The Effects of FTO on the Proliferation and Differentiation of Rabbit Preadipocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Meat Rabbit Sourcing and Adipose Tissue Sampling
2.2. Cell Culture and Induced Differentiation
2.3. Plasmid Construction and Cell Transfection
2.4. Oil Red O Staining Protocol
2.5. CCK-8 Viability Assay
2.6. Gene Expression Profiling: RNA to qPCR
2.7. Western Blot Analysis
2.8. RNA Sequencing and Bioinformatics Analysis
2.9. Statistical Analysis
3. Results
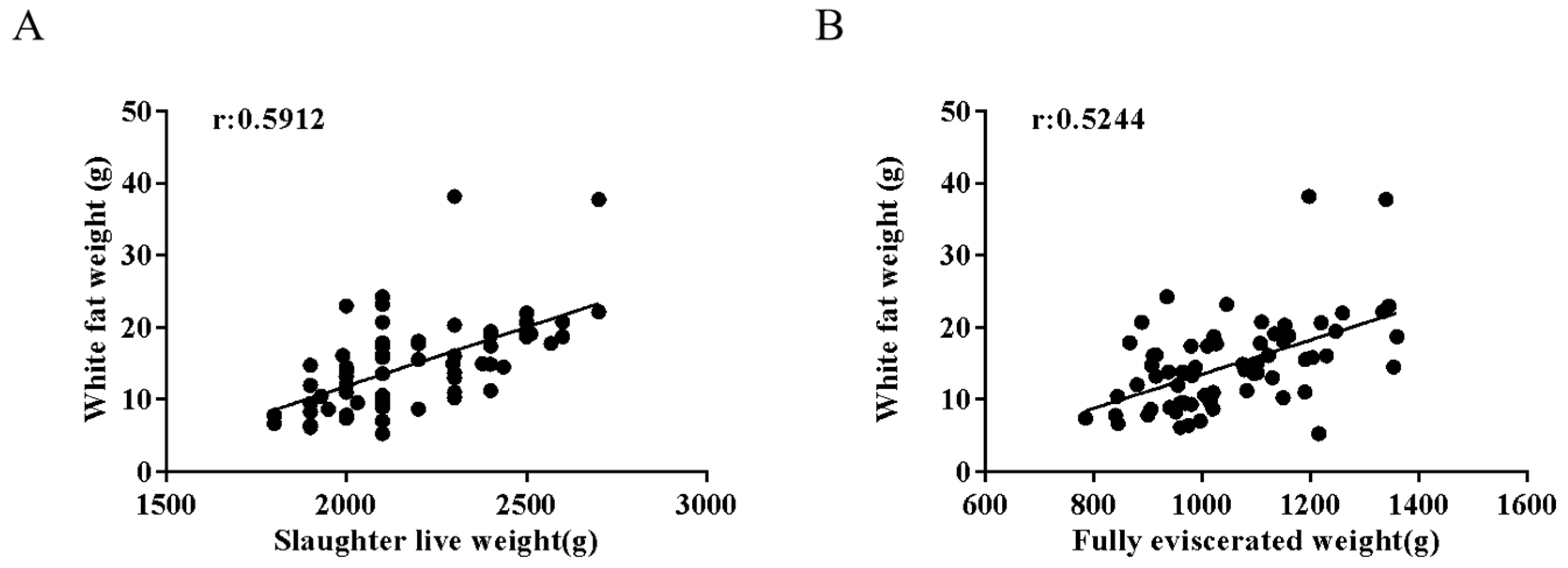
3.1. Effects of Live Weight Before Slaughter-on-Slaughter Traits and Meat Quality Traits in Meat Rabbits
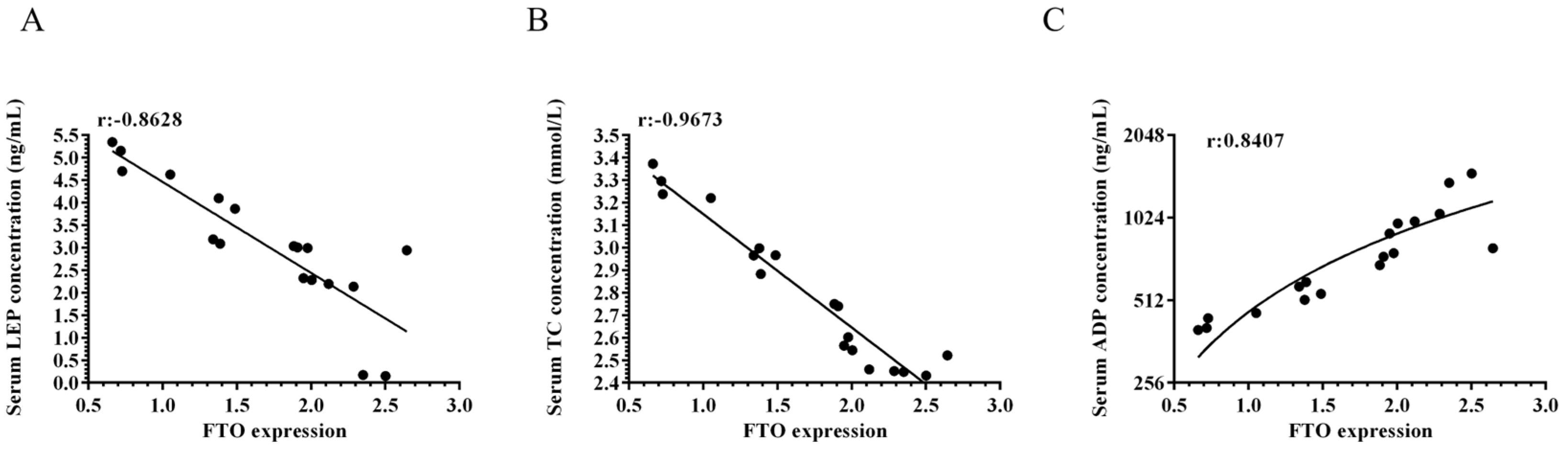
3.2. Serum Adiponectin, Leptin, and Total Cholesterol Levels in Meat Rabbits
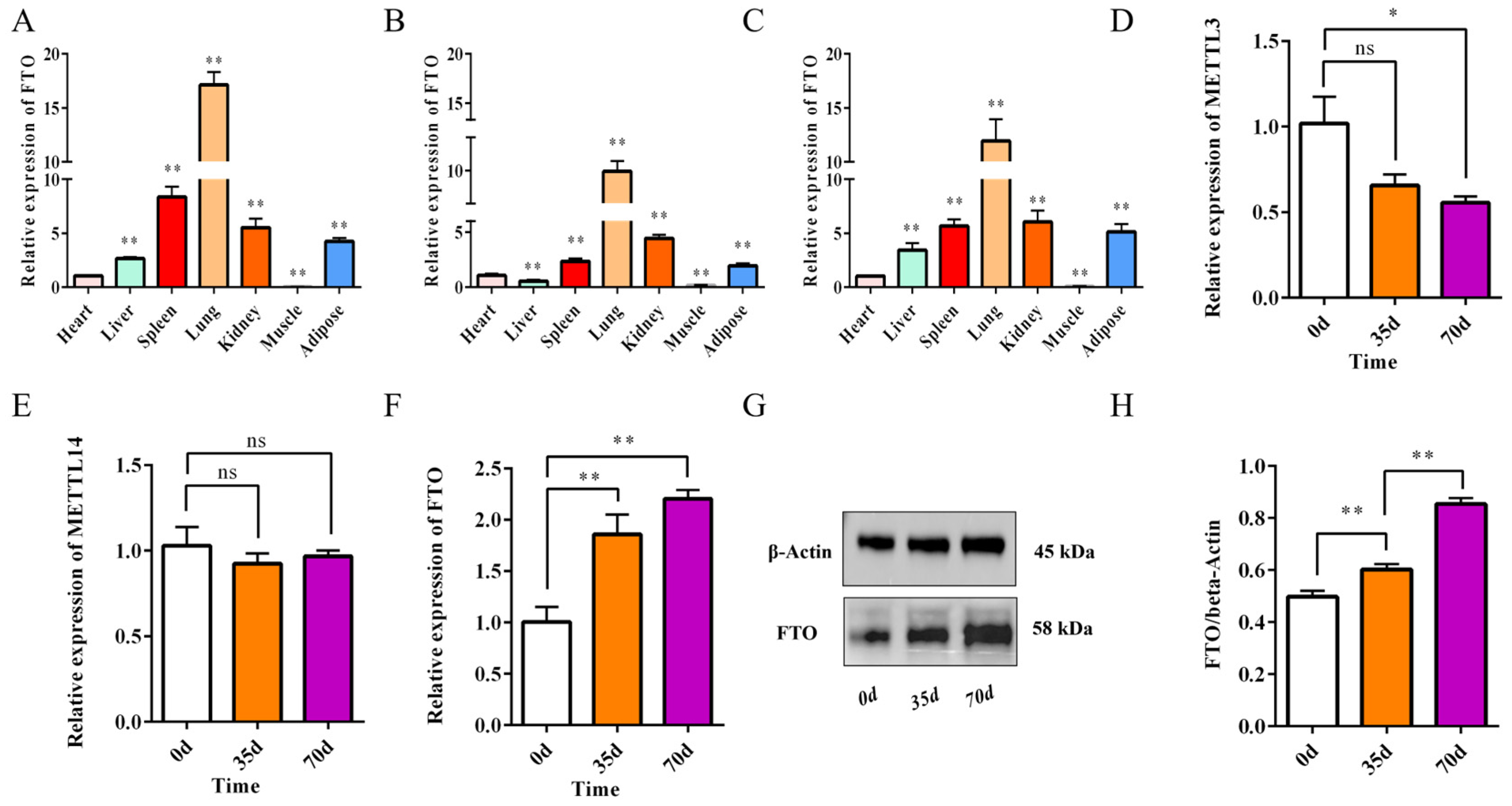
3.3. FTO Is Associated with Adipose Tissue Development
3.4. Establishment of a Preadipocyte Differentiation Model in Meat Rabbits
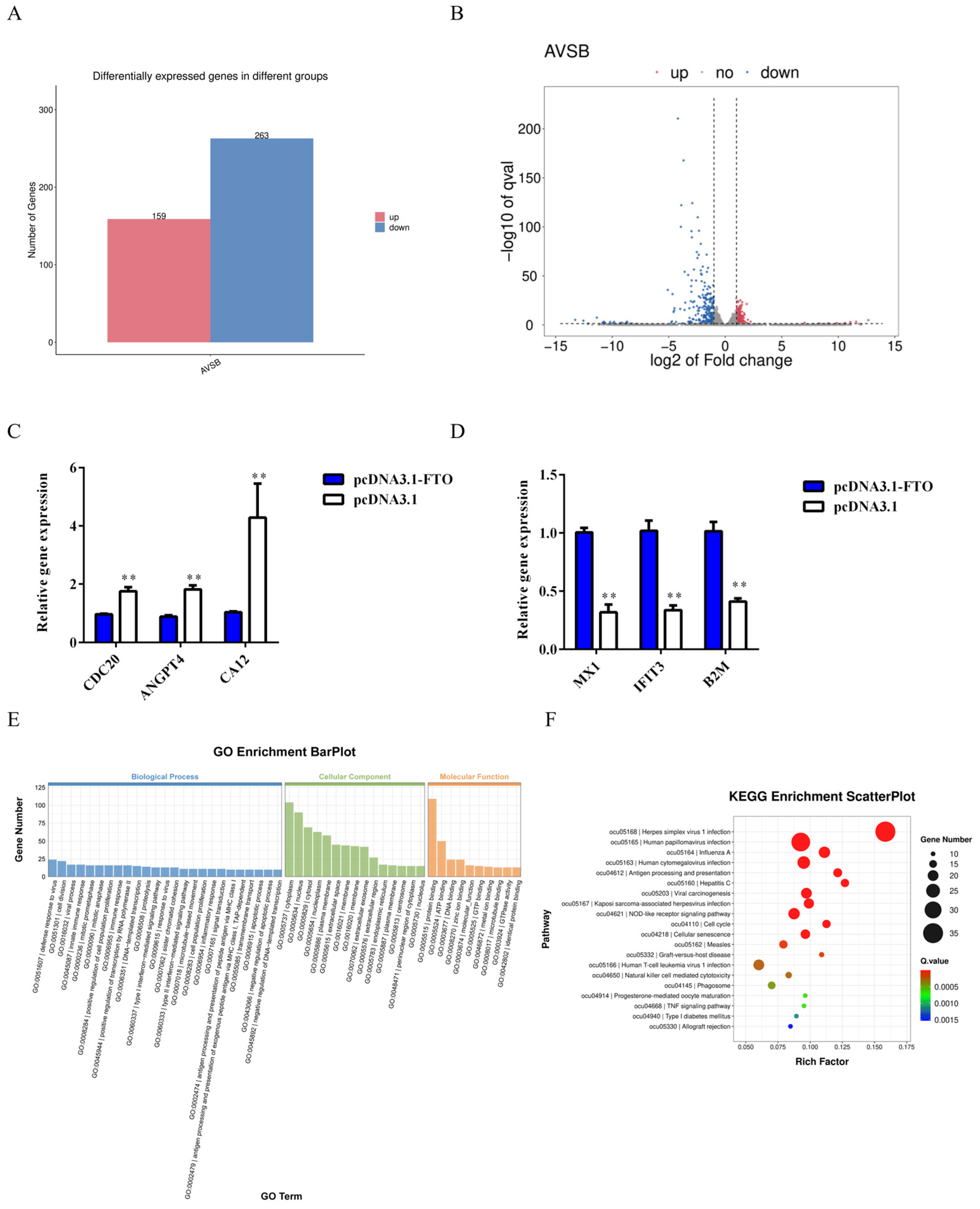
3.5. Analysis of Differentially Expressed Genes
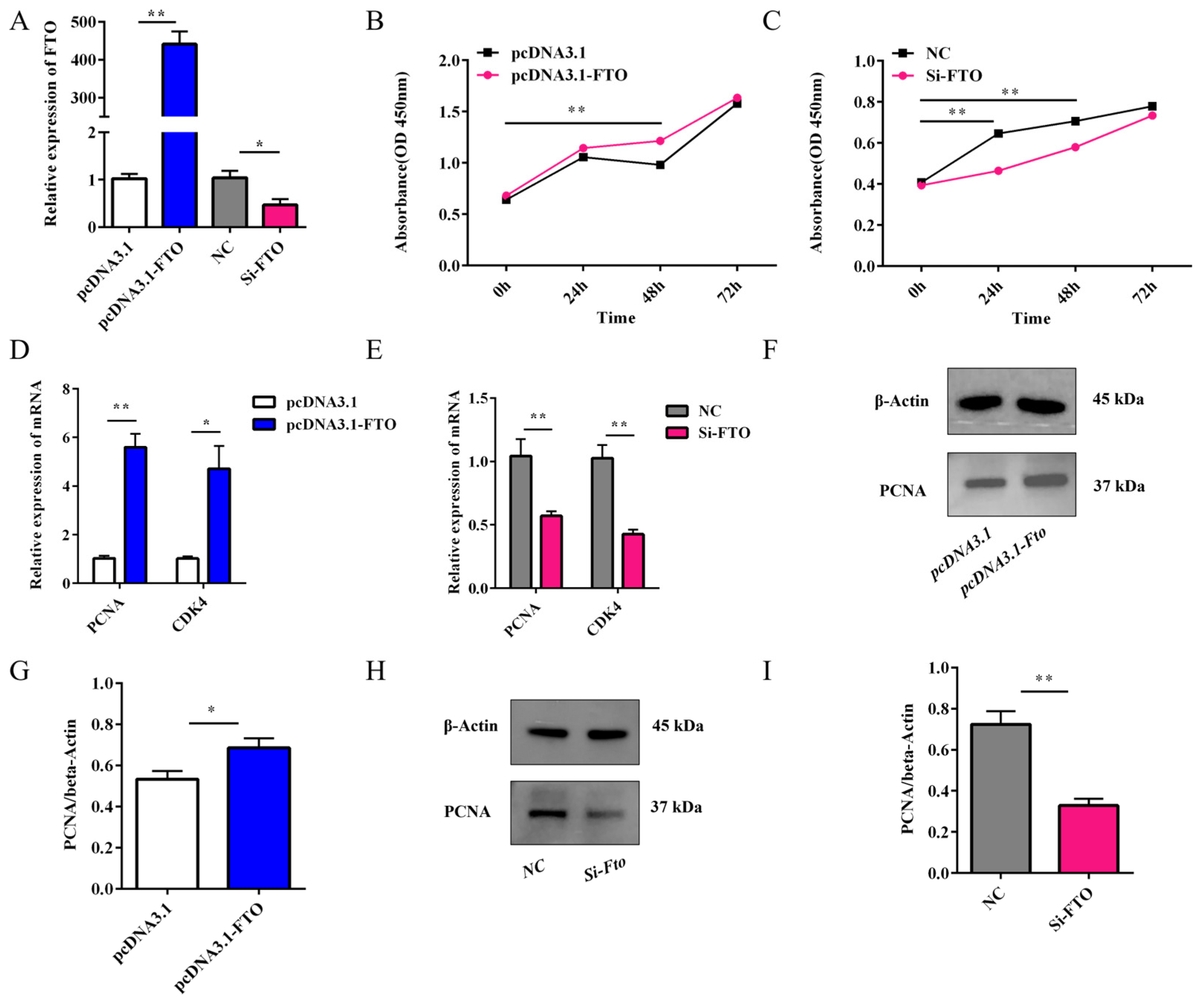
3.6. Effect of FTO on the Proliferation of Rabbit Preadipocytes
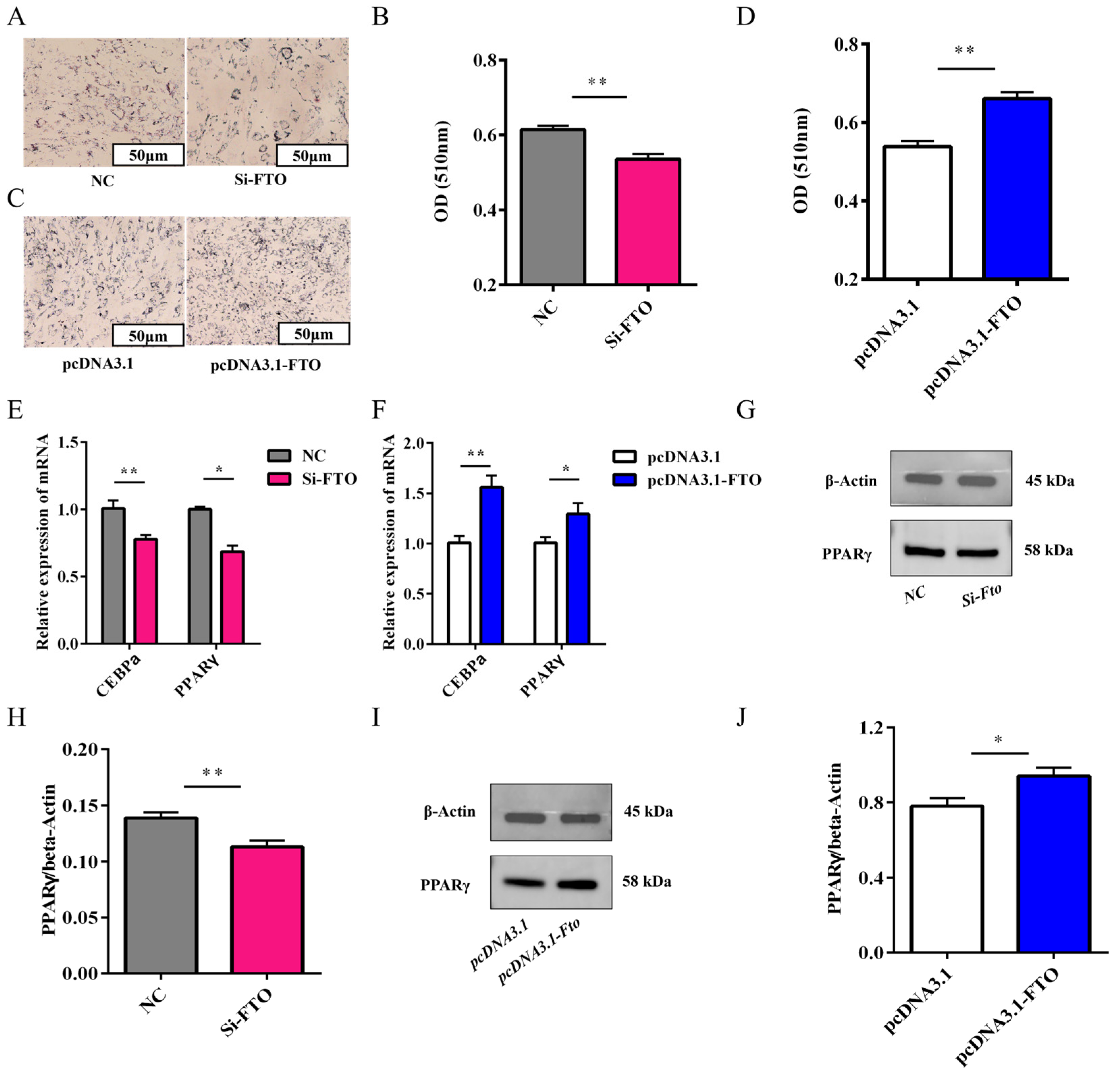
3.7. Effect of FTO on the Differentiation of Rabbit Preadipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDK4 | Cyclin-Dependent Kinase 4 |
| C/EBPα | CCAAT/Enhancer-Binding Protein Alpha |
