Effect of Dead-Cell Limosilactobacillus ingluviei on Hematological Parameters and Jejunal Transcriptome Profile in Calves During the Weaning Period
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Treatments and Management
2.3. Sampling Method
2.4. RNA Extraction and RNA-Seq Library Construction
2.5. Transcriptome Sequencing and Data Analysis
2.6. Validation of DEGs by Quantitative Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
3.1. Effect of DCLI on Blood Metabolic Parameters in Calves
3.2. Effect of DCLI on Complete Blood Count in Calves
3.3. Quality of RNA-Seq Reads
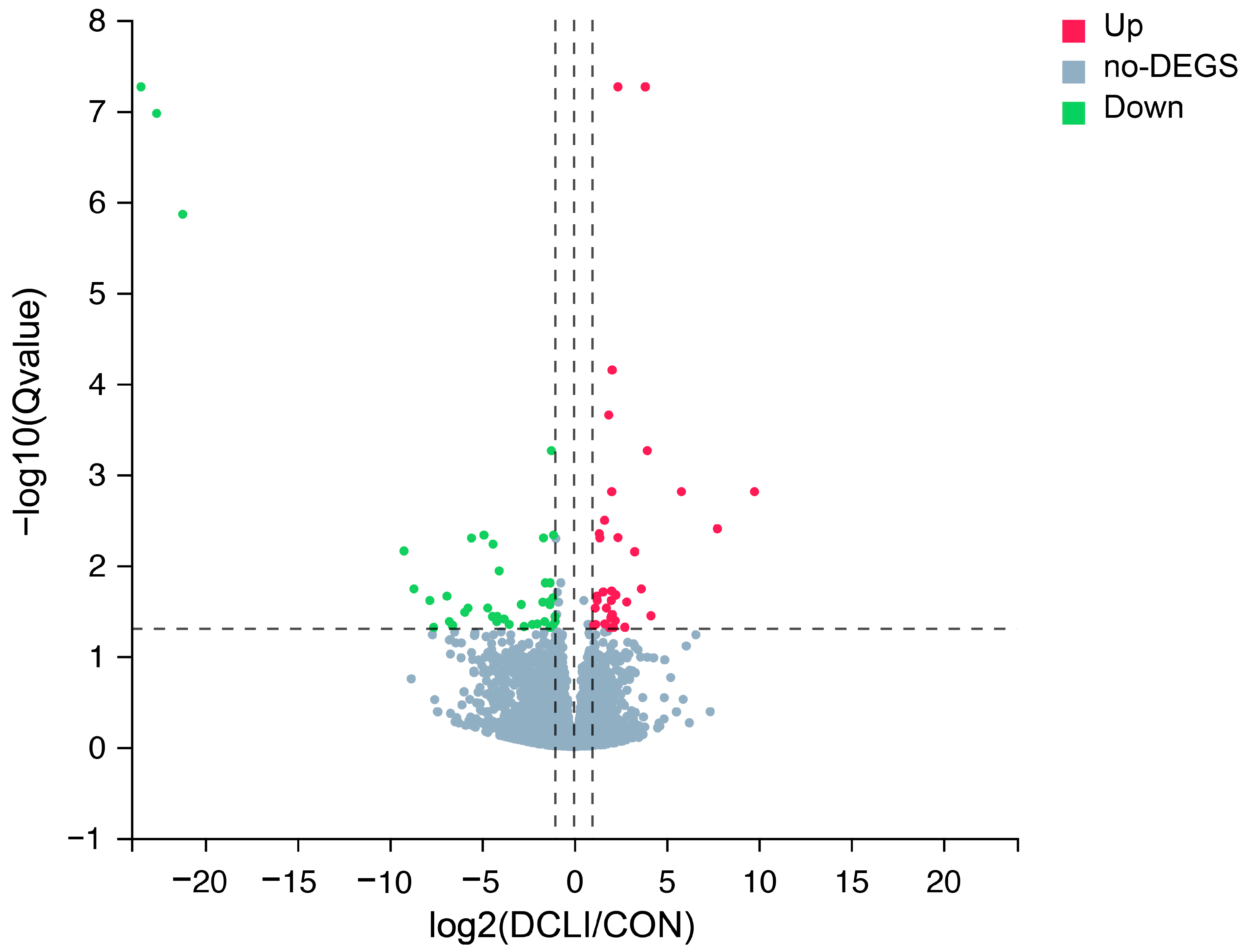
3.4. DEGs Analysis
3.5. GO and KEGG Enrichment Analysis of DEGs
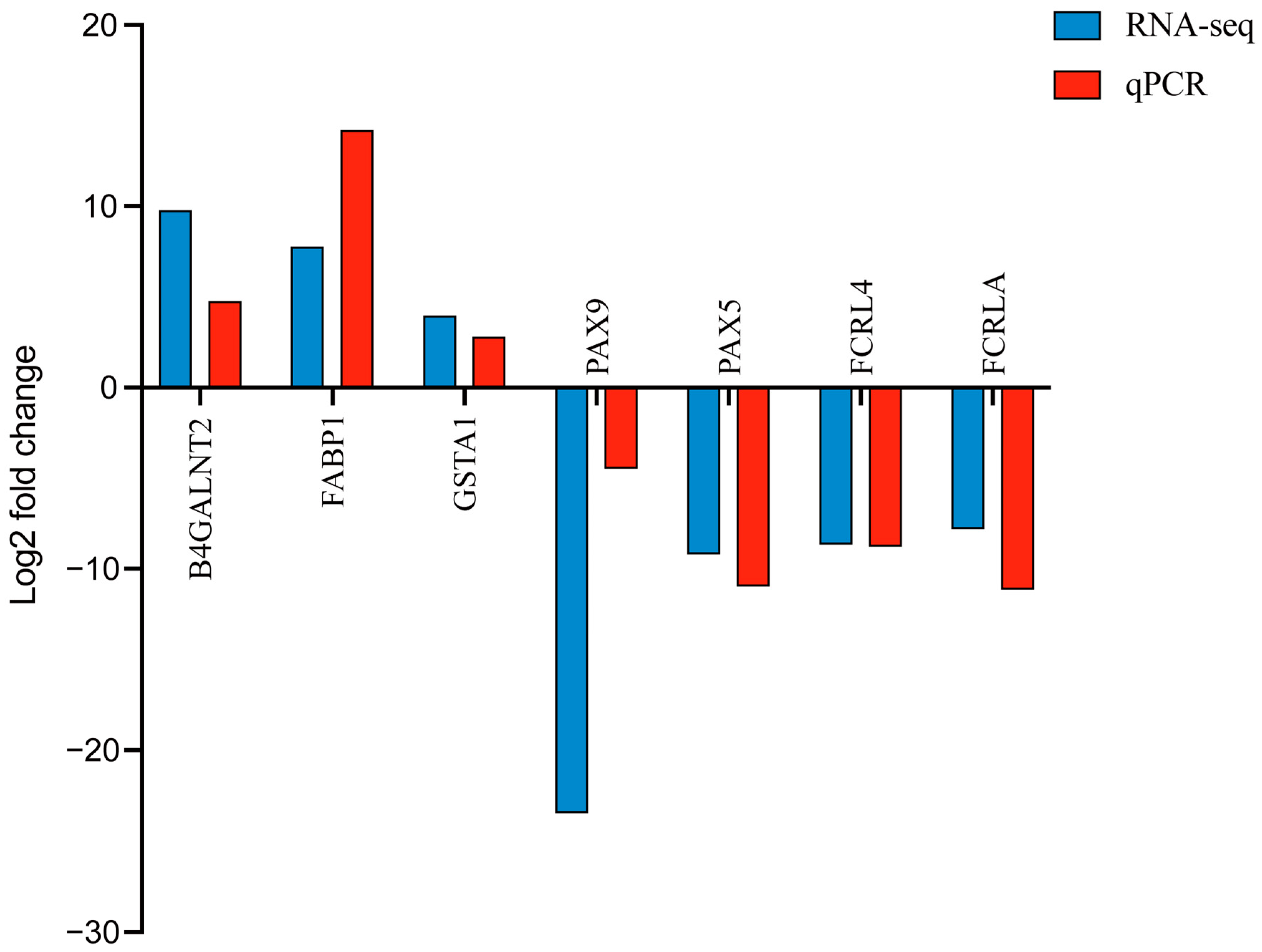
3.6. qPCR Validation of RNA-Seq
4. Discussion
4.1. DCLI on Blood Parameters in Calves
4.2. DEGs in the Jejunum Epithelium
4.3. GO and KEGG Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, A.; Heinrichs, A. Invited review: The importance of colostrum in the newborn dairy calf. J. Dairy Sci. 2022, 105, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Aghakeshmiri, F.; Azizzadeh, M.; Farzaneh, N.; Gorjidooz, M. Effects of neonatal diarrhea and other conditions on subsequent productive and reproductive performance of heifer calves. Vet. Res. Commun. 2017, 41, 107–112. [Google Scholar] [CrossRef]
- Okawa, T.; Nagai, M.; Hase, K. Dietary intervention impacts immune cell functions and dynamics by inducing metabolic rewiring. Front. Immunol. 2021, 11, 623989. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, M.; Mohanta, R.K.; Patra, A.K. Probiotics in livestock and poultry nutrition and health. In Advances in Probiotics for Sustainable Food and Medicine; Springer: Singapore, 2021; pp. 149–179. [Google Scholar] [CrossRef]
- Marteau, P.; Shanahan, F. Basic aspects and pharmacology of probiotics: An overview of pharmacokinetics, mechanisms of action and side-effects. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 725–740. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet. Res. 2019, 15, 315. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.-Y.; Tan, Y.; Zhang, H. Heat-killed Bifidobacterium bifidum B1628 may alleviate dextran sulfate sodium-induced colitis in mice, and the anti-inflammatory effect is associated with gut microbiota modulation. Nutrients 2022, 14, 5233. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lim, B.; Kim, J.-M.; Kil, D.Y. Integrated transcriptome analysis for the hepatic and jejunal mucosa tissues of broiler chickens raised under heat stress conditions. J. Anim. Sci. Biotechnol. 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Cantet, J.M.; Yu, Z.; Ríus, A.G. Heat Stress-Mediated Activation of Immune–Inflammatory Pathways. Antibiotics 2021, 10, 1285. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, Y.; Penner, G.; Oba, M.; Guan, L. Transcriptome analysis of ruminal epithelia revealed potential regulatory mechanisms involved in host adaptation to gradual high fermentable dietary transition in beef cattle. BMC Genom. 2017, 18, 976. [Google Scholar] [CrossRef]
