Lipidomic Landscapes of Cryopreserved Sperm from Alpine and Spanish–Creole Bucks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Sample Preparation, Cryopreservation, and Determination of Post-Thaw Semen Parameters
2.2. Extraction of Lipids from Sperm Samples
2.2.1. Sample Preparation
2.2.2. Lipid Extraction Protocol
2.2.3. Preparation of Internal and External Standards
2.2.4. Preparation of Fatty Acid Methyl Esters (FAME)
2.2.5. Gas Chromatography–Mass Spectrophotometry (GC-MS) Analysis
2.3. Statistical Analysis
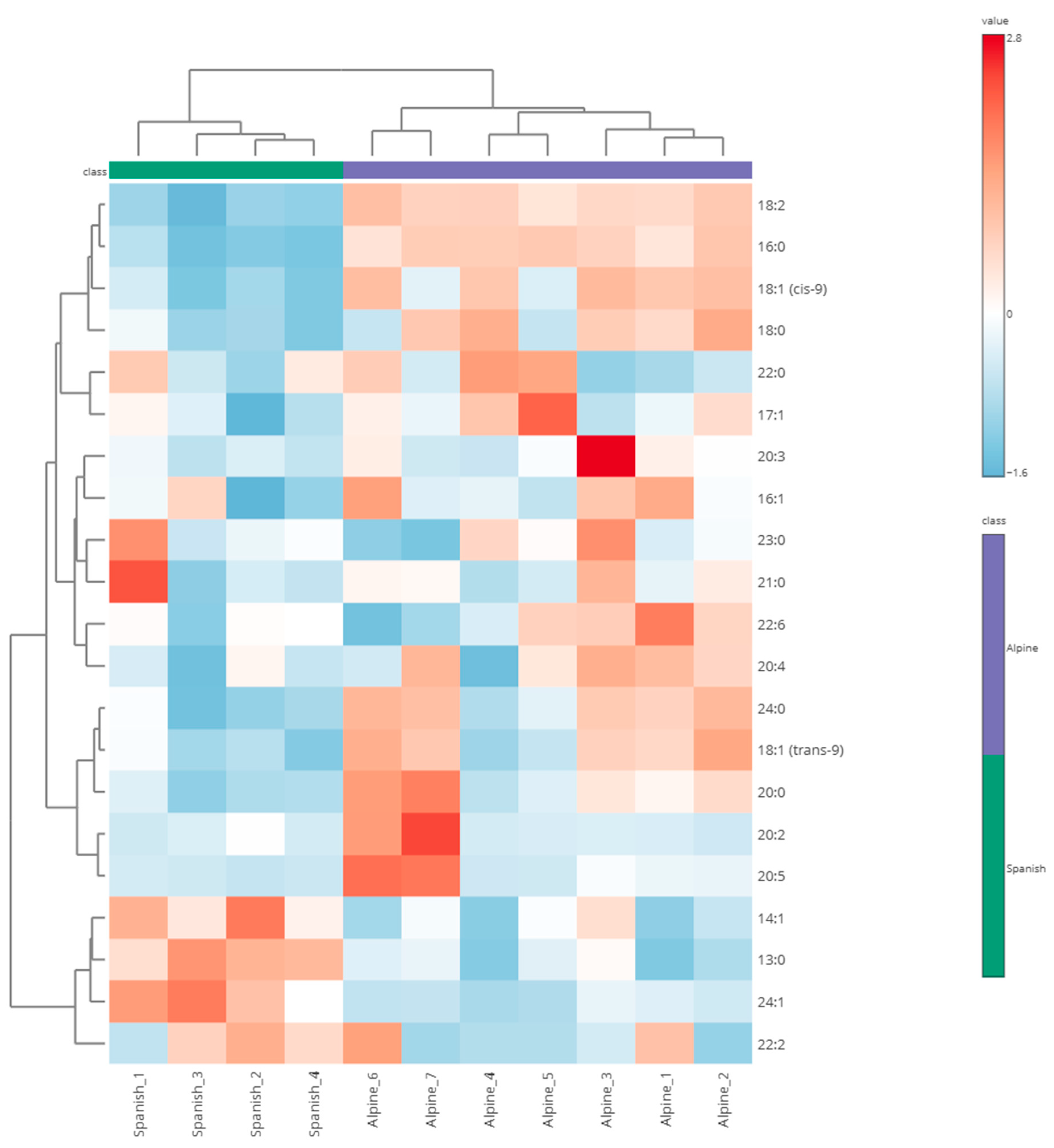
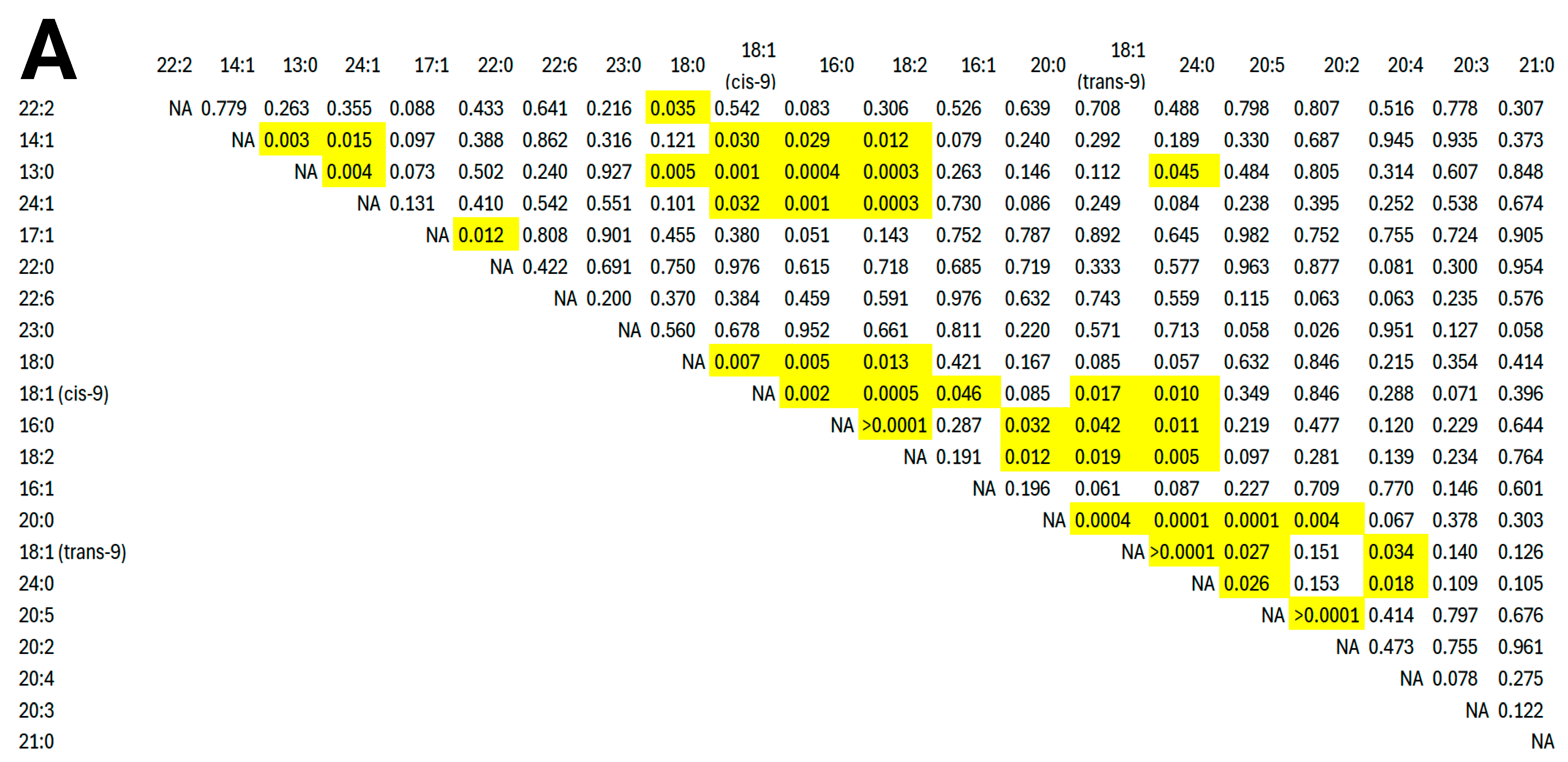
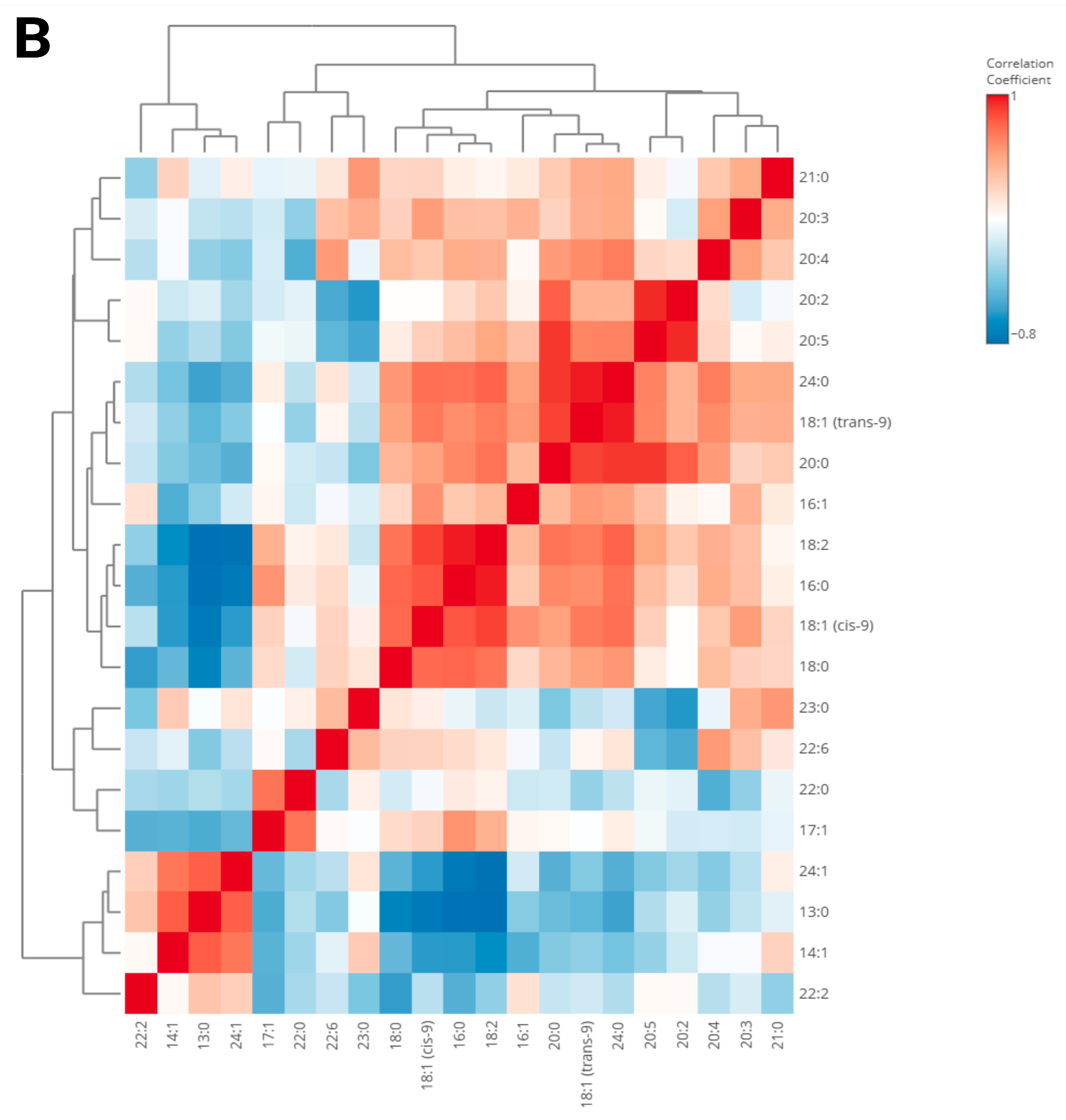
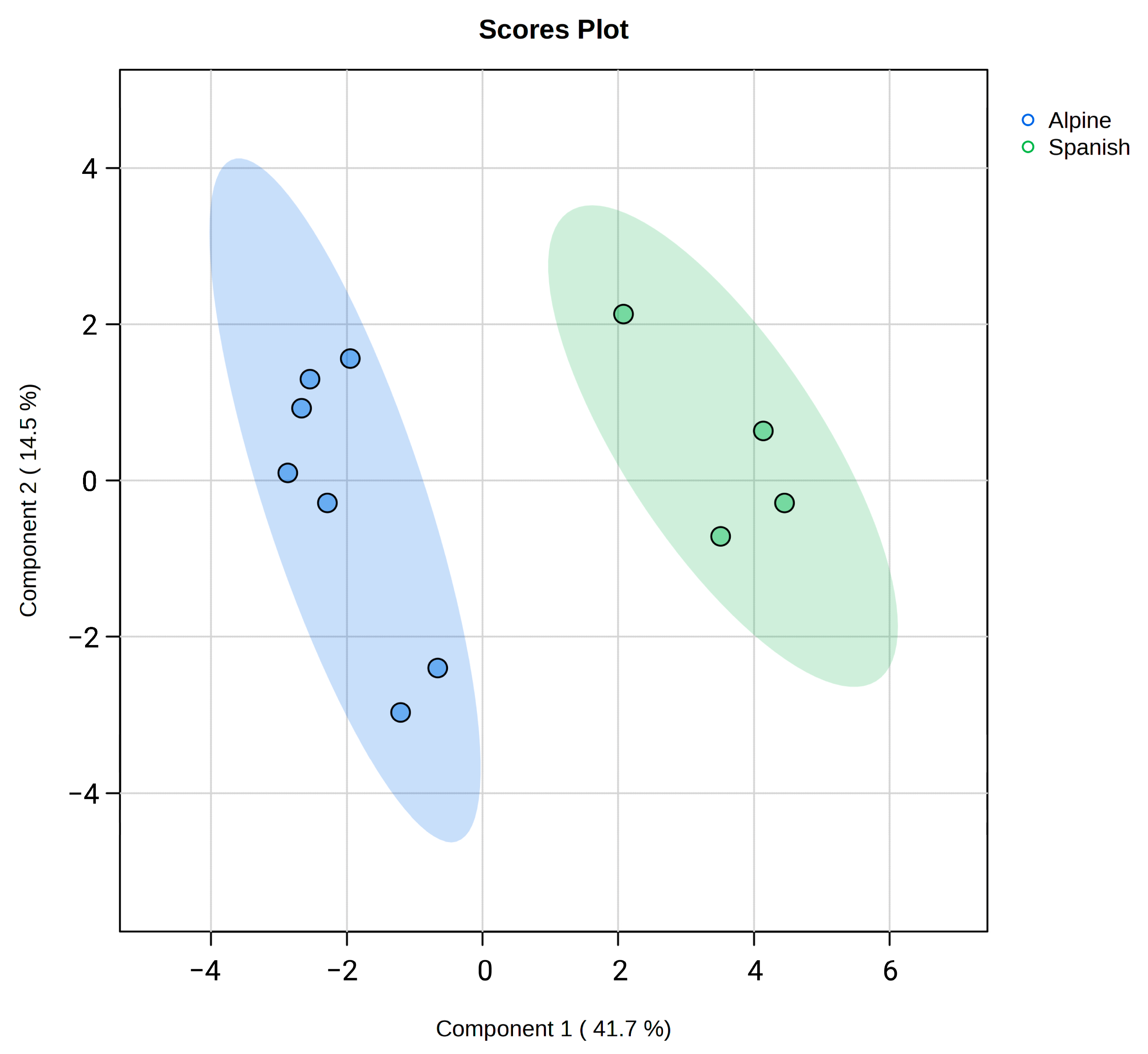
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodu, M.; Hitit, M.; Memili, E. Harnessing the value of fertility biomarkers in bull sperm for buck sperm. Anim. Reprod. Sci. 2025, 272, 107643. [Google Scholar] [CrossRef]
- Alemayehu, G.; Mamo, G.; Alemu, B.; Desta, H.; Wieland, B. Towards objective measurement of reproductive performance of traditionally managed goat flocks in the drylands of Ethiopia. Trop. Anim. Health Prod. 2021, 53, 156. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, J.A.; Martin, G.B. Alternative methods for control of reproduction in small ruminants: A focus on the needs of grazing industries. Anim. Front. 2015, 5, 57–65. [Google Scholar] [CrossRef]
- Nissen, H.P.; Kreysel, H.W. Analysis of phospholipids in human semen by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1983, 276, 29–35. [Google Scholar] [CrossRef]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Bodu, M.; Hitit, M.; Greenwood, O.C.; Murray, R.D.; Memili, E. Extender development for optimal cryopreservation of buck sperm to increase reproductive efficiency of goats. Front. Vet. Sci. 2025, 12, 1554771. [Google Scholar] [CrossRef]
- Lucio, C.; Brito, M.; Angrimani, D.; Belaz, K.; Morais, D.; Zampieri, D.; Losano, J.; Assumpção, M.; Nichi, M.; Eberlin, M.; et al. Lipid composition of the canine sperm plasma membrane as markers of sperm motility. Reprod. Domest. Anim. 2017, 52, 208–213. [Google Scholar] [CrossRef]
- van der Horst, G.; Maree, L. Origin, Migration, and Reproduction of Indigenous Domestic Animals with Special Reference to Their Sperm Quality. Animals 2022, 12, 657. [Google Scholar] [CrossRef]
- Pan, X.; Kang, G.; Yang, N.; Zhang, X.; Shao, L.; Zhai, P.; Feng, Q.; Zhang, X.; Li, J.; Wang, X.; et al. A Sperm Quality Detection System Based on Microfluidic Chip and Micro-Imaging System. Front. Vet. Sci. 2022, 9, 916861. [Google Scholar] [CrossRef]
- Hitit, M.; Özbek, M.; Ayaz-Guner, S.; Guner, H.; Oztug, M.; Bodu, M.; Kirbas, M.; Bulbul, B.; Bucak, M.N.; Ataman, M.B.; et al. Proteomic fertility markers in ram sperm. Anim. Reprod. Sci. 2021, 235, 106882. [Google Scholar] [CrossRef]
- Kameni, S.L.; Dlamini, N.H.; Feugang, J.M. Exploring the Full Potential of Sperm Function With Nanotechnology Tools. Anim. Reprod. 2024, 21, e20240033. [Google Scholar] [CrossRef] [PubMed]