| PCNA | Proliferating Cell Nuclear Antigen |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| β-actin | Beta-Actin |
References
- Kumar, P.; Sharma, N.; Narnoliya, L.K.; Verma, A.K.; Umaraw, P.; Mehta, N.; Ismail-Fitry, M.R.; Kaka, U.; Yong-Meng, G.; Lee, S.J.; et al. Improving quality and consumer acceptance of rabbit meat: Prospects and challenges. Meat Sci. 2025, 219, 109660. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Havrysh, O.; Korzeniowska, M.; Getya, A. Potential and limitations of rabbit meat in maintaining food security in Ukraine. Meat Sci. 2023, 204, 109293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Kim, H.J.; Jayasena, D.D.; Jo, C. On-Farm and Processing Factors Affecting Rabbit Carcass and Meat Quality Attributes. Food Sci. Anim. Resour. 2023, 43, 197–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, G. RNA m6A modification, signals for degradation or stabilisation? Biochem. Soc. Trans. 2024, 52, 707–717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- An, Y.; Duan, H. The role of m6A RNA methylation in cancer metabolism. Mol. Cancer 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ming, X.; Chen, S.; Li, H.; Wang, Y.; Zhou, L.; Lv, Y. m6A RNA Methylation and Implications for Hepatic Lipid Metabolism. DNA Cell Biol. 2024, 43, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, L.; Luo, E.; Hou, J.; Yan, G.; Wang, D.; Qiao, Y.; Tang, C. Role of m6A RNA methylation in cardiovascular disease (Review). Int. J. Mol. Med. 2020, 46, 1958–1972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Wu, R.; Liu, Y.; Zhao, Y.; Bi, Z.; Yao, Y.; Liu, Q.; Shi, H.; Wang, F.; Wang, Y. m6A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy 2020, 16, 1221–1235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azzam, S.K.; Alsafar, H.; Sajini, A.A. FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 3800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Z.; Yu, G.L.; Zhu, X.; Peng, T.H.; Lv, Y.C. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: Implications in lipid metabolic disorders. Genes Dis. 2021, 9, 51–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, R.; Guo, G.; Bi, Z.; Liu, Y.; Zhao, Y.; Chen, N.; Wang, F.; Wang, Y.; Wang, X. m(6)A methylation modulates adipogenesis through JAK2-STAT3-C/EBPbeta signaling. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, C.; Wang, N.; Li, S.; Liu, Q.; Sun, Z.; Wang, K.; Yu, S.-C.; Yang, Q. NADP modulates RNA m(6)A methylation and adipogenesis via enhancing FTO activity. Nat. Chem. Biol. 2020, 16, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Sun, C.; Yan, Y.; Niu, Z.; Li, Y.; Xu, X.; Zhang, J.; Wu, Y.; Li, Y.; Wang, L.; et al. Aberrant elevation of FTO levels promotes liver steatosis by decreasing the m6A methylation and increasing the stability of SREBF1 and ChREBP mRNAs. J. Mol. Cell Biol. 2023, 14, mjac061. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, C.; Guo, T.; Liu, J.; Yang, B.; Lu, Z. The molecular regulated mechanism of METTL3 and FTO in lipid metabolism of Hu sheep. Genomics 2024, 116, 110945. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Jiang, H. Molecular and Cellular Mechanisms of Intramuscular Fat Development and Growth in Cattle. Int. J. Mol. Sci. 2024, 25, 2520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, S.B.; Crouse, J.D. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J. Nutr. 1984, 114, 792–800. [Google Scholar] [CrossRef]
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality—A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, T.; Tian, X.; Li, D.; He, Y.; Yang, P.; Cheng, Y.; Zhao, X.; Sun, J.; Yang, G. Transcriptome, proteome and metabolome analysis provide insights on fat deposition and meat quality in pig. Food Res. Int. 2023, 166, 112550. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, Y.; Luo, H.; Pang, W.; Martin, G.B. Fat deposition and partitioning for meat production in cattle and sheep. Anim. Nutr. 2024, 17, 376–386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duo, T.; Liu, X.; Mo, D.; Bian, Y.; Cai, S.; Wang, M.; Li, R.; Zhu, Q.; Tong, X.; Liang, Z.; et al. Single-base editing in IGF2 improves meat production and intramuscular fat deposition in Liang Guang Small Spotted pigs. J. Anim. Sci. Biotechnol. 2023, 14, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, D.; Su, R.; Zhang, Y.; Wang, B.; Hou, Y.; Luo, Y.; Sun, L.; Guo, Y.; Jin, Y. Impact of dietary Lactobacillus supplementation on intramuscular fat deposition and meat quality of Sunit sheep. J. Food Biochem. 2022, 46, e14207. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, K.; Pilžys, T.; Garbicz, D.; Marcinkowski, M.; Skorobogatov, O.; Dylewska, M.; Gajewski, Z.; Grzesiuk, E.; Zabielski, R. Intracellular and tissue specific expression of FTO protein in pig: Changes with age, energy intake and metabolic status. Sci. Rep. 2020, 10, 13029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, M.; Ji, W.; Yang, X.; Tian, K.; Ma, X.; Yu, S.; Chen, L.; Zhao, X. The role of m6A demethylases in lung cancer: Diagnostic and therapeutic implications. Front. Immunol. 2023, 14, 1279735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, G.; Cue, L.; Puckett, Y. Endometrial Hyperplasia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Pan, Y.; Cao, S.; Tang, J.; Arroyo, J.P.; Terker, A.S.; Wang, Y.; Niu, A.; Fan, X.; Wang, S.; Zhang, Y.; et al. Cyclooxygenase-2 in adipose tissue macrophages limits adipose tissue dysfunction in obese mice. J. Clin. Investig. 