- Jin, C.; Wu, S.; Liang, Z.; Zhang, J.; Lei, X.; Bai, H.; Liang, G.; Su, X.; Chen, X.; Wang, P. Multi-omics reveal mechanisms of high enteral starch diet mediated colonic dysbiosis via microbiome-host interactions in young ruminant. Microbiome 2024, 12, 38. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Reverter, A.; Keogh, K.; Alexandre, P.A.; Afonso, J.; Palhares, J.C.P.; Cardoso, T.F.; Malheiros, J.M.; Bruscadin, J.J.; de Oliveira, P.S.N. Transcriptional response to an alternative diet on liver, muscle, and rumen of beef cattle. Sci. Rep. 2024, 14, 13682. [Google Scholar] [CrossRef]
- Sirisopapong, M.; Shimosato, T.; Okrathok, S.; Khempaka, S. Assessment of lactic acid bacteria isolated from the chicken digestive tract for potential use as poultry probiotics. Anim. Biosci. 2023, 36, 1209. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, M.; Sirisopapong, M.; Namai, F.; Ishida, M.; Okrathok, S.; Shigemori, S.; Ogita, T.; Sato, T.; Khempaka, S.; Shimosato, T. Lactobacillus ingluviei C37 from chicken inhibits inflammation in LPS-stimulated mouse macrophages. Anim. Sci. J. 2020, 91, e13436. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Nydam, D.; Godden, S.; Bristol, L.; Kryzer, A.; Ranum, J.; Schaefer, D. Brix refractometry in serum as a measure of failure of passive transfer compared to measured immunoglobulin G and total protein by refractometry in serum from dairy calves. Vet. J. 2016, 211, 82–87. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.; Renaud, D.; Steele, M.; Cangiano, L.; Olmeda, M.; Villot, C.; Chevaux, E.; Yu, J.; Hernandez, L.; Frizzarini, W. Effects of weaning and inactivated Lactobacillus helveticus supplementation on dairy calf behavioral and physiological indicators of affective state. J. Dairy Sci. 2024, 107, 11363–11380. [Google Scholar] [CrossRef]
- Thorsteinsson, M.M.; Canibe, N.; Vestergaard, M. Effect of dietary supplementation of Saccharomyces cerevisiae and a postbiotic from Lactobacillus acidophilus on the concentration of organic acids, biogenic amines, and microbiota in the small intestine and colon of rosé veal calves. J. Anim. Feed. Sci. 2020, 29, 345–351. [Google Scholar] [CrossRef]
- Van Niekerk, J.; Middeldorp, M.; Guan, L.; Steele, M. Preweaning to postweaning rumen papillae structural growth, ruminal fermentation characteristics, and acute-phase proteins in calves. J. Dairy Sci. 2021, 104, 3632–3645. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Chen, Y.; Liang, G.; Goonewardene, L.A.; Guan, L.L. Heat-treated colostrum feeding promotes beneficial bacteria colonization in the small intestine of neonatal calves. J. Dairy Sci. 2015, 98, 8044–8053. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C.; Niu, X.; Zhang, Z.; Li, F.; Li, F. An intensive milk replacer feeding program benefits immune response and intestinal microbiota of lambs during weaning. BMC Vet. Res. 2018, 14, 366. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Meale, S.J.; Chaucheyras-Durand, F.; Berends, H.; Steele, M.A. From pre-to postweaning: Transformation of the young calf’s gastrointestinal tract. J. Dairy Sci. 2017, 100, 5984–5995. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. Invited review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef]
- Hickey, M.-C.; Drennan, M.; Earley, B. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J. Anim. Sci. 2003, 81, 2847–2855. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mamuad, L.L.; Yang, C.-J.; Kim, S.-H.; Ha, J.K.; Lee, W.-S.; Cho, K.-K.; Lee, S.-S. Hemato-biochemical and cortisol profile of Holstein growing-calves supplemented with vitamin C during summer season. Asian-Australas. J. Anim. Sci. 2012, 25, 361. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhang, C.; Chi, H.; Liu, C.; Li, A.; Yu, W. Postbiotics derived from Lactobacillus plantarum 1.0386 ameliorate lipopolysaccharide-induced tight junction injury via MicroRNA-200c-3p mediated activation of the MLCK-MLC pathway in Caco-2 cells. Food Funct. 2022, 13, 11008–11020. [Google Scholar] [CrossRef]
- Lázaro, Á.; Vila-Donat, P.; Manyes, L. Emerging mycotoxins and preventive strategies related to gut microbiota changes: Probiotics, prebiotics, and postbiotics–a systematic review. Food Funct. 2024, 15, 8998–9023. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Gao, Z.; Li, Q.; Qiu, X.; Wu, F.; Guan, T.; Cao, B.; Su, H. Effects of compound probiotics on growth performance, rumen fermentation, blood parameters, and health status of neonatal Holstein calves. J. Dairy Sci. 2022, 105, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Altindag, A. Hematological Parameters of Tench (Tinca tinca L.) after Acute and Chronic Exposure to Lethal and Sublethal Mercury Treatments. Bull. Environ. Contam. Toxicol. 2004, 73, 911–918. [Google Scholar] [CrossRef]
- Masmeijer, C.; Deprez, P.; van Leenen, K.; De Cremer, L.; Cox, E.; Devriendt, B.; Pardon, B. Arrival cortisol measurement in veal calves and its association with body weight, protein fractions, animal health and performance. Prev. Vet. Med. 2021, 187, 105251. [Google Scholar] [CrossRef] [PubMed]
- Peschle, C.; Sasso, G.; Mastroberardino, G.; Condorelli, M. The mechanism of endocrine influences on erythropoiesis. J. Lab. Clin. Med. 1971, 78, 20–29. [Google Scholar]
- Probo, M.; Veronesi, M.C. Clinical Scoring Systems in the Newborn Calf: An Overview. Animals 2022, 12, 3013. [Google Scholar] [CrossRef] [PubMed]
- Atata, J.; Esievo, K.; Adamu, S.; Abdulsalam, H.; Avazi, D.; Ajadi, A. Haemato-biochemical studies of dogs with haemorrhage-induced dehydration. Comp. Clin. Pathol. 2019, 28, 129–135. [Google Scholar] [CrossRef]