- Sigit, B.; Panjono; Riyan Nugroho, A. The Effect of Sanrego Wood (Lunasia amara Blanco) Extract Addition to the Andromed® Diluent on Sperm Quality of Belgian Blue Crossbreeds Bull. In Proceedings of the 2nd International Conference on Smart and Innovative Agriculture (ICoSIA 2021), Yogyakarta, Indonesia, 10 March 2022; pp. 371–375. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Kataoka, S.; Kinukawa, M.; Uchiyama, K.; Kambe, J.; Watanabe, G.; Jin, W.; Nagaoka, K. L-amino acid oxidase 1 in sperm is associated with reproductive performance in male mice and bulls. Biol. Reprod. 2021, 104, 1154–1161. [Google Scholar] [CrossRef]
- Evans, H.C.; Dinh, T.T.N.; Ugur, M.R.; Hitit, M.; Sajeev, D.; Kaya, A.; Topper, E.; Nicodemus, M.C.; Smith, G.D.; Memili, E. Lipidomic markers of sperm cryotolerance in cattle. Sci. Rep. 2020, 10, 20192. [Google Scholar] [CrossRef]
- Lopes César, M.; Maicon Pereira, L.; Emmanuel Emydio Gomes, P.; Rafael Alexandre, M. Alternative Animal Feeding for Intensive Livestock Farming Systems and Their Impact on Reproductive Performance of Ruminants. In Intensive Animal Farming; Shumaila, M., Muhammad, A., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 4. [Google Scholar]
- Congras, A.; Yerle-Bouissou, M.; Pinton, A.; Vignoles, F.; Liaubet, L.; Ferchaud, S.; Acloque, H. Sperm DNA Methylation Analysis in Swine Reveals Conserved and Species-Specific Methylation Patterns and Highlights an Altered Methylation at the GNAS Locus in Infertile Boars1. Biol. Reprod. 2014, 91, 119610. [Google Scholar] [CrossRef] [PubMed]
- Namani, S.C.; Kolikapongu, R.S.; Chelkapally, S.C.; Heikal, R.; Neha, A.; Shaik, A.; Pech Cervantes, A.A.; Whitley, N.C.; Kouakou, B.; Terrill, T.H.; et al. 379 Impacts of Serecia Lespedeza and Black Seed Meal Dietary Supplementation on Sperm Quality and Fertilityvariables of Male Goats. J. Anim. Sci. 2023, 101, 302–303. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Hosseini, S.Y.; Dadkhah, F.; Asgari, M.A. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: A comparison between fertile and infertile men. Clin. Nutr. 2010, 29, 100–105. [Google Scholar] [CrossRef]
- Furimsky, A.; Vuong, N.; Xu, H.; Kumarathasan, P.; Xu, M.; Weerachatyanukul, W.; Bou Khalil, M.; Kates, M.; Tanphaichitr, N. Percoll Gradient-Centrifuged Capacitated Mouse Sperm Have Increased Fertilizing Ability and Higher Contents of Sulfogalactosylglycerolipid and Docosahexaenoic Acid-Containing Phosphatidylcholine Compared to Washed Capacitated Mouse Sperm1. Biol. Reprod. 2005, 72, 574–583. [Google Scholar] [CrossRef]
