Livestock Animal Hair as an Indicator of Environmental Heavy Metals Pollution in Central Albania
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
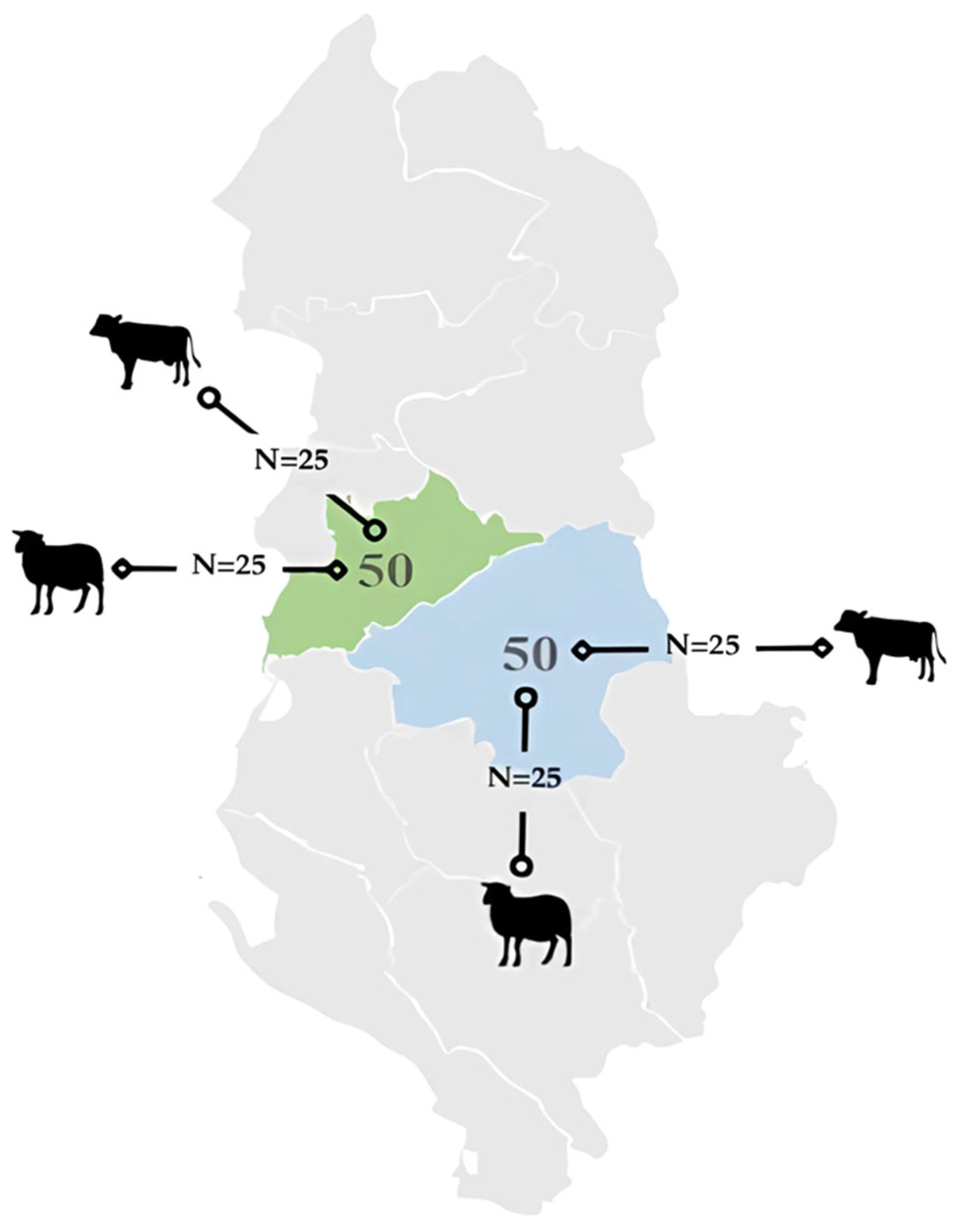
2.1. Study Area and Animals
2.2. Hair Collection and Analysis
2.3. Statistical Analysis
3. Results
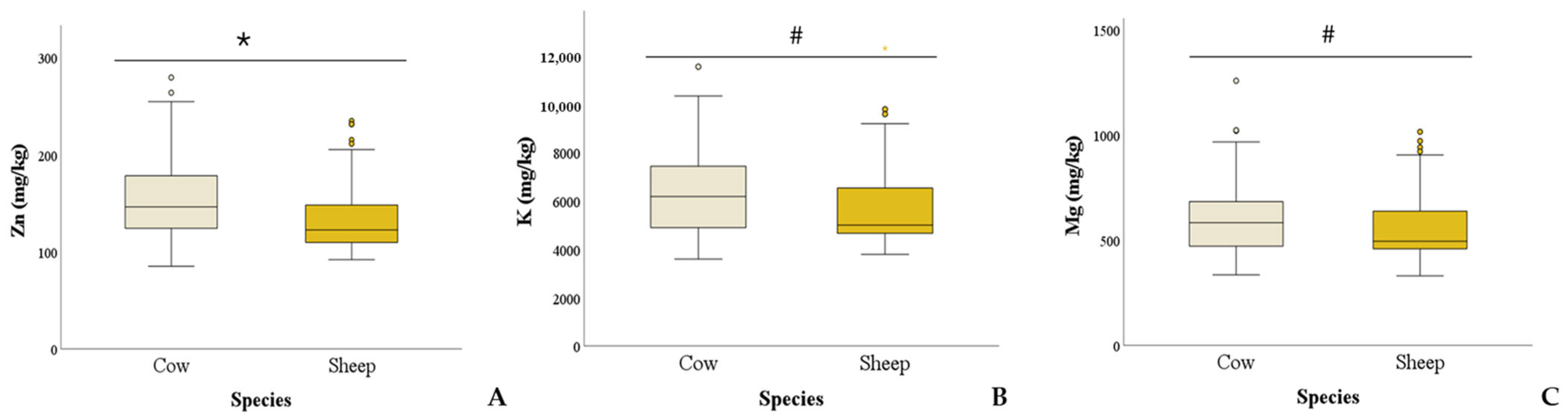
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malmsten, A.; Dalin, A.-M.; Pettersson, J.; Persson, S. Concentrations of Cadmium, Lead, Arsenic, and Some Essential Metals in Wild Boar from Sweden. Eur. J. Wildl. Res. 2021, 67, 18. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Tadayon, F.; Jamshidi, R.; Tadayon, A.; Ostovar, R. Quantification of Toxic Elements in Tail Hair of Cows as an Indicator of Environmental Exposure in Different Areas from Iran. E3S Web Conf. 2013, 1, 41029. [Google Scholar] [CrossRef]
- Dasharathy, S.; Arjunan, S.; Maliyur Basavaraju, A.; Murugasen, V.; Ramachandran, S.; Keshav, R.; Murugan, R. Mutagenic, Carcinogenic, and Teratogenic Effect of Heavy Metals. Evid.-Based Complement. Altern. Med. 2022, 2022, 8011953. [Google Scholar] [CrossRef] [PubMed]
- Bíreš, J.; Dianovský, J.; Bartko, P.; Juhásová, Z. Effects on Enzymes and the Genetic Apparatus of Sheep after Administration of Samples from Industrial Emissions. Biometals 1995, 8, 53–58. [Google Scholar] [CrossRef]
- Tahir, I.; Alkheraije, K.A. A Review of Important Heavy Metals Toxicity with Special Emphasis on Nephrotoxicity and Its Management in Cattle. Front. Vet. Sci. 2023, 10, 1149720. [Google Scholar] [CrossRef]
- Rhind, S.M.; Evans, N.P.; Bellingham, M.; Sharpe, R.M.; Cotinot, C.; Mandon-Pepin, B.; Loup, B.; Sinclair, K.D.; Lea, R.G.; Pocar, P.; et al. Effects of Environmental Pollutants on the Reproduction and Welfare of Ruminants. Animal 2010, 4, 1227–1239. [Google Scholar] [CrossRef][Green Version]
- Patra, R.C.; Swarup, D.; Naresh, R.; Kumar, P.; Nandi, D.; Shekhar, P.; Roy, S.; Ali, S.L. Tail Hair as an Indicator of Environmental Exposure of Cows to Lead and Cadmium in Different Industrial Areas. Ecotoxicol. Environ. Saf. 2007, 66, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Gugliandolo, E.; Nava, V.; Piccione, G.; Giannetto, C.; Licata, P. Bioaccumulation of Mineral Elements in Different Biological Substrates of Athletic Horse from Messina, Italy. Animals 2020, 10, 1877. [Google Scholar] [CrossRef]