- Ayyat, M.S.; El-Nagar, H.A.; Wafa, W.M.; Abd El-Latif, K.M.; Mahgoub, S.; Al-Sagheer, A.A. Comparable evaluation of nutritional benefits of Lactobacillus plantarum and Bacillus toyonensis probiotic supplementation on growth, feed utilization, health, and fecal microbiota in pre-weaning male calves. Animals 2023, 13, 3422. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Paape, M.; Bannerman, D.; Zhao, X.; Lee, J.-W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef]
- Scapini, P.; Cassatella, M.A. Social networking of human neutrophils within the immune system. Blood J. Am. Soc. Hematol. 2014, 124, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Cai, N.; Zhou, Y.; Liu, Y.; Hu, J.; Li, Y.; Yi, S.; Song, W.; Kang, L.; He, H. Acute stress induces an inflammation dominated by innate immunity represented by neutrophils in mice. Front. Immunol. 2022, 13, 1014296. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M. Postbiotic L. plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, H.; He, Z.; Fischer-Tlustos, A.; Ma, T.; Steele, M.; Guan, L.L. Transcriptome analysis revealed that delaying first colostrum feeding postponed ileum immune system development of neonatal calves. Genomics 2021, 113, 4116–4125. [Google Scholar] [CrossRef]
- Kelly, D.; Coutts, A. Development of digestive and immunological function in neonates: Role of early nutrition. Livest. Prod. Sci. 2000, 66, 161–167. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Shuai, J.; Chen, J.; Zhang, Z.; Fang, W. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int. J. Food Microbiol. 2007, 115, 307–312. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Hayashi, H.; Maruyama, S.; Fukuoka, M.; Kozakai, T.; Nakajima, K.; Onaga, T.; Kato, S. Fatty acid-binding protein expression in the gastrointestinal tract of calves and cows. Anim. Sci. J. 2013, 84, 35–41. [Google Scholar] [CrossRef]
- Wang, G.; Bonkovsky, H.L.; de Lemos, A.; Burczynski, F.J. Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 2015, 56, 2238–2247. [Google Scholar] [CrossRef]
- Yan, J.; Gong, Y.; She, Y.-M.; Wang, G.; Roberts, M.S.; Burczynski, F.J. Molecular mechanism of recombinant liver fatty acid binding protein’s antioxidant activity. J. Lipid Res. 2009, 50, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Habashy, W.S.; Milfort, M.C.; Fuller, A.L.; Attia, Y.A.; Rekaya, R.; Aggrey, S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017, 61, 2111–2118. [Google Scholar] [CrossRef]
- Elbaz, A.M.; El-Sonousy, N.K.; Arafa, A.S.; Sallam, M.; Ateya, A.; Abdelhady, A.Y. Oregano essential oil and Bacillus subtilis role in enhancing broiler’s growth, stress indicators, intestinal integrity, and gene expression under high stocking density. Sci. Rep. 2024, 14, 25411. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, L.; Zhou, Y.; Tang, L.; Zeng, Z.; Wang, Q.; Zou, P.; Yu, D.; Li, W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021, 7, 829–840. [Google Scholar] [CrossRef]