- Sellem, E.; Jammes, H.; Schibler, L. Sperm-borne sncRNAs: Potential biomarkers for semen fertility? Reprod. Fertil. Dev. 2022, 34, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.C.; Dinh, T.T.N.; Hardcastle, M.L.; Gilmore, A.A.; Ugur, M.R.; Hitit, M.; Jousan, F.D.; Nicodemus, M.C.; Memili, E. Advancing Semen Evaluation Using Lipidomics. Front. Vet. Sci. 2021, 8, 601794. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, Y.; Wang, J.; Liu, J.; Zhang, J.; Zhang, C.; Zhou, L.; Cao, J.; Jiang, L. Lipidomic and transcriptomic characteristics of boar seminal plasma extracellular vesicles associated with sperm motility. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2025, 1870, 159561. [Google Scholar] [CrossRef]
- Prasinou, P.; De Amicis, I.; Fusaro, I.; Bucci, R.; Cavallini, D.; Parrillo, S.; Caputo, M.; Gramenzi, A.; Carluccio, A. The Lipidomics of Spermatozoa and Red Blood Cells Membrane Profile of Martina Franca Donkey: Preliminary Evaluation. Animals 2023, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, H.; Xiong, Y.; Sun, P.; Xue, Y.; Li, K. Lipidomics profiles of human spermatozoa: Insights into capacitation and acrosome reaction using UPLC-MS-based approach. Front. Endocrinol. 2023, 14, 1273878. [Google Scholar] [CrossRef]
- Furse, S.; Kusinski, L.C.; Ray, A.; Glenn-Sansum, C.; Williams, H.E.L.; Koulman, A.; Meek, C.L. Relative Abundance of Lipid Metabolites in Spermatozoa across Three Compartments. Int. J. Mol. Sci. 2022, 23, 11655. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, V.; Narwade, B.; Mohanty, T.; Atreja, S. Comparative Quality Assessment of Buffalo (Bubalus bubalis) Semen Chilled (5 °C) in Egg Yolk- and Soya Milk–Based Extenders. Reprod. Domest. Anim. 2012, 47, 596–600. [Google Scholar] [CrossRef]
- Macías García, B.; González Fernández, L.; Ortega Ferrusola, C.; Morillo Rodríguez, A.; Gallardo Bolaños, J.M.; Rodríguez Martinez, H.; Tapia, J.A.; Morcuende, D.; Peña, F.J. Fatty acids and plasmalogens of the phospholipids of the sperm membranes and their relation with the post-thaw quality of stallion spermatozoa. Theriogenology 2011, 75, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Badyakar, D.; Chakrabarty, J. Role of Membrane Lipid Fatty Acids in Sperm Cryopreservation. Adv. Androl. 2014, 2014, 190542. [Google Scholar] [CrossRef]
- Zadeh Hashem, E.; Haddad, R.; Eslami, M. Evaluation of ram semen enrichment with oleic acid on different spermatozoa parameters during low temperature liquid storage. Small Rumin. Res. 2017, 150, 30–39. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances. Animals 2021, 11, 1932. [Google Scholar] [CrossRef]
- Bucak, M.N.; Karaşör, Ö.F.; Sarı, A.; Bodu, M.; Ili, P.; Narlıçay, S.; Ataman, M.B.; Sari, F. Lipid mixtures (from a liposome kit) and melatonin improve post-thawed Angora goat sperm parameters. Cryobiology 2024, 115, 104897. [Google Scholar] [CrossRef]
- Li, C.; Liang, J.; Allai, L.; Badaoui, B.; Shao, Q.; Ouyang, Y.; Wu, G.; Quan, G.; Lv, C. Integrating proteomics and metabolomics to evaluate impact of semen collection techniques on the quality and cryotolerance of goat semen. Sci. Rep. 2024, 14, 29489. [Google Scholar] [CrossRef]