- Miroshnikov, S.; Zavyalov, O.; Frolov, A.; Sleptsov, I.; Sirazetdinov, F.; Poberukhin, M. The Content of Toxic Elements in Hair of Dairy Cows as an Indicator of Productivity and Elemental Status of Animals. Environ. Sci. Pollut. Res. 2019, 26, 18554–18564. [Google Scholar] [CrossRef]
- Salih, Z.; Aziz, F. Heavy Metal Accumulation in Dust andWorkers’ Scalp Hair as a Bioindicator forAir Pollution from a Steel Factory. Pol. J. Environ. Stud. 2020, 29, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Agradi, S.; Munga, A.; Barbato, O.; Palme, R.; Tarhan, D.; Bilgiç, B.; Dokuzeylül, B.; Ercan, A.M.; Or, M.E.; Brecchia, G.; et al. Goat Hair as a Bioindicator of Environmental Contaminants and Adrenal Activation during Vertical Transhumance. Front. Vet. Sci. 2023, 10, 1274081. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N.; Soltan, M.E. Animal Hair as Biological Indicator for Heavy Metal Pollution in Urban and Rural Areas. Environ. Monit. Assess. 2005, 110, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Draghi, S.; Agradi, S.; Riva, F.; Tarhan, D.; Bilgiç, B.; Dokuzeylül, B.; Ercan, A.M.; Or, M.E.; Brecchia, G.; Vigo, D.; et al. Roe Deer (Capreolus Capreolus) Hair as a Bioindicator for the Environmental Presence of Toxic and Trace Elements. Toxics 2023, 11, 49. [Google Scholar] [CrossRef]
- Shallari, S.; Schwartz, C.; Hasko, A.; Morel, J.L. Heavy Metals in Soils and Plants of Serpentine and Industrial Sites of Albania. Sci. Total Environ. 1998, 209, 133–142. [Google Scholar] [CrossRef]
- Gjoka, F.; Duering, R.-A.; Siemens, J. Background Concentrations and Spatial Distribution of Heavy Metals in Albania’s Soils. Environ. Monit. Assess. 2022, 194, 115. [Google Scholar] [CrossRef] [PubMed]
- Ozuni, E.; Andoni, E.; Castrica, M.; Balzaretti, C.M.; Brecchia, G.; Agradi, S.; Curone, G.; Di Cesare, F.; Fehri, N.E.; Luke, B.; et al. Human Exposure to Heavy Metals and Possible Public Health Risks via Consumption of Mussels M. Galloprovincialis from the Albanian Sea Cost. Chemosphere 2024, 368, 143689. [Google Scholar] [CrossRef]