- Byrne, G.; Ahmad-Villiers, S.; Du, Z.; McGregor, C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation 2018, 25, e12394. [Google Scholar] [CrossRef]
- CAPON, C.; MAES, E.; MICHALSKI, J.-C.; LEFFLER, H.; KIM, Y.S. Sda-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem. J. 2001, 358, 657–664. [Google Scholar] [CrossRef]
- Jiang, Q.; Palombo, V.; Sherlock, D.N.; Vailati-Riboni, M.; D’Andrea, M.; Yoon, I.; Loor, J.J. Alterations in ileal transcriptomics during an intestinal barrier challenge in lactating Holstein cows fed a Saccharomyces cerevisiae fermentation product identify potential regulatory processes. J. Anim. Sci. 2023, 101, skad277. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Marques, M.; Serrano, C.; Cardoso, A.R.; Salazar, R.; Seixas, S.; Amorim, A.; Azevedo, L.; Prata, M.J. GBA3: A polymorphic pseudogene in humans that experienced repeated gene loss during mammalian evolution. Sci. Rep. 2020, 10, 11565. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Yang, H.; Mao, X.; Wang, J.; Gao, W. Effect of Natural β-Glucosidase Inhibitors in Reducing Toxicity of Amygdalin in Persicae Semen. Phytother. Res. 2017, 31, 771–777. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, C.C.; Townsend, D.M.; Tew, K.D. Glutathione S-transferase polymorphisms: Cancer incidence and therapy. Oncogene 2006, 25, 1639–1648. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, J.-Z.; Singhal, S.S.; Saini, M.; Pandya, U.; Awasthi, S.; Awasthi, Y.C. Role of glutathione S-transferases in protection against lipid peroxidation: Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J. Biol. Chem. 2001, 276, 19220–19230. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Yang, Y.; Sharma, A.; Awasthi, S.; Awasthi, Y.C. Antioxidant role of glutathione S-transferases: Protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid. Redox Signal. 2004, 6, 289–300. [Google Scholar] [CrossRef]
- Romero, L.; Andrews, K.; Ng, L.; O’Rourke, K.; Maslen, A.; Kirby, G. Human GSTA1-1 reduces c-Jun N-terminal kinase signalling and apoptosis in Caco-2 cells. Biochem. J. 2006, 400, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ma, X.; Luo, X.; Zhang, Y.; He, Y.; Dai, Z.; Yang, Y.; Wu, G.; Wu, Z. l-Glutamine Attenuates Apoptosis in Porcine Enterocytes by Regulating Glutathione-Related Redox Homeostasis. J. Nutr. 2018, 148, 526–534. [Google Scholar] [CrossRef]
- Yu, J.; Li, D.; Jiang, H. Emerging role of ONECUT2 in tumors. Oncol. Lett. 2020, 20, 328. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Song, X.; Guo, R.; Zhao, W.; Liu, C.; Chen, X.; Song, Q.; Wu, B.; Deng, N. Targeting ONECUT2 inhibits tumor angiogenesis via down-regulating ZKSCAN3/VEGFA. Biochem. Pharmacol. 2024, 225, 116315. [Google Scholar] [CrossRef]