- Öztürk, A.E.; Bodu, M.; Bucak, M.N.; Ağır, V.; Özcan, A.; Keskin, N.; İli, P.; Topraggaleh, T.R.; Sidal, H.; Başpınar, N. The synergistic effect of trehalose and low concentrations of cryoprotectants can improve post-thaw ram sperm parameters. Cryobiology 2020, 95, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, R.; Wang, Z.; Liu, H.; Wang, Z.; Zhang, W.; Zhang, Y.; Su, R.; Liu, Z.; Liu, Y.; et al. Evaluation of lipidomic change in goat sperm after cryopreservation. Front. Vet. Sci. 2022, 9, 1004683. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Scoggin, K.; Ball, B.A.; Troedsson, M.H.; Squires, E.L. Lipidomics of equine sperm and seminal plasma: Identification of amphiphilic (O-acyl)-ω-hydroxy-fatty acids. Theriogenology 2016, 86, 1212–1221. [Google Scholar] [CrossRef]
- Araujo, P.; Nguyen, T.-T.; Frøyland, L.; Wang, J.; Kang, J.X. Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J. Chromatogr. A 2008, 1212, 106–113. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60, 481–492. [Google Scholar] [CrossRef]
- Purdy, P. A review on goat sperm cryopreservation. Small Rumin. Res. 2006, 63, 215–225. [Google Scholar] [CrossRef]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Hu, J.H.; Zhao, X.L.; Tian, W.Q.; Zan, L.S.; Li, Q.W. Effects of vitamin E supplementation in the extender on frozen-thawed bovine semen preservation. Animal 2011, 5, 107–112. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zhu, Z. Carboxylated ε-Poly-L-Lysine Supplementation of the Freezing Extender Improves the Post-Thawing Boar Sperm Quality. Animals 2022, 12, 1726. [Google Scholar] [CrossRef]
- Zhang, W.; Min, L.; Li, Y.; Lang, Y.; Hoque, S.A.M.; Adetunji, A.O.; Zhu, Z. Beneficial Effect of Proline Supplementation on Goat Spermatozoa Quality during Cryopreservation. Animals 2022, 12, 2626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, W.; Liu, Y.; Liu, Y.; Liang, H.; Xu, Q.; Liu, Z.; Weng, X. Plasma membrane lipid composition and metabolomics analysis of Yorkshire boar sperms with high and low resistance to cryopreservation. Theriogenology 2023, 206, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, Y.-J.; Ho Kang, B.; Park, C.-K. Effect of nicotinic acid on the plasma membrane function and polyunsaturated fatty acids composition during cryopreservation in boar sperm. Reprod. Domest. Anim. 2019, 54, 1251–1257. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, W.H.; Cheong, H.T.; Yang, B.K.; Park, C.K. Effect of Alpha-Linolenic Acid with Bovine Serum Albumin or Methyl-Beta-Cyclodextrin on Membrane Integrity and Oxidative Stress of Frozen-Thawed Boar Sperm. Dev. Reprod. 2019, 23, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Carro, M.D.L.M.; Ramírez-Vasquez, R.R.A.; Peñalva, D.A.; Buschiazzo, J.; Hozbor, F.A. Desmosterol Incorporation Into Ram Sperm Membrane Before Cryopreservation Improves in vitro and in vivo Fertility. Front. Cell Dev. Biol. 2021, 9, 660165. [Google Scholar] [CrossRef]