- Akkaya, E.; Muratoglu, K.; Tarhan, D.; Ozsobaci, N.P.; Ercan, A.M.; Colak, H.; Hampikyan, H.; Bingol, E.B.; Or, M.E.; Andoni, E.; et al. Determination of Heavy Metal Levels and Assessment of L. Monocytogenes and Salmonella Spp. Presence in Fishery Products and Mussels from the Marmara Region, Türkiye. Toxics 2025, 13, 153. [Google Scholar] [CrossRef]
- European Neighbourhood Policy and Enlargement Negotiations (DG NEAR). Albania 2024 Report; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Perillo, L.; Arfuso, F.; Piccione, G.; Dara, S.; Tropia, E.; Cascone, G.; Licitra, F.; Monteverde, V. Quantification of Some Heavy Metals in Hair of Dairy Cows Housed in Different Areas from Sicily as a Bioindicator of Environmental Exposure—A Preliminary Study. Animals 2021, 11, 2268. [Google Scholar] [CrossRef]
- Liu, Z. Lead Poisoning Combined with Cadmium in Sheep and Horses in the Vicinity of Non-Ferrous Metal Smelters. Sci. Total Environ. 2003, 309, 117–126. [Google Scholar] [CrossRef]
- Patra, R.C.; Swarup, D.; Sharma, M.C.; Naresh, R. Trace Mineral Profile in Blood and Hair from Cattle Environmentally Exposed to Lead and Cadmium Around Different Industrial Units. J. Vet. Med. Ser. A 2006, 53, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Marshinskaia, O.V.; Kazakova, T.V.; Notova, S.V. Elemental Status of Farm Animals from Different Regions with Different Environmental Loads. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012199. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary Steps of Ecophysiological Adaptation and Diversification of Ruminants: A Comparative View of Their Digestive System. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Instituti i Statistikës (INSTAT). Popullsia Në 1 Janar Sipas Qarqeve dhe Gjinisë 2001–2020; Instituti i Statistikës (INSTAT): Tirana, Albania, 2021. [Google Scholar]
- Pardo, C.; Bellati, A.; Polverino, G.; Canestrelli, D. The Dark Side of Organic Farming: Copper Sulphate Compromises the Life History and Behaviour of the Walking Stick Insect, Bacillus Rossius. Sci. Total Environ. 2024, 942, 173626. [Google Scholar] [CrossRef]
