Factor Analysis of Semen Quality in Chicken and Its Impact on Fertility
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection and Quality Assessment
2.3. Fertility Analysis of Beijing-You Roosters
2.4. Statistical Analysis
3. Results
3.1. Analysis of Semen Quality Traits of Seven Breeds
3.2. Correlation Analysis of Semen Quality Traits
3.3. Factor Analysis of Semen Quality Traits
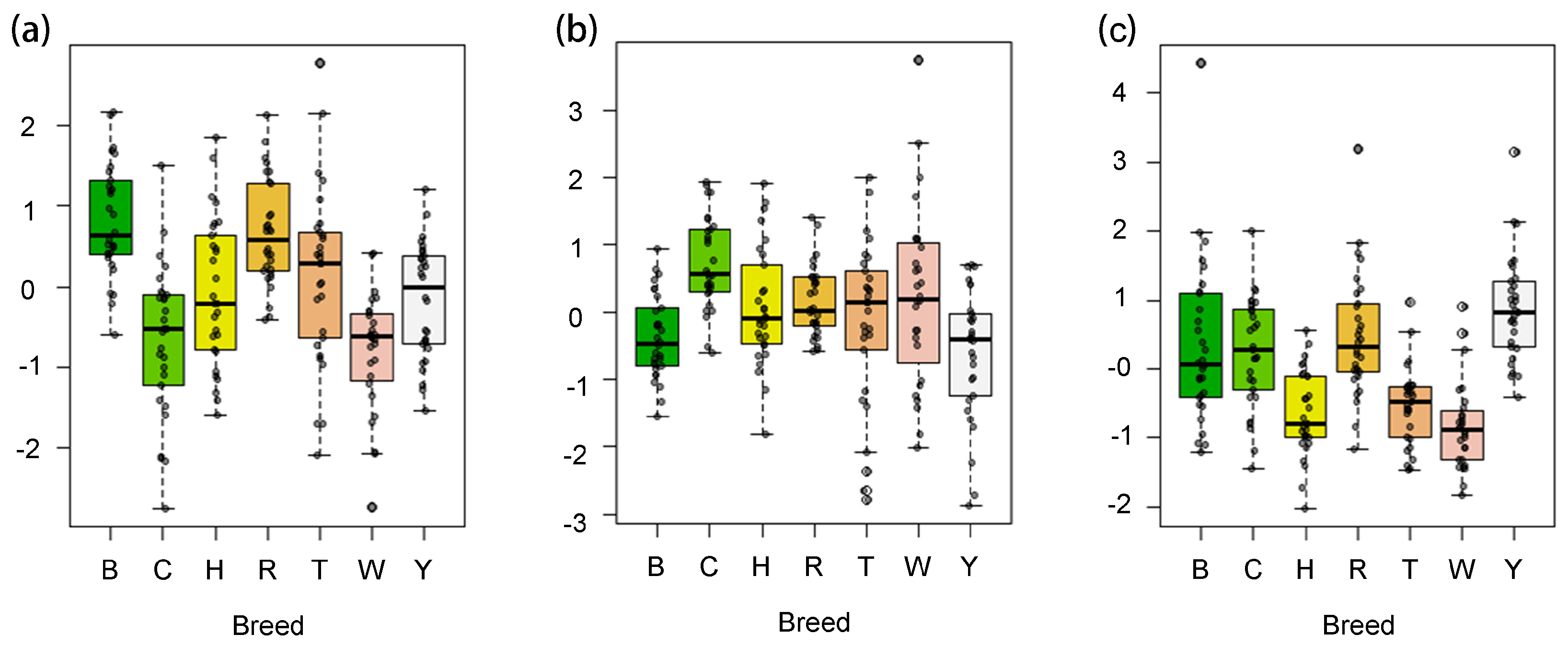
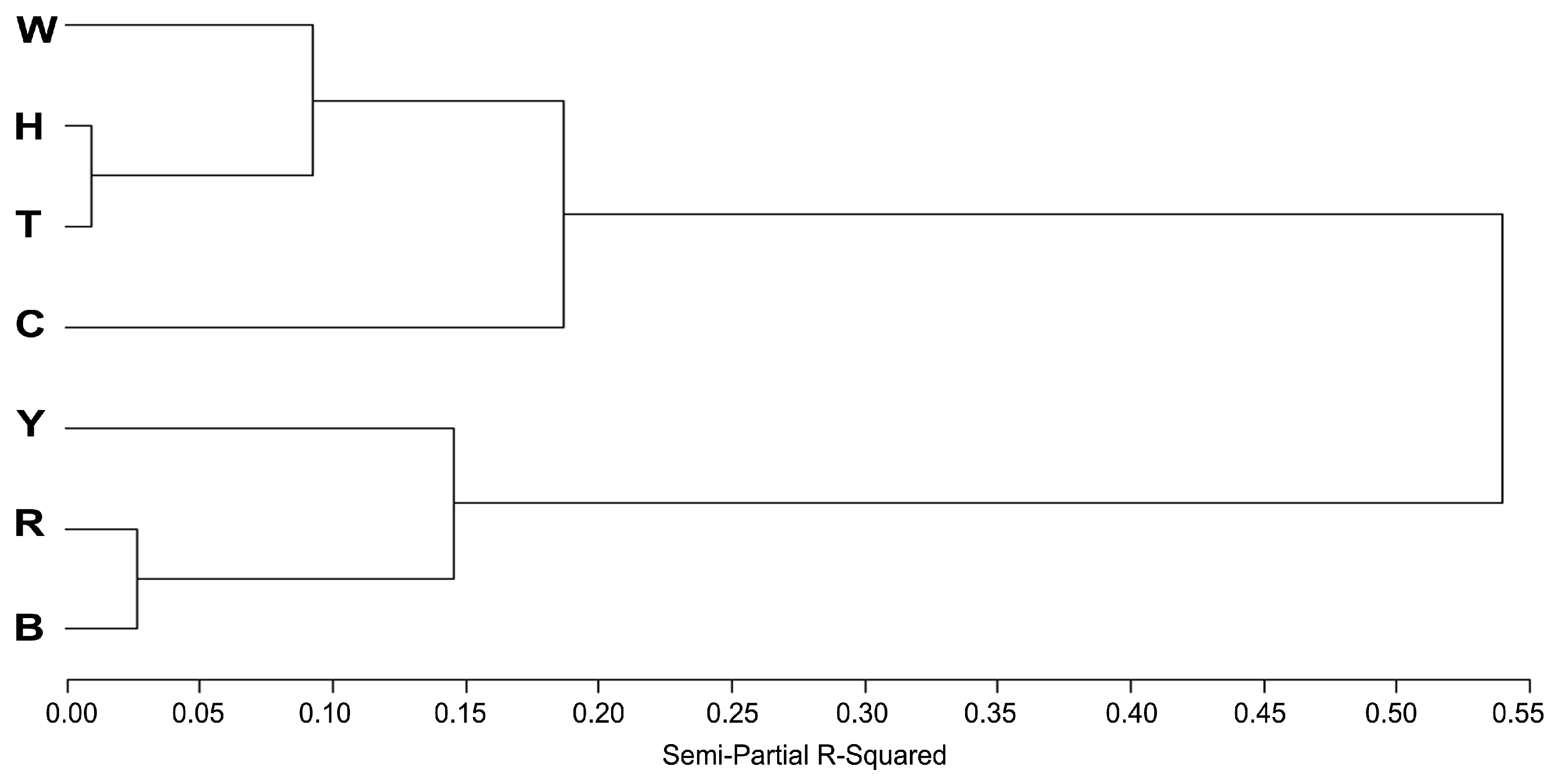
3.4. Analysis of Semen Factor Scores Among Seven Breeds
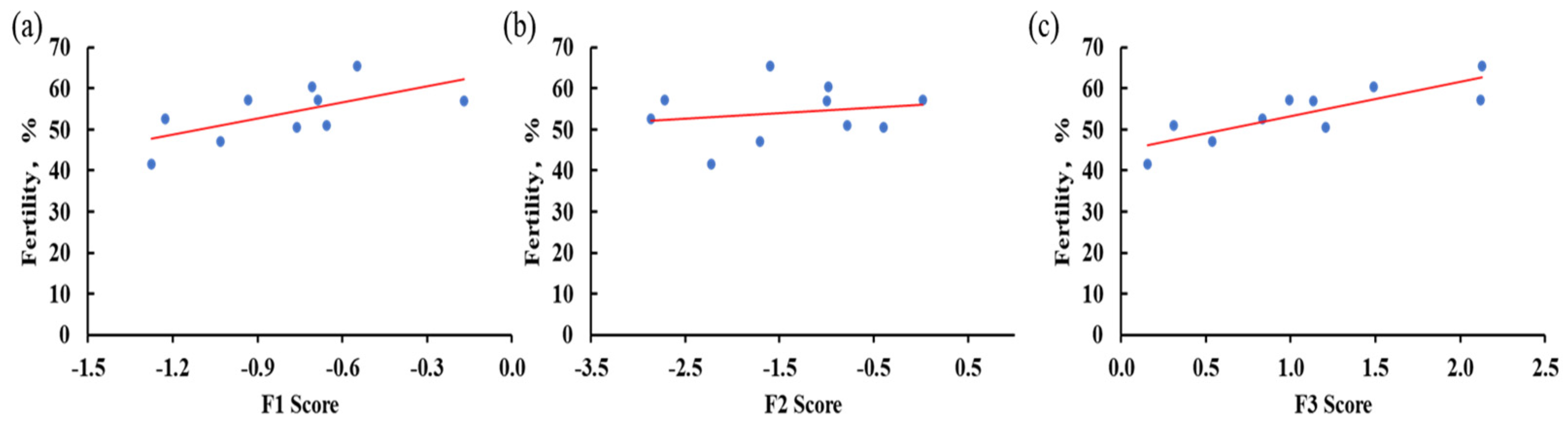
3.5. Correlation Among Fertility, Semen Quality Traits, and Their Factor Scores in Beijing-You
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial insemination |
| CASA | computer-aided sperm analysis |
| VOL | semen volume |
| CON | sperm concentration |
| MOT | sperm motility |
| TSC | total sperm count |
| ESC | effective sperm count |
| VCL | curvilinear velocity |
| VSL | straight-line velocity |
| VAP | average path velocity |
| LIN | linearity |
| WOB | wobble |
| STR | straightness |
| ALH | amplitude of lateral head |
| BCF | beat cross frequency |
| PCA | principal components analysis |
| FA | factor analysis |
| ANOVA | one-way analysis of variance |
| KMO | Kaiser–Meyer–Olkin |
References
- Dhama, K.; Singh, R.P.; Karthik, K.; Chakrabort, S.; Tiwari, R.; Wani, M.Y.; Mohan, J. Artificial insemination in poultry and possible transmission of infectious pathogens: A review. Asian J. Anim. Vet. Adv. 2014, 9, 211–228. [Google Scholar] [CrossRef]
- Mohan, J.; Sharma, S.K.; Kolluri, G.; Dhama, K. History of artificial insemination in poultry, its components and significance. World’s Poult. Sci. J. 2018, 74, 475–488. [Google Scholar] [CrossRef]
- Mortimer, S.; Horst, G.D.; Mortimer, D. The future of computer-aided sperm analysis. Asian J. Androl. 2015, 17, 545. [Google Scholar] [CrossRef]
- Sayed, M.; Abouelezz, F.; Abdel-Wahab, A.A. Analysis of sperm motility, velocity and morphometry of three egyptian indigenous chicken strains. Egypt. Poult. Sci. J. 2017, 37, 1173–1185. [Google Scholar]