- Carro, M.; Luquez, J.M.; Peñalva, D.A.; Buschiazzo, J.; Hozbor, F.A.; Furland, N.E. PUFA-rich phospholipid classes and subclasses of ram spermatozoa are unevenly affected by cryopreservation with a soybean lecithin-based extender. Theriogenology 2022, 186, 122–134. [Google Scholar] [CrossRef]
- Macías García, B.; González Fernández, L.; Ortega Ferrusola, C.; Salazar-Sandoval, C.; Morillo Rodríguez, A.; Rodríguez Martinez, H.; Tapia, J.; Morcuende, D.; Peña, F. Membrane Lipids of the Stallion Spermatozoon in Relation to Sperm Quality and Susceptibility to Lipid Peroxidation. Reprod. Domest. Anim. 2011, 46, 141–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, N.; Cao, J.; Zhang, J.; Liu, J.; Zhang, C.; Jiang, L. Proteomics and Metabolic Characteristics of Boar Seminal Plasma Extracellular Vesicles Reveal Biomarker Candidates Related to Sperm Motility. J. Proteome Res. 2024, 23, 3764–3779. [Google Scholar] [CrossRef]
- Islam, M.M.; Umehara, T.; Tsujita, N.; Shimada, M. Saturated fatty acids accelerate linear motility through mitochondrial ATP production in bull sperm. Reprod. Med. Biol. 2021, 20, 289–298. [Google Scholar] [CrossRef]
- Klaiwattana, P.; Srisook, K.; Srisook, E.; Vuthiphandchai, V.; Neumvonk, J. Effect of cryopreservation on lipid composition and antioxidant enzyme activity of seabass (Lates calcarifer) sperm. Iran. J. Fish. Sci. 2016, 15, 157–169. [Google Scholar]
- Chakrabarty, J.; Banerjee, D.; Pal, D.; De, J.; Ghosh, A.; Majumder, G.C. Shedding off specific lipid constituents from sperm cell membrane during cryopreservation. Cryobiology 2007, 54, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Paventi, G.; Di Iorio, M.; Rusco, G.; Sobolev, A.P.; Cerolini, S.; Antenucci, E.; Spano, M.; Mannina, L.; Iaffaldano, N. The Effect of Semen Cryopreservation Process on Metabolomic Profiles of Turkey Sperm as Assessed by NMR Analysis. Biology 2022, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Kogan, T.; Grossman Dahan, D.; Laor, R.; Argov-Argaman, N.; Zeron, Y.; Komsky-Elbaz, A.; Kalo, D.; Roth, Z. Association between Fatty Acid Composition, Cryotolerance and Fertility Competence of Progressively Motile Bovine Spermatozoa. Animals 2021, 11, 2948. [Google Scholar] [CrossRef]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage1The present trial is part of an International Patent Application n. PCT/GB97/01735.1. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, Y.-J.; Kang, B.H.; Yun, Y.S.; Park, C.-K. The relationship between acrosome reaction and polyunsaturated fatty acid composition in boar sperm. Reprod. Domest. Anim. 2020, 55, 624–631. [Google Scholar] [CrossRef]
- Beirão, J.; Zilli, L.; Vilella, S.; Cabrita, E.; Schiavone, R.; Herráez, M.P. Improving Sperm Cryopreservation with Antifreeze Proteins: Effect on Gilthead Seabream (Sparus aurata) Plasma Membrane Lipids1. Biol. Reprod. 2012, 86, 093401. [Google Scholar] [CrossRef]