- Reis, L.S.L.S.; Pardo, P.E.; Camargos, A.S.; Oba, E. Mineral Element and Heavy Metal Poisoning in Animals. J. Med. Med. Sci. 2010, 1, 560–579. [Google Scholar]
- Draghi, S.; Fehri, N.E.; Ateş, F.; Özsobacı, N.P.; Tarhan, D.; Bilgiç, B.; Dokuzeylül, B.; Yaramış, Ç.P.; Ercan, A.M.; Or, M.E.; et al. Use of Hair as Matrix for Trace Elements Biomonitoring in Cattle and Roe Deer Sharing Pastures in Northern Italy. Animals 2024, 14, 2209. [Google Scholar] [CrossRef]
- Mandal, T.; Dey, R.; Datta, B.; Patra, P.; Sarkar, S.; Chakraborty, A.; Bhar, M.; Majumdar, D. Effect of Environmental Exposure of Arsenic on Cattle and Poultry in Nadia District, West Bengal, India. Toxicol. Int. 2012, 19, 59. [Google Scholar] [CrossRef]
- Das, A.; Joardar, M.; Chowdhury, N.R.; De, A.; Mridha, D.; Roychowdhury, T. Arsenic Toxicity in Livestock Growing in Arsenic Endemic and Control Sites of West Bengal: Risk for Human and Environment. Environ. Geochem. Health 2021, 43, 3005–3025. [Google Scholar] [CrossRef]
- Maji, C.; Sarkar, S.; Biswas, S.; Patra, P.H.; Datta, B.K.; Bandyopadhyay, S.; Biswas, T.K.; Jana, C.; Mandal, T.K. Experimental Assessment of Arsenic Toxicity in Garole Sheep in India. Emerg. Contam. 2016, 2, 128–134. [Google Scholar] [CrossRef]
- Keshavarzi, B.; Seradj, A.; Akbari, Z.; Moore, F.; Shahraki, A.R.; Pourjafar, M. Chronic Arsenic Toxicity in Sheep of Kurdistan Province, Western Iran. Arch. Environ. Contam. Toxicol. 2015, 69, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-Y.; Yu, S.-D.; Hong, Y.-S. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef] [PubMed]
| Element | Wavelength (nm) | LoD (mg·kg−1) | LoQ (mg·kg−1) | RSD (%) | R2 |
|---|---|---|---|---|---|
| Al | 396.153 | 0.644 | 2.147 | 0.93 | 0.999891 |
| As | 188.979 | 0.007 | 0.023 | 0.85 | 0.999902 |
| B | 249.677 | 0.401 | 1.337 | 0.99 | 0.999915 |
| Ca | 317.933 | 0.894 | 2.980 | 0.94 | 0.999896 |
| Cd | 228.802 | 0.005 | 0.017 | 0.87 | 0.999919 |
| Cr | 267.716 | 0.009 | 0.030 | 0.97 | 0.999873 |
| Cu | 327.393 | 0.095 | 0.317 | 1.01 | 0.999924 |
| Fe | 238.204 | 0.341 | 1.137 | 1.12 | 0.999849 |
| K | 766.490 | 1.358 | 4.527 | 1.24 | 0.999899 |
| Mg | 285.213 | 0.512 | 1.707 | 0.88 | 0.999928 |
| Mn | 257.610 | 0.099 | 0.330 | 1.05 | 0.999812 |