- Dusing, M.R.; Maier, E.A.; Aronow, B.J.; Wiginton, D.A. Onecut-2 knockout mice fail to thrive during early postnatal period and have altered patterns of gene expression in small intestine. Physiol. Genom. 2010, 42, 115–125. [Google Scholar] [CrossRef]
- Blake, J.A.; Ziman, M.R. Pax genes: Regulators of lineage specification and progenitor cell maintenance. Development 2014, 141, 737–751. [Google Scholar] [CrossRef]
- Delogu, A.; Schebesta, A.; Sun, Q.; Aschenbrenner, K.; Perlot, T.; Busslinger, M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity 2006, 24, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Calderón, L.; Schindler, K.; Malin, S.G.; Schebesta, A.; Sun, Q.; Schwickert, T.; Alberti, C.; Fischer, M.; Jaritz, M.; Tagoh, H.; et al. Pax5 regulates B cell immunity by promoting PI3K signaling via PTEN down-regulation. Sci. Immunol. 2021, 6, eabg5003. [Google Scholar] [CrossRef]
- Rodrigo, I.; Hill, R.E.; Balling, R.; Münsterberg, A.; Imai, K. Pax1 and Pax9 activate Bapx1 to induce chondrogenic differentiation in the sclerotome. Development 2003, 130, 473–482. [Google Scholar] [CrossRef]
- Borges, G.H.; Lins-Candeiro, C.L.; Henriques, I.V.; de Brito Junior, R.B.; Pithon, M.M.; Paranhos, L.R. Exploring the genetics, mechanisms, and therapeutic innovations in non-syndromic tooth agenesis. Morphologie 2025, 109, 100941. [Google Scholar] [CrossRef]
- Yu, M.; Wong, S.W.; Han, D.; Cai, T. Genetic analysis: Wnt and other pathways in nonsyndromic tooth agenesis. Oral Dis. 2019, 25, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB signaling pathway during inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef]
- Bovo, S.; Ribani, A.; Schiavo, G.; Taurisano, V.; Bertolini, F.; Fornasini, D.; Frabetti, A.; Fontanesi, L. Genome-wide association studies for diarrhoea outcomes identified genomic regions affecting resistance to a severe enteropathy in suckling rabbits. J. Anim. Breed. Genet. 2024, 141, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.p.; Jiang, Y.f.; Liu, J.w.; Tian, C.j.; Lin, Y.z.; Yang, Y.z.; Zhang, Z.k.; Fang, Y.l.; Huang, B.; Lin, H. Fc receptor-like A promotes malignant behavior in renal cell carcinoma and correlates with tumor immune infiltration. Cancer Med. 2024, 13, e70072. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.W.; Krueger, P.D.; Davis, R.S.; Pierce, S.K. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood J. Am. Soc. Hematol. 2011, 118, 6332–6341. [Google Scholar] [CrossRef]
- Mahata, S.K.; Ying, W.; Muntjewerff, E.M.; Mahata, S.; Nicolasen, M.J.; Das, S.; Bandyopadhyay, G.K.; van den Bogaart, G. Catestatin Improves Insulin Sensitivity by Promoting M1-M2 Polarization and Inhibiting Obesity-Induced Macrophage Infiltration and Gluconeogenesis in Liver. FASEB J. 2019, 33, 834.13. [Google Scholar] [CrossRef]