- Akbarzadeh-Jahromi, M.; Jafari, F.; Parsanezhad, M.E.; Alaee, S. Evaluation of supplementation of cryopreservation medium with gallic acid as an antioxidant in quality of post-thaw human spermatozoa. Andrologia 2022, 54, e14571. [Google Scholar] [CrossRef]
- Li, C.; Lv, C.; Larbi, A.; Liang, J.; Yang, Q.; Wu, G.; Quan, G. Revisiting the Injury Mechanism of Goat Sperm Caused by the Cryopreservation Process from a Perspective of Sperm Metabolite Profiles. Int. J. Mol. Sci. 2024, 25, 9112. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Wu, C.; Qiu, S.; Chen, X.; Cai, B.; Xie, H. Freeze-thawing impairs the motility, plasma membrane integrity and mitochondria function of boar spermatozoa through generating excessive ROS. BMC Vet. Res. 2021, 17, 127. [Google Scholar] [CrossRef]
- Maldjian, A.; Pizzi, F.; Gliozzi, T.; Cerolini, S.; Penny, P.; Noble, R. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Theriogenology 2005, 63, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, J.; Nartey, M.A.; Mo, X.; Ye, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Fang, Y.; Zhou, G. Ram sperm cryopreservation disrupts metabolism of unsaturated fatty acids. Theriogenology 2023, 204, 8–17. [Google Scholar] [CrossRef]
- Leão, B.C.S.; Rocha-Frigoni, N.A.S.; Cabral, E.C.; Coelho, M.B.; Ferreira, C.R.; Eberlin, M.N.; Accorsi, M.F.; Nogueira, É.; Mingoti, G.Z. Improved embryonic cryosurvival observed after in vitro supplementation with conjugated linoleic acid is related to changes in the membrane lipid profile. Theriogenology 2015, 84, 127–136. [Google Scholar] [CrossRef]
- Melville, D.F.; Johnston, S.D.; Miller, R.R. Flying-fox (Pteropus spp.) sperm membrane fatty acid composition, its relationship to cold shock injury and implications for cryopreservation success. Cryobiology 2012, 65, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, H.; Ghallab, A.; Abou-Ahmed, M. Influence of linoleic acid supplementation on the quality of frozen-thawed ram semen. Vet. Med. J. 2024, 63, 21–25. [Google Scholar] [CrossRef]
- Büyükleblebici, S.; Taşdemir, U.; Tuncer, P.B.; Durmaz, E.; Özgürtaş, T.; Büyükleblebici, O.; Coşkun, E.; Gürcan, İ.S. Can linoleic acid improve the quality of frozen thawed bull sperm? Cryoletters 2014, 35, 473–481. [Google Scholar] [PubMed]
- Díaz, R.; Quiñones, J.; Short, S.; Contreras, P.; Ulloa-Rodríguez, P.; Cancino-Baier, D.; Sepúlveda, N.; Valdebenito, I.; Farías, J.G. Effect of exogenous lipids on cryotolerance of Atlantic salmon (Salmo salar) spermatozoa. Cryobiology 2021, 98, 25–32. [Google Scholar] [CrossRef]
- Soares, M.P.; Brandelli, A.; Celeghini, E.C.C.; de Arruda, R.P.; Rodriguez, S.A.F. Effect of cis-9,trans-11 and trans-10,cis-12 isomers of conjugated linoleic acid on the integrity and functionality of cryopreserved bovine spermatozoa. Cryobiology 2013, 67, 102–105. [Google Scholar] [CrossRef]
- Andersen, J.M.; Rønning, P.O.; Herning, H.; Bekken, S.D.; Haugen, T.B.; Witczak, O. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology 2016, 4, 857–865. [Google Scholar] [CrossRef]