| Ni | 231.604 | 0.013 | 0.043 | 0.91 | 0.999894 |
| Pb | 220.353 | 0.011 | 0.037 | 0.89 | 0.999884 |
| Zn | 213.857 | 0.152 | 0.507 | 1.11 | 0.999918 |
| Element | Quality Control (QC) | Expected Concentration (ppb) | Measured Concentration (n = 3) (ppb) | Recovery (%) |
|---|---|---|---|---|
| Al, As, B, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Ni, Pb, Zn | QC-1 QC-2 QC-3 | 25 250 1000 | 25 251 999 | 100 100.4 99.9 |
| Elements | Parameter | County | p Value * | |
|---|---|---|---|---|
| Elbasan | Tirana | |||
| Al | Mean ± SD 95%CI for mean Md Min–Max | 151 ± 50 130–172 136 90–313 | 140 ± 38 124–156 137 85–224 | 0.449 |
| As | Mean ± SD 95%CI for mean Md Min–Max | 1.73 ± 0.84 1.38–2.07 1.55 0.46–4.03 | 1.39 ± 0.89 1.02–1.75 1.22 0.46–3.43 | 0.084 |
| B | Mean ± SD 95%CI for mean Md Min–Max | 1067 ± 288 947–1186 978 684–1720 | 1048 ± 377 893–1203 916 636–2045 | 0.659 |
| Ca | Mean ± SD 95%CI for mean Md Min–Max | 2605 ± 822 2266–2944 2267 1615–4736 | 2380 ± 838 2034–2726 2248 1408–4507 | 0.262 |
| Cd | Mean ± SD 95%CI for mean Md Min–Max | 2.00 ± 0.98 1.59–2.40 1.82 0.61–4.37 | 1.71 ± 0.76 1.39–2.02 1.58 0.68–3.75 | 0.330 |
| Cr | Mean ± SD 95%CI for mean Md Min–Max | 4.71 ± 1.97 3.89–5.52 4.24 2.39–11.77 | 4.23 ± 1.97 3.42–5.05 3.62 2.51–10.01 | 0.276 |
| Cu | Mean ± SD 95%CI for mean Md Min–Max | 15.58 ± 4.78 13.61–17.56 14.06 9.67–27.31 | 14.14 ± 5.00 12.07–16.20 12.69 9.79–27.13 | 0.209 |
| Fe | Mean ± SD 95%CI for mean Md Min–Max | 432 ± 119 382–480 404.14 266–802 | 384 ± 115 337–432 370 227–634 | 0.124 |
| K | Mean ± SD 95%CI for mean Md Min–Max | 6107 ± 1966 5295–6918 5434 3846–12329 | 5489 ± 1633 4815–6163 4878 3798–9803 | 0.204 |
| Mg | Mean ± SD 95%CI for mean Md Min–Max | 585 ± 153 521–648 521 367–1016 | 538 ± 188 460–615 473 331–972 | 0.190 |
| Mn | Mean ± SD 95%CI for mean Md Min–Max | 5.63 ± 2.03 4.79–6.47 4.92 2.96–12.38 | 4.83 ± 1.82 4.08–5.58 4.27 2.80–9.58 | 0.097 |
| Ni | Mean ± SD 95%CI for mean Md Min–Max | 3.01 ± 1.30 2.47–3.54 2.46 1.33–6.58 | 2.67 ± 1.13 2.20–3.13 2.24 1.33–5.20 | 0.300 |
| Pb | Mean ± SD 95%CI for mean Md Min–Max | 2.63 ± 1.08 2.18–3.08 2.39 1.13–6.00 | 2.27 ± 1.18 1.78–2.76 2.11 0.97–4.80 | 0.130 |