2022, 132, e152391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, G.; Wang, W.; Tu, M.; Zhao, B.; Han, J.; Li, J.; Pan, Y.; Zhou, J.; Ma, W.; Liu, Y.; et al. Deciphering adipose development: Function, differentiation and regulation. Dev. Dyn. 2024, 253, 956–997. [Google Scholar] [CrossRef] [PubMed]
- Fooks, A.N.; Beppu, L.Y.; Frias, A.B.; D’Cruz, L.M. Adipose tissue regulatory T cells: Differentiation and function. Int. Rev. Immunol. 2023, 42, 323–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Luo, Y.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. FTO Promotes Adipogenesis through Inhibition of the Wnt/β-catenin Signaling Pathway in Porcine Intramuscular Preadipocytes. Anim. Biotechnol. 2017, 28, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, W.; Wang, Z.; Nie, Q. m6A demethylase FTO regulate CTNNB1 to promote adipogenesis of chicken preadipocyte. J. Anim. Sci. Biotechnol. 2022, 13, 147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, G.; Ai, Y.; Zhu, T.; Li, J.; Ren, Z. FTO promoted adipocyte differentiation by regulating ADRB1 gene through m6A modification in Hycole rabbits. Anim. Biotechnol. 2023, 34, 2565–2570. [Google Scholar] [CrossRef] [PubMed]