- Ferguson, C.S.; Tyndale, R.F. Cytochrome P450 enzymes in the brain: Emerging evidence of biological significance. Trends Pharmacol. Sci. 2011, 32, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Antioxidant, anti-inflammatory and immunomodulatory roles of vitamins in COVID-19 therapy. Eur. J. Med. Chem. 2022, 232, 114175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shang, T.; Huang, Y.; Wang, S.; Liu, H.; Wang, J.; Wang, Y.; Ji, H.; Zhang, R. Gene expression profile changes in the jejunum of weaned piglets after oral administration of Lactobacillus or an antibiotic. Sci. Rep. 2017, 7, 15816. [Google Scholar] [CrossRef]

| Time 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 76 | Day 83 | Day 90 | p Value 2 | |||||||
| Item 3 | CON | DCLI | CON | DCLI | CON | DCLI | SEM | Group | Time | G × T |
| Albumin, g/dL | 2.79 | 2.87 | 2.91 | 2.97 | 3.14 | 3.34 | 0.069 | 0.433 | 0.078 | 0.299 |
| Globulin, g/dL | 3.31 | 2.76 | 3.61 | 3.01 | 3.87 | 3.37 | 0.087 | 0.008 | 0.006 | 0.596 |
| AGR | 0.85 | 1.04 | 0.81 | 0.99 | 0.82 | 1.01 | 0.029 | 0.027 | 0.091 | 0.921 |
| Total protein, g/dL | 6.10 | 5.53 | 6.53 | 5.63 | 7.01 | 6.71 | 0.117 | 0.027 | 0.002 | 0.252 |
| Time 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 76 | Day 83 | Day 90 | p Value 3 | |||||||
| Item 1 | CON | DCLI | CON | DCLI | CON | DCLI | SEM | Group | Time | G × T |
| RBC, ×106 cells/mm3 | 8.13 | 8.19 | 8.64 | 8.26 | 8.43 | 8.21 | 0.222 | 0.723 | 0.563 | 0.213 |
| HGB, g/dL | 7.77 | 7.89 | 9.07 | 9.26 | 8.74 | 8.90 | 0.230 | 0.740 | 0.036 | 0.930 |
| HCT, % | 24.43 | 25.71 | 27.43 | 28.57 | 27.57 | 27.36 | 0.612 | 0.555 | 0.137 | 0.248 |
| WBC, ×103 cells/mm3 | 10.23 | 7.70 | 11.29 | 8.50 | 12.44 | 9.77 | 0.385 | 0.006 | 0.155 | 0.938 |
| Neu, ×103 cells/mm3 | 3.31 | 1.95 | 4.40 | 2.59 | 5.11 | 3.10 | 0.243 | 0.003 | 0.192 | 0.411 |
| Lymp, ×103 cells/mm3 | 6.69 | 5.44 | 6.37 | 5.63 | 6.65 | 5.96 | 0.226 | 0.126 | 0.614 | 0.370 |
| NLR | 0.54 | 0.36 | 0.69 | 0.46 | 0.77 | 0.51 | 0.036 | 0.009 | 0.686 | 0.605 |
| Sample Name | Raw Reads (Million) | Clean Reads (Million) | GC Content (%) | Clean Reads Q30 (%) 1 | Total Mapping (%) |
|---|---|---|---|---|---|
| CON1 | 45.44 | 43.87 | 52.10 | 95.88 | 97.27 |
| CON2 | 47.19 | 45.17 | 50.95 | 96.12 | 97.22 |
| CON3 | 45.44 | 44.38 | 50.27 | 95.93 | 97.69 |
| CON4 | 47.19 | 44.57 | 50.67 | 96.22 | 98.04 |
| DCLI1 | 47.19 | 45.15 | 50.44 | 96.06 | 97.11 |
| DCLI2 | 45.44 | 44.30 | 49.26 | 95.87 | 98.01 |
| DCLI3 | 45.44 | 44.08 | 49.78 | 95.74 | 97.67 |
| DCLI4 | 45.44 | 43.90 | 50.68 | 95.70 | 97.23 |
| Average | 46.10 | 44.43 | 50.52 | 95.94 | 97.53 |
| Gene ID | Symbol 1 | Log2 Fold Change | Q Value 2 | Regulated |
|---|---|---|---|---|
| 508108 | B4GALNT2 | 9.7874 | 1.55 × 10−3 | Up |
| 327700 | FABP1 | 7.7692 | 3.97 × 10−3 | Up |
| 539625 | GBA3 | 5.8228 | 1.55 × 10−3 | Up |
| 786706 | - | 4.1697 | 3.61 × 10−2 | Up |
| 777644 | GSTA1 | 3.9776 | 5.49 × 10−4 | Up |