| Zn | Mean ± SD 95%CI for mean Md Min–Max | 140 ± 38 124–155 134 92–235 | 135 ± 41 118–151 122 93–232 | 0.562 |
| Elements | Parameter | County | p Value * | |
| Elbasan | Tirana | |||
| Al | Mean ± SD 95%CI for mean Md Min–Max | 165 ± 64 132–159 150 93–307 | 151 ± 53 129–173 139 95–321 | 0.461 |
| As | Mean ± SD 95%CI for mean Md Min–Max | 2.08 ± 1.41 1.45–1.21 1.67 0.54–5.80 | 1.51 ± 0.76 1.19–1.81 1.28 0.596–3.37 | 0.219 |
| B | Mean ± SD 95%CI for mean Md Min–Max | 1235 ± 453 972–1152 1241 647–2121 | 1075 ± 252 971–1179 1035 673–1601 | 0.252 |
| Ca | Mean ± SD 95%CI for mean Md Min–Max | 2764 ± 921 2249–2689 2916 1569–4613 | 2557 ± 708 2264–2850 2591 1438–3993 | 0.458 |
| Cd | Mean ± SD 95%CI for mean Md Min–Max | 2.36 ± 1.37 1.63–2.07 2.27 0.72–5.88 | 2.00 ± 0.79 1.68–2.32 2.00 0.78–4.05 | 0.585 |
| Cr | Mean ± SD 95%CI for mean Md Min–Max | 5.58 ± 2.74 3.87–4.89 5.19 2.43–12.06 | 4.53 ± 1.59 3.87–5.18 4.49 2.44–7.73 | 0.179 |
| Cu | Mean ± SD 95%CI for mean Md Min–Max | 17.84 ± 7.75 13.63–16.34 17.08 9.07–36.53 | 15.84 ± 4.48 14.00–17.69 14.93 9.76–25.99 | 0.489 |
| Fe | Mean ± SD 95%CI for mean Md Min–Max | 463 ± 168 375–446 461 241–810 | 437 ± 133 381–492 400 258–752 | 0.681 |
| K | Mean ± SD 95%CI for mean Md Min–Max | 6746 ± 2358 5373–6287 6638 3603–11566 | 6171 ± 1537 5536–6805 6094 3883–9813 | 0.497 |
| Mg | Mean ± SD 95%CI for mean Md Min–Max | 653 ± 246 509–597 660 365–1258 | 567 ± 114 520–614 579 336–836 | 0.282 |
| Mn | Mean ± SD 95%CI for mean Md Min–Max | 6.20 ± 2.72 4.75–5.72 5.98 2.77–12.52 | 5.63 ± 1.52 5.00–6.26 5.30 3.30–9.69 | 0.667 |
| Ni | Mean ± SD 95%CI for mean Md Min–Max | 3.42 ± 1.73 2.51–3.11 3.19 1.31–7.47 | 2.96 ± 0.98 2.55–3.36 2.96 1.51–5.64 | 0.543 |
| Pb | Mean ± SD 95%CI for mean Md Min–Max | 3.02 ± 1.63 2.07–2.70 3.18 0.93–6.94 | 2.50 ± 1.03 2.08–2.93 2.30 1.15–4.83 | 0.417 |
| Zn | Mean ± SD 95%CI for mean Md Min–Max | 162 ± 57 131–153 151 85–280 | 150 ± 35 135–164 142 103–232 | 0.571 |
| Al | As | B | Ca | Cd | Cr | Cu | Fe | K | Mg | Mn | Ni | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.900 | -- | |||||||||||
| B | 0.869 | 0.890 | -- | ||||||||||
| Ca | 0.824 | 0.874 | 0.844 | -- | |||||||||