- Agarwal, A.; Sharma, R.K.; Nelson, D.R. New semen quality scores developed by principal component analysis of semen characteristics. J. Androl. 2003, 24, 343–352. [Google Scholar] [CrossRef]
- Baldassini, W.A.; Chardulo, L.; Silva, J.; Malheiros, J.M.; Dias, V.; Espigolan, R.; Baldi, F.S.; Albuquerque, L.G.; Fernandes, T.T.; Padilha, M. Meat quality traits of nellore bulls according to different degrees of backfat thickness: A multivariate approach. Anim. Prod. Sci. 2016, 57, 363–370. [Google Scholar] [CrossRef]
- Patel, N.; Bergamaschi, M.; Magro, L.; Petrini, A.; Bittante, G. Relationships of a detailed mineral profile of meat with animal performance and beef quality. Animals 2019, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Markos, S.; Belay, B.; Dessie, T. Morphometric differentiation of three chicken ecotypes of ethiopia using multivariate analysis. PLoS ONE 2024, 19, e295134. [Google Scholar] [CrossRef]
- Budaev, S.V. Using principal components and factor analysis in animal behaviour research: Caveats and guidelines. Ethology 2010, 116, 472–480. [Google Scholar] [CrossRef]
- Martinez-Pastor, F.; Tizado, E.J.; Garde, J.J.; Anel, L.; de Paz, P. Statistical series: Opportunities and challenges of sperm motility subpopulation analysis. Theriogenology 2011, 75, 783–795. [Google Scholar] [CrossRef]
- Garcia-Herreros, M. Sperm subpopulations in avian species: A comparative study between the rooster (gallus domesticus) and guinea fowl (numida meleagris). Asian J. Androl. 2016, 18, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Gacem, S.; Valverde, A.; Catalan, J.; Yanez Ortiz, I.; Soler, C.; Miro, J. A new approach of sperm motility subpopulation structure in donkey and horse. Front. Vet. Sci. 2021, 8, 651477. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.N.; Morán, J.M.; Peña, F.J. Two-step cluster procedure after principal component analysis identifies sperm subpopulations in canine ejaculates and its relation to cryoresistance. J. Androl. 2006, 27, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.B.; Crispín, R.H.; Mendoza, M.M.; Gallegos, O.H.; Martínez, A.A. Use of multivariate statistics to identify unreliable data obtained using casa. Syst. Biol. Reprod. Med. 2013, 59, 164–171. [Google Scholar] [CrossRef]