| Name | Primer Sequences |
|---|---|
| Si-FTO-F | GUGAAAGGUUCUACUAUAA |
| Si-FTO-R | UUAUAGUAGAACCUUUCAC |
| Name | Primer Sequence |
|---|---|
| GAPDH-F | CTGACCTGCCGCCTGGAGAAAG |
| GAPDH-R | CCTGTTGCTGTAGCCAAATTCGTTG |
| FTO-F | CGCGAGCGTGAAGCTAAGAAA |
| FTO-R | TTATGGAGCTCCTCGGACAC |
| PPARγ-F | CGCTGATGCACTGCCTATGA |
| PPARγ-R | AGAGGTCCACAGAGCTGATTCC |
| C/EBPα-F | CAAGAACAGCAACGAGTACCG |
| C/EBPα-R | GTCACTGGTCAACTCCAGCAC |
| CDK4-F | AGTTTCTAAGCGGCCTGGAT |
| CDK4-R | AACTTCAGGAGCTCGGTACC |
| PCNA-F | TTGCACGTATATGCCGAGACC |
| PCNA-R | GGTGAACAGGCTCATTCATCTCT |
| METTL3-F | GTCCCCAACCTTCCGTAGTG |
| METTL3-R | TGTGCTAGACTTGGAGCCAC |
| METTL14-F | GATAGCCGCTTGCAGGAGAT |
| METTL14-R | TGCTGTTTAGCACAGCACCT |
| Antibody | Company | Accession Number | Dilution Ratio |
|---|---|---|---|
| anti-PCNA | Zenbio (Chengdu, China) | R25293 | 1:3000 |
| anti-PPARγ | Nature Biosciences (Shanghai, China) | #51311 | 1:1000 |
| anti-β-Actin | GenuIN (Hefei, China) | #2885 | 1:5000 |
| anti-FTO | Proteintech (WuHan, China) | 27226-1-AP | 1:1500 |
| Slaughtering Traits | Slaughter Liveweight (g) | Fully Eviscerated Weight (g) | Eviscerated Yield (%) | Semi-Eviscerated Yield (%) | Abdominal and Renal Fat Weights (g) |
|---|---|---|---|---|---|
| Low-BW Group (n = 35) | 2007.00 ± 14.26 | 944.80 ± 8.87 | 47.05 ± 0.13 | 51.45 ± 0.13 | 11.80 ± 0.57 |
