive enteropathy (n = 5) Immunosuppressants-responsive enteropathy (n = 15) Control (n = 68) | Dogs | Erythrocyte membrane phospholipids | CIEs: SFA:↓ C16:0, ↑ C18:0; PUFA: ↓ LA, ↑ C20:3n − 6, ↑ EPA, ↑ DHA, ↑ n − 3, ↓ SFA/MUFA, ↓ n − 6/n − 3, ↑ elongase (C18/C16), Δ6-desaturase, ↓ Δ5-desaturase, ↓ Δ9-desaturase. No statistical differences between CE groups. | [106] |
| Food-responsive enteropathy (n = 9) Control (n = 6) | Dogs | Plasma | FRE: ↑ C20:3n − 6, ↓ C20:5n − 3, ↓ C22:5n − 3, ↓ ∑PUFA | [93] |
| Chronic enteropathy (n = 13) Control (n = 20) | Dogs (Yorkshire terrier) | Plasma | CIEs: ↓ C18:1, ↓ PUFA (↓ C20:2n − 6, ↓ C20:3n − 6) | [114] |
| Chronic enteropathy (n = 55) Control (n = 204) | Dogs | Serum | CIEs: ↓ SFA, ↓ C16:0, ↓ C18:1, ↓ PUFA, (LA, n − 6) | [107] |
| Chronic enteropathy (n = 41): Food-responsive enteropathy (n = 17) Inflammatory Intestinal Disease (n = 15) T-phenotype small cell lymphoma (n = 9) Control (n = 43) | Cats | Erythrocyte membrane phospholipids | CIEs: PUFA: ↑ C22:5n − 3, ↑ DHA, ↑ n − 3 PUFA; ↓ LA, ratio n − 6/n − 3, ↑ Δ6-desaturase No changes between CE groups. | [108] |
| Disease | Species | Sample | Findings | Reference |
|---|---|---|---|---|
| Chronic enteropathy (n = 15) Control (n = 15) | Dogs | Feces | CIEs: ↑ cisteine, ↑ glycine, ↑ phenylalanine, ↑ valine, ↑ leucine, ↑ lysine ↓ metabolites derived from tryptophan | [125] |
| Inflammatory Intestinal Disease (n = 9) Control (n = 13) | Dogs | Feces | IBD: ↑ proline, ↑ valine, ↑ leucine, ↑ isoleucine, ↑ alanine, ↑ tryptophan, ↑ asparagine, ↑ aspartic acid, ↑ cisteine, ↑ cystine, ↑ glutamic acid, ↑ glycine, ↑ methionine, ↑ phenylalanine, ↑ serine, ↑ threonine | [166] |
| Food-responsive enteropathy (n = 9) Control (n = 6) | Dogs | Feces | FRE: ↓ phenylalanine, ↑ cystine, | [149] |
| Food-responsive enteropathy (n = 35) Immunosuppressant-responsive enteropathy (n = 18) Control (n = 22) | Dogs | Feces | IRE vs. control: ↑ Tyrosine, ↑ Threonine; FRE vs. control: ↑ Tyrosine; FRE vs. IRE: no changes | [162] |
| Inflammatory Intestinal Disease (n = 11) T-phenotype small cell lymphoma (n = 11) Control (n = 14) | Cats | Feces | IBD, lymphoma: ↑ aspartate, ↑ cisteine, ↑ phenylalanine, ↑ leucine, ↑ valine ↓ Metabolites derived from tryptophan Alteration in amino acid metabolism | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, A.I.; Higueras, C.; Olmeda, P.; Sainz, A.; Gálvez, B.G.; Larrosa, M. Markers of Gut Health in Small Animals: Focus on Fatty Acids and Amino Acids as Indicators of Intestinal Functionality and Microbiome Activity. Animals 2025, 15, 1927. https://doi.org/10.3390/ani15131927
Rey AI, Higueras C, Olmeda P, Sainz A, Gálvez BG, Larrosa M. Markers of Gut Health in Small Animals: Focus on Fatty Acids and Amino Acids as Indicators of Intestinal Functionality and Microbiome Activity. Animals. 2025; 15(13):1927. https://doi.org/10.3390/ani15131927
Chicago/Turabian StyleRey, Ana I., Cristina Higueras, Patricia Olmeda, Angel Sainz, Beatriz G. Gálvez, and Mar Larrosa. 2025. "Markers of Gut Health in Small Animals: Focus on Fatty Acids and Amino Acids as Indicators of Intestinal Functionality and Microbiome Activity" Animals 15, no. 13: 1927. https://doi.org/10.3390/ani15131927
APA StyleRey, A. I., Higueras, C., Olmeda, P., Sainz, A., Gálvez, B. G., & Larrosa, M. (2025). Markers of Gut Health in Small Animals: Focus on Fatty Acids and Amino Acids as Indicators of Intestinal Functionality and Microbiome Activity. Animals, 15(13), 1927. https://doi.org/10.3390/ani15131927