- Joliffe, I.T.; Morgan, B.J. Principal component analysis and exploratory factor analysis. Stat. Methods Med. Res. 1992, 1, 69–95. [Google Scholar] [CrossRef]
- Burrows, W.H.; Quinn, J.P. The collection of spermatozoa from the domestic fowl and turkey. Poult. Sci. 1937, 16, 19–24. [Google Scholar] [CrossRef]
- W.H.O. Who laboratory manual for the examination and processing of human semen. In WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Kirby, J.D.; Froman, D.P. Analysis of poultry fertility data. Poult. Sci. 1990, 69, 1764–1768. [Google Scholar] [CrossRef]
- King, L.M.; Holsberger, D.R.; Donoghue, A.M. Correlation of casa velocity and linearity parameters with sperm mobility phenotype in turkeys. J. Androl. 2000, 21, 65–71. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain variations in pig fertility1. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef]
- Simonik, O.; Sichtar, J.; Krejcarkova, A.; Rajmon, R.; Stadnik, L.; Beran, J.; Dolezalova, M.; Biniova, Z. Computer assisted sperm analysis the relationship to bull field fertility, possible errors and their impact on outputs: A review. Indian J. Anim. Sci. 2015, 85, 3–11. [Google Scholar] [CrossRef]
- Peters, S.O.; Shoyebo, O.D.; Ilori, B.M.; Ozoje, M.O.; Ikeobi, C.; Adebambo, O.A. Semen quality traits of seven strains of chickens raised in the humid tropics. Int. J. Poult. Sci. 2008, 7, 949–953. [Google Scholar] [CrossRef]
- Alquezar-Baeta, C.; Gimeno-Martos, S.; Miguel-Jimenez, S.; Santolaria, P.; Yaniz, J.; Palacin, I.; Casao, A.; Cebrian-Perez, J.A.; Muino-Blanco, T.; Perez-Pe, R. Opencasa: A new open-source and scalable tool for sperm quality analysis. PLoS Comput. Biol. 2019, 15, e1006691. [Google Scholar] [CrossRef] [PubMed]