| 539937 | ARL14 | 3.8660 | 5.44 × 10−8 | Up |
| 782542 | ONECUT2 | 3.6487 | 1.83 × 10−2 | Up |
| 511869 | TM4SF5 | 3.2932 | 7.12 × 10−3 | Up |
| 505865 | FOLH1B | 2.8627 | 2.54 × 10−2 | Up |
| 538670 | FAM151A | 2.7599 | 4.83 × 10−2 | Up |
| 414732 | GATM | 2.3843 | 4.97 × 10−3 | Up |
| 525682 | NOTUM | 2.3823 | 5.44 × 10−8 | Up |
| 786760 | BTN3A3 | 2.2747 | 2.13 × 10−2 | Up |
| 514667 | MST1 | 2.2436 | 4.08 × 10−2 | Up |
| 100336768 | ROS1 | 2.1404 | 4.85 × 10−2 | Up |
| 407225 | MOGAT1 | 2.0725 | 3.49 × 10−2 | Up |
| 511097 | SLC46A1 | 2.0696 | 7.13 × 10−5 | Up |
| 100300004 | GLTPD2 | 2.0471 | 1.55 × 10−3 | Up |
| 282605 | FAM13A | 2.0424 | 1.92 × 10−2 | Up |
| 513137 | TMEM72 | 2.0322 | 3.76 × 10−2 | Up |
| 540196 | PAX9 | −23.4653 | 5.44 × 10−8 | Down |
| 112444345 | - | −22.6113 | 1.07 × 10−7 | Down |
| 112446673 | - | −21.2060 | 1.37 × 10−6 | Down |
| 538371 | PAX5 | −9.2089 | 6.98 × 10−3 | Down |
| 534753 | FCRL4 | −8.6701 | 1.83 × 10−2 | Down |
| 782871 | FCRLA | −7.8139 | 2.45 × 10−2 | Down |
| 531420 | GP2 | −7.6012 | 4.85 × 10−2 | Down |
| 504258 | SIGLEC10 | −6.8829 | 2.20 × 10−2 | Down |
| 407126 | CR2 | −6.7550 | 4.18 × 10−2 | Down |
| 107131854 | - | −6.5830 | 4.60 × 10−2 | Down |
| 408008 | KCNN1 | −5.9122 | 3.30 × 10−2 | Down |
| 512439 | HBA | −5.7393 | 2.97 × 10−2 | Down |
| 112445446 | - | −5.5555 | 5.03 × 10−3 | Down |
| 493988 | SLC14A1 | −4.8759 | 4.67 × 10−3 | Down |
| 101908107 | P2RY8 | −4.6693 | 2.97 × 10−2 | Down |
| 515911 | STRA6 | −4.4179 | 3.68 × 10−2 | Down |
| 782922 | - | −4.3880 | 5.88 × 10−3 | Down |
| 616320 | SLC9B2 | −4.1830 | 4.18 × 10−2 | Down |
| 100296105 | - | −4.1574 | 3.68 × 10−2 | Down |
| 506550 | TSPAN1 | −4.0508 | 1.16 × 10−2 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, C.; Srisaikham, S.; Tian, X.; Lounglawan, P. Effect of Dead-Cell Limosilactobacillus ingluviei on Hematological Parameters and Jejunal Transcriptome Profile in Calves During the Weaning Period. Animals 2025, 15, 1905. https://doi.org/10.3390/ani15131905
Ban C, Srisaikham S, Tian X, Lounglawan P. Effect of Dead-Cell Limosilactobacillus ingluviei on Hematological Parameters and Jejunal Transcriptome Profile in Calves During the Weaning Period. Animals. 2025; 15(13):1905. https://doi.org/10.3390/ani15131905
Chicago/Turabian StyleBan, Chao, Supreena Srisaikham, Xingzhou Tian, and Pipat Lounglawan. 2025. "Effect of Dead-Cell Limosilactobacillus ingluviei on Hematological Parameters and Jejunal Transcriptome Profile in Calves During the Weaning Period" Animals 15, no. 13: 1905. https://doi.org/10.3390/ani15131905
APA StyleBan, C., Srisaikham, S., Tian, X., & Lounglawan, P. (2025). Effect of Dead-Cell Limosilactobacillus ingluviei on Hematological Parameters and Jejunal Transcriptome Profile in Calves During the Weaning Period. Animals, 15(13), 1905. https://doi.org/10.3390/ani15131905