| Cd | 0.793 | 0.774 | 0.829 | 0.716 | -- | ||||||||
| Cr | 0.835 | 0.897 | 0.888 | 0.882 | 0.799 | -- | |||||||
| Cu | 0.870 | 0.930 | 0.894 | 0.866 | 0.774 | 0.873 | -- | ||||||
| Fe | 0.864 | 0.910 | 0.877 | 0.837 | 0.793 | 0.895 | 0.902 | -- | |||||
| K | 0.829 | 0.868 | 0.877 | 0.810 | 0.838 | 0.896 | 0.871 | 0.872 | -- | ||||
| Mg | 0.790 | 0.882 | 0.846 | 0.824 | 0.765 | 0.861 | 0.876 | 0.872 | 0.873 | -- | |||
| Mn | 0.814 | 0.870 | 0.878 | 0.832 | 0.834 | 0.880 | 0.857 | 0.884 | 0.882 | 0.888 | -- | ||
| Ni | 0.838 | 0.864 | 0.890 | 0.800 | 0.836 | 0.805 | 0.911 | 0.844 | 0.859 | 0.833 | 0.870 | -- | |
| Pb | 0.815 | 0.861 | 0.847 | 0.845 | 0.814 | 0.868 | 0.854 | 0.863 | 0.836 | 0.819 | 0.840 | 0.808 | -- |
| Zn | 0.781 | 0.815 | 0.845 | 0.743 | 0.784 | 0.793 | 0.861 | 0.860 | 0.840 | 0.818 | 0.870 | 0.893 | 0.773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrica, M.; Andoni, E.; Quattrone, A.; Koleci, X.; Ozuni, E.; Zalla, P.; Postoli, R.; Menchetti, L.; Bilgiç, B.; Tarhan, D.; et al. Livestock Animal Hair as an Indicator of Environmental Heavy Metals Pollution in Central Albania. Animals 2025, 15, 1898. https://doi.org/10.3390/ani15131898
Castrica M, Andoni E, Quattrone A, Koleci X, Ozuni E, Zalla P, Postoli R, Menchetti L, Bilgiç B, Tarhan D, et al. Livestock Animal Hair as an Indicator of Environmental Heavy Metals Pollution in Central Albania. Animals. 2025; 15(13):1898. https://doi.org/10.3390/ani15131898
Chicago/Turabian StyleCastrica, Marta, Egon Andoni, Alda Quattrone, Xhelil Koleci, Enkeleda Ozuni, Pellumb Zalla, Rezart Postoli, Laura Menchetti, Bengü Bilgiç, Duygu Tarhan, and et al. 2025. "Livestock Animal Hair as an Indicator of Environmental Heavy Metals Pollution in Central Albania" Animals 15, no. 13: 1898. https://doi.org/10.3390/ani15131898
APA StyleCastrica, M., Andoni, E., Quattrone, A., Koleci, X., Ozuni, E., Zalla, P., Postoli, R., Menchetti, L., Bilgiç, B., Tarhan, D., Yalcin, I. E., Dova, I., Fehri, N. E., Or, M. E., Munga, A., Beqiraj, D., Curone, G., & Agradi, S. (2025). Livestock Animal Hair as an Indicator of Environmental Heavy Metals Pollution in Central Albania. Animals, 15(13), 1898. https://doi.org/10.3390/ani15131898