- Jambui, M.; Honaker, C.F.; Siegel, P.B. Correlated responses to long-term divergent selection for 8-week body weight in female white plymouth rock chickens: Sexual maturity. Poult. Sci. 2017, 96, 3844–3851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Li, Y.; Bai, H.; Xue, F.; Xu, S.; Xu, H.; Shi, L.; Yang, N.; Chen, J. Analyses of long non-coding rna and mrna profiling using rna sequencing in chicken testis with extreme sperm motility. Sci. Rep. 2017, 7, 9055. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Li, Y.; Bai, H.; Xu, S.; Xu, H.; Ni, A.; Yang, N.; Chen, J. Identification and differential expression of micrornas in the testis of chicken with high and low sperm motility. Theriogenology 2018, 122, 94–101. [Google Scholar] [CrossRef]
- Amin, A.M.S.; Salem, M.M.I.; Ashour, A.F.; EL Nagar, A.G. Principal component analysis of phenotypic and breeding value data for semen traits in egyptian buffalo bulls. Trop. Anim. Health Prod. 2024, 56, 135. [Google Scholar] [CrossRef]
- Wang, N.; Song, M.; Gu, H.; Gao, Y.; Yu, G.; Lv, F.; Shi, C.; Wang, S.; Sun, L.; Xiao, Y.; et al. A factor analysis model for rapid evaluation of the semen quality of fertile men in china. J. Multidiscip. Healthc. 2022, 15, 431–441. [Google Scholar] [CrossRef]
- Tabatabaei, S. The effect of spermatozoa number on fertility rate of chicken in artificial insemination programs. J. Anim. Vet. Adv. 2010, 9, 1717–1719. [Google Scholar] [CrossRef]
- Tanga, B.M.; Qamar, A.Y.; Raza, S.; Bang, S.; Fang, X.; Yoon, K.; Cho, J. Semen evaluation: Methodological advancements in sperm quality-specific fertility assessment-a review. Anim. Biosci. 2021, 34, 1253–1270. [Google Scholar] [CrossRef]
- Tamargo, C.; Garriga, F.; Yeste, M.; Pinart, E.; Muiño, R.; Carbajo, M.T.; Hidalgo, C.O. Predictive indicators of cryotolerance and fertility in bovine sperm: Evaluating fresh semen quality to improveai outcomes with frozen–thawed sperm. Reprod. Domest. Anim. 2024, 59, e14742. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; García, W.; Tabarez, A.; Palomo, M.J. The contribution of different sperm parameters to better explain ram semen cryoresistance. Vet. J. 2024, 306, 106194. [Google Scholar] [CrossRef] [PubMed]