| High-BW Group (n = 35) | 2421.60 ± 20.65 ** | 1156.20 ± 15.88 * | 47.68 ± 0.23 * | 52.23 ± 0.26 * | 18.42 ± 0.93 ** |
| Meat Traits | Index Drip Loss (%) | Cooking Rate (%) | Leg Muscle pH | Abdominal and Renal Fat Weights (g) |
|---|---|---|---|---|
| Low-BW Group (n = 35) | 7.17 ± 0.12 | 64.50 ± 0.14 | 5.83 ± 0.02 | 11.80 ± 0.57 |
| High-BW Group (n = 35) | 6.55 ± 0.09 ** | 64.97 ± 0.12 * | 5.62 ± 0.02 ** | 18.42 ± 0.93 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sun, W.; Jia, X.; Wang, J.; Yu, H.; Lai, S. The Effects of FTO on the Proliferation and Differentiation of Rabbit Preadipocytes. Animals 2025, 15, 1909. https://doi.org/10.3390/ani15131909
Wang J, Sun W, Jia X, Wang J, Yu H, Lai S. The Effects of FTO on the Proliferation and Differentiation of Rabbit Preadipocytes. Animals. 2025; 15(13):1909. https://doi.org/10.3390/ani15131909
Chicago/Turabian StyleWang, Jiaxue, Wenqiang Sun, Xianbo Jia, Jie Wang, Hengwei Yu, and Songjia Lai. 2025. "The Effects of FTO on the Proliferation and Differentiation of Rabbit Preadipocytes" Animals 15, no. 13: 1909. https://doi.org/10.3390/ani15131909
APA StyleWang, J., Sun, W., Jia, X., Wang, J., Yu, H., & Lai, S. (2025). The Effects of FTO on the Proliferation and Differentiation of Rabbit Preadipocytes. Animals, 15(13), 1909. https://doi.org/10.3390/ani15131909