- Güngör, İ.H.; Koca, R.H.; Cinkara, S.D.; Acısu, T.C.; Erişir, F.E.; Arkalı, G.; Kaya, Ş.Ö.; Sönmez, M.; Gür, S.; Yılmaz, Ö.; et al. Changes in fatty acids, vitamins, cholesterol and amino acid profiles of ram semen by freeze-thawing process. Reprod. Biol. 2025, 25, 100953. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef] [PubMed]
- Argov-Argaman, N.; Mahgrefthe, K.; Zeron, Y.; Roth, Z. Season-induced variation in lipid composition is associated with semen quality in Holstein bulls. Reproduction 2013, 145, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Feng, C.; Liu, R.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Lu, H.; Zhang, T.; Zeng, W. Exogenous Oleic Acid and Palmitic Acid Improve Boar Sperm Motility via Enhancing Mitochondrial Β-Oxidation for ATP Generation. Animals 2020, 10, 591. [Google Scholar] [CrossRef]
- Benko, F.; Árvay, J.; Jančo, I.; Ďuračka, M.; Mohammadi-Sangcheshmeh, A.; Lukáč, N.; Ivanič, P.; Tvrdá, E. In vitro versus cryo-induced capacitation of bovine spermatozoa, part 3: Compositional and molecular changes to the plasma membrane. Cryobiology 2024, 117, 104972. [Google Scholar] [CrossRef]
- Gautier, C.; Aurich, C. “Fine feathers make fine birds”—The mammalian sperm plasma membrane lipid composition and effects on assisted reproduction. Anim. Reprod. Sci. 2022, 246, 106884. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Zou, S.; Chen, X.; Pan, B.; Ning, A.; Qin, J.; Wei, Y.; Du, K.; Ye, J.; Liang, Q.; et al. Abnormalities in mitochondrial energy metabolism induced by cryopreservation negatively affect goat sperm motility. Front. Vet. Sci. 2024, 11, 1514362. [Google Scholar] [CrossRef]
- Setiawan, R.; Christi, R.F.; Alhuur, K.R.G.; Widyastuti, R.; Solihati, N.; Rasad, S.D.; Hidajat, K.; Do, D.N. Impact of glucose and pyruvate on adenosine triphosphate production and sperm motility in goats. Anim. Biosci. 2024, 37, 631–639. [Google Scholar] [CrossRef]

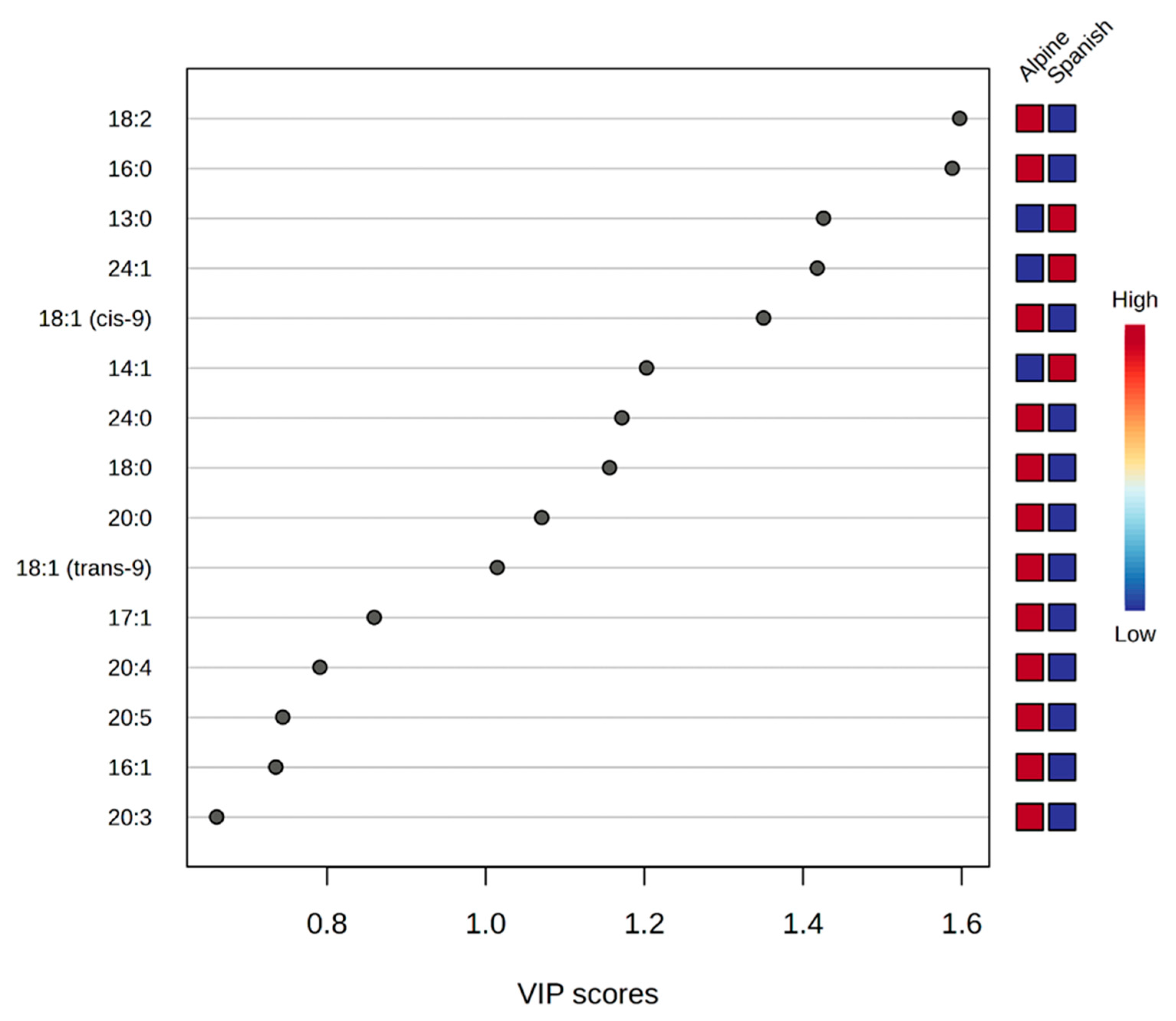
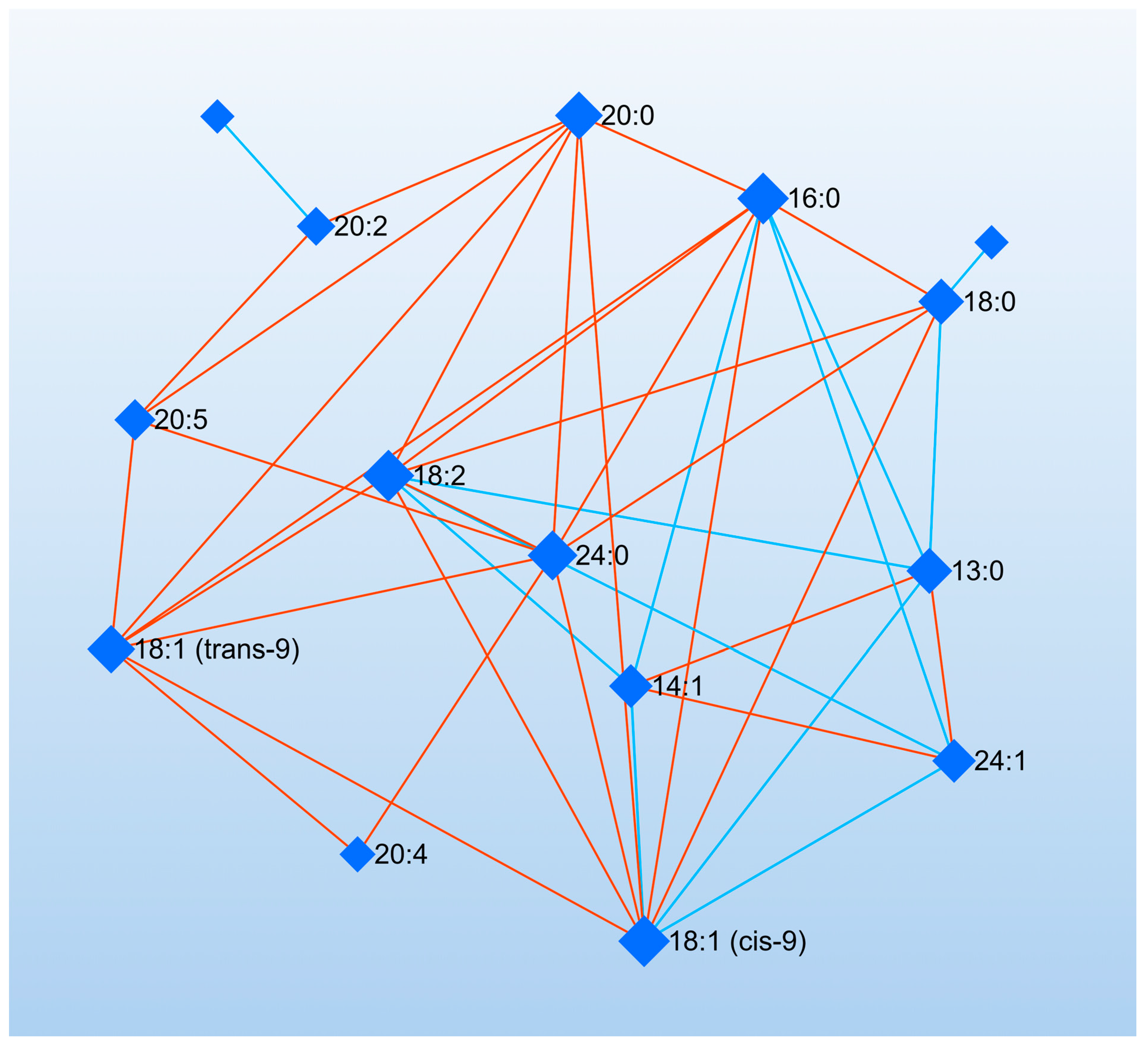
| Parameters | Alpine Mean ± SEM | Spanish–Creole Mean ± SEM | p-Values |
|---|---|---|---|
| Total Motility (%) | 60.28 ± 0.81 | 60.82 ± 0.97 | 0.67 |
| Progressive Motility (%) | 36.3 ± 2.03 | 40.88 ± 1.65 | 0.109 |
| VAP (µm/s) | 104.68 ± 6.38 | 100.28 ± 6.13 | 0.633 |
| VCL (µm/s) | 214.34 ± 13.15 | 197.61 ± 13.99 | 0.398 |
| VSL (µm/s) | 79.28 ± 5.09 | 72.51 ± 3.6 | 0.318 |
| ALH (µm) | 10.01 ± 0.48 | 9.53 ± 0.62 | 0.537 |
| BCF (Hz) * | 33.29 ± 0.88 | 29.67 ± 1.11 | 0.017 |
| LIN (%) | 40.66 ± 1.54 | 39.86 ± 1.35 | 0.709 |
| STR (%) | 76.08 ± 1.3 | 74.34 ± 1.81 | 0.432 |
| AREA (µm2) * | 17.65 ± 0.11 | 18.48 ± 0.17 | <0.001 |
| WOB (%) | 52.01 ± 1.05 | 52.57 ± 0.72 | 0.683 |
| ELONGATION | 0.48 ± 0.01 | 0.48 ± 0.01 | 0.886 |
| Fatty Acid | Alpine Mean (ng/mL) ± SEM | Spanish–Creole Mean (ng/mL) ± SEM | Fold Change (FC) | −log10 (p-Value) | FDR-Corrected p-Value |
|---|---|---|---|---|---|