- Mavi, G.K.; Dubey, P.P.; Cheema, R.S.; Dash, S.K.; Bansal, B.K. Comparative analysis of semen quality parameters and their relationship with fertility in different genetic groups of layer chicken. Indian J. Anim. Res. 2019, 53, 1269–1274. [Google Scholar] [CrossRef]
- Tesfay, H.H.; Sun, Y.; Li, Y.; Shi, L.; Fan, J.; Wang, P.; Zong, Y.; Ni, A.; Ma, H.; Mani, A.I.; et al. Comparative studies of semen quality traits and sperm kinematic parameters in relation to fertility rate between 2 genetic groups of breed lines. Poult. Sci. 2020, 99, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, H.; Obara, H.; Ayustawati; Hirano, Y.; Suzuki, T.; Ohno, A.; Takamizawa, S.; Suzuki, M. Prediction of pregnancy by intrauterine insemination using casa estimates and strict criteria in patients with male factor infertility. Int. J. Androl. 2004, 27, 63–68. [Google Scholar] [CrossRef]
- Inanç, M.E.; Cil, B.; Tekin, K.; Alemdar, H.; Daşkin, A. The combination of casa kinetic parameters and fluorescein staining as a fertility tool in cryopreserved bull semen. Turk. J. Vet. Anim. Sci. 2018, 42, 452–458. [Google Scholar] [CrossRef]

| Traits | B | C | R | W | H | T | Y | Pooled-SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| VOL, μL | 465.67 b | 305.41 c | 467.00 b | 216.79 d | 268.97 cd | 290.69 cd | 558.47 a | 76.06 | <0.01 |
| CON, 108/mL | 18.70 c | 28.82 a | 22.18 bc | 18.50 c | 21.41 bc | 22.91 b | 24.10 b | 3.58 | <0.01 |
| MOT, % | 69.21 a | 60.27 ab | 69.62 a | 48.67 cd | 42.64 d | 43.15 d | 57.85 bc | 9.46 | <0.01 |
| TSC, ×108 | 8.97 b | 8.92 b | 10.15 b | 4.07 c | 6.08 c | 6.42 c | 13.47 a | 2.28 | <0.01 |
| ESC, ×108 | 6.40 a | 5.57 a | 7.29 a | 2.08 b | 2.59 b | 2.74 b | 7.45 a | 1.71 | <0.01 |
| pH | 7.10 cd | 7.08 cd | 7.20 bc | 7.52 a | 7.36 ab | 7.30 b | 6.99 d | 0.18 | <0.01 |
| VCL, μm/s | 52.09 b | 60.11 a | 56.24 a | 56.80 a | 59.01 a | 57.03 a | 50.79 b | 3.40 | <0.01 |
| VSL, μm/s | 28.39 a | 24.12 cd | 29.32 a | 22.38 d | 25.98 bc | 27.48 ab | 24.28 cd | 2.00 | <0.01 |
| VAP, μm/s | 39.52 b | 54.22 a | 43.74 b | 51.27 a | 41.78 b | 40.33 b | 40.23 b | 3.72 | <0.01 |
| ALH, μm | 5.09 b | 5.81 a | 5.49 a | 5.55 a | 5.76 a | 5.57 a | 4.96 b | 0.33 | <0.01 |
| BCF, Hz | 2.32 c | 2.51 bc | 2.33 c | 2.77 ab | 2.70 ab | 2.87 a | 2.56 abc | 0.92 | <0.01 |
| LIN, % | 54.56 a | 41.09 c | 52.20 a | 40.27 c | 44.84 b | 48.03 b | 47.84 b | 3.26 | <0.01 |
| WOB, % | 75.87 c | 89.68 a | 77.78 c | 89.35 a | 79.11 bc | 78.97 bc | 83.98 b | 4.20 | <0.01 |
| STR, % | 73.01 a | 46.61 d | 68.22 ab | 45.66 d | 60.16 c | 67.92 b | 61.36 c | 5.85 | <0.01 |
| Factors | Unrotated | ||
|---|---|---|---|
| Eigenvalue | Percentage of Variance, % | Cumulative Percentage of Variance, % | |
| Factor 1 | 4.39 | 31.34 | 31.34 |
| Factor 2 | 3.26 | 23.28 | 54.62 |
| Factor 3 | 2.97 | 21.19 | 75.82 |
| Traits | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| VSL | 0.92 | 0.34 | −0.03 |
| LIN | 0.88 | −0.32 | 0.06 |
| STR | 0.82 | −0.33 | −0.03 |
| MOT | 0.51 | 0.35 | 0.47 |
| WOB | −0.82 | 0.30 | 0.22 |
| BCF | −0.58 | −0.47 | −0.23 |
| VCL | 0.00 | 0.93 | −0.17 |
| VAP | −0.34 | 0.86 | 0.00 |
| ALH | 0.03 | 0.93 | −0.16 |
| TSC | −0.17 | −0.11 | 0.94 |
| ESC | 0.10 | 0.11 | 0.94 |
| VOL | 0.06 | −0.27 | 0.78 |
| CON | −0.38 | 0.24 | 0.56 |
| pH | −0.05 | 0.13 | −0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, Y.; Ni, A.; Tesfay, H.H.; Isa, A.M.; Zong, Y.; Ma, H.; Yuan, J.; Chen, J. Factor Analysis of Semen Quality in Chicken and Its Impact on Fertility. Animals 2025, 15, 1906. https://doi.org/10.3390/ani15131906
Li Y, Sun Y, Ni A, Tesfay HH, Isa AM, Zong Y, Ma H, Yuan J, Chen J. Factor Analysis of Semen Quality in Chicken and Its Impact on Fertility. Animals. 2025; 15(13):1906. https://doi.org/10.3390/ani15131906
Chicago/Turabian StyleLi, Yunlei, Yanyan Sun, Aixin Ni, Hailai Hagos Tesfay, Adamu Mani Isa, Yunhe Zong, Hui Ma, Jingwei Yuan, and Jilan Chen. 2025. "Factor Analysis of Semen Quality in Chicken and Its Impact on Fertility" Animals 15, no. 13: 1906. https://doi.org/10.3390/ani15131906
APA StyleLi, Y., Sun, Y., Ni, A., Tesfay, H. H., Isa, A. M., Zong, Y., Ma, H., Yuan, J., & Chen, J. (2025). Factor Analysis of Semen Quality in Chicken and Its Impact on Fertility. Animals, 15(13), 1906. https://doi.org/10.3390/ani15131906