| 13:0 * | 11.23 ± 1.10 | 20.54 ± 1.19 | 0.547 | 3.369 | 0.003 |
| 16:0 *+ | 166.21 ± 3.42 | 76.84 ± 7.13 | 2.163 | 6.382 | <0.001 |
| 18:0 * | 46.52 ± 2.96 | 33.36 ± 2.40 | 1.395 | 1.837 | 0.038 |
| 20:0 | 2.06 ± 0.12 | 1.61 ± 0.06 | 1.283 | 1.551 | 0.066 |
| 21:0 | 9.59 ± 0.02 | 9.59 ± 0.08 | 1.000 | 0.014 | 0.968 |
| 22:0 | 10.62 ± 0.02 | 10.61 ± 0.02 | 1.001 | 0.111 | 0.814 |
| 23:0 | 16.51 ± 0.08 | 16.57 ± 0.10 | 0.996 | 0.184 | 0.724 |
| 24:0 * | 18.04 ± 0.05 | 17.80 ± 0.05 | 1.014 | 1.896 | 0.038 |
| Total SFA * | 280.78 ± 5.29 | 186.91 ± 8.67 | 1.502 | 5.395 | <0.001 |
| 14:1 * | 86.45 ± 5.18 | 116.36 ± 8.06 | 0.743 | 2.020 | 0.033 |
| 16:1 | 11.63 ± 1.33 | 8.09 ± 2.04 | 1.437 | 0.786 | 0.245 |
| 17:1 | 14.37 ± 0.85 | 11.90 ± 0.91 | 1.207 | 1.020 | 0.182 |
| 18:1 (cis-9) *+ | 11.57 ± 0.90 | 5.66 ± 0.74 | 2.044 | 2.785 | 0.007 |
| 24:1 * | 23.86 ± 0.18 | 27.07 ± 0.77 | 0.881 | 3.298 | 0.003 |
| Total cis-MUFA * | 147.89 ± 4.69 | 169.09 ± 8.02 | 0.875 | 1.445 | 0.036 |
| 18:1 (trans-9) | 63.14 ± 4.52 | 47.45 ± 3.33 | 1.331 | 1.389 | 0.086 |
| Total trans-MUFA | 63.14 ± 4.52 | 47.45 ± 3.33 | 1.331 | 1.389 | 0.086 |
| 18:2 *+ | 94.45 ± 2.01 | 40.12 ± 3.47 | 2.354 | 6.862 | <0.001 |
| 20:2 | 18.48 ± 0.60 | 17.71 ± 0.16 | 1.044 | 0.430 | 0.459 |
| 20:3 | 13.17 ± 0.42 | 12.42 ± 0.12 | 1.061 | 0.666 | 0.302 |
| 20:4 | 20.88 ± 0.53 | 19.56 ± 0.46 | 1.067 | 0.885 | 0.228 |
| 20:5 | 18.74 ± 0.68 | 17.31 ± 0.05 | 1.082 | 0.801 | 0.245 |
| 22:2 | 9.33 ± 0.11 | 9.53 ± 0.11 | 0.979 | 0.582 | 0.344 |
| 22:6 | 74.23 ± 5.90 | 68.21 ± 4.24 | 1.088 | 0.301 | 0.583 |
| Total PUFA * | 249.27 ± 4.53 | 184.85 ± 8.17 | 1.349 | 4.459 | <0.001 |
| Total Fatty Acid * | 741.09 ± 11.43 | 588.30 ± 23.17 | 1.260 | 4.050 | <0.001 |
| Saturation Index * | 0.61 ± 0.02 | 0.47 ± 0.01 | 1.310 | 3.508 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodu, M.; Hitit, M.; Woldesenbet, S.; Uğur, M.R.; Erdoğan, Z.; Greenwood, O.C.; Murray, R.D.; Cervantes, A.P.; Memili, E. Lipidomic Landscapes of Cryopreserved Sperm from Alpine and Spanish–Creole Bucks. Animals 2025, 15, 1897. https://doi.org/10.3390/ani15131897
Bodu M, Hitit M, Woldesenbet S, Uğur MR, Erdoğan Z, Greenwood OC, Murray RD, Cervantes AP, Memili E. Lipidomic Landscapes of Cryopreserved Sperm from Alpine and Spanish–Creole Bucks. Animals. 2025; 15(13):1897. https://doi.org/10.3390/ani15131897
Chicago/Turabian StyleBodu, Mustafa, Mustafa Hitit, Selamawit Woldesenbet, Muhammet Raşit Uğur, Zeynep Erdoğan, Olivia Chika Greenwood, Raheem Davian Murray, Andres Pech Cervantes, and Erdoğan Memili. 2025. "Lipidomic Landscapes of Cryopreserved Sperm from Alpine and Spanish–Creole Bucks" Animals 15, no. 13: 1897. https://doi.org/10.3390/ani15131897
APA StyleBodu, M., Hitit, M., Woldesenbet, S., Uğur, M. R., Erdoğan, Z., Greenwood, O. C., Murray, R. D., Cervantes, A. P., & Memili, E. (2025). Lipidomic Landscapes of Cryopreserved Sperm from Alpine and Spanish–Creole Bucks. Animals, 15(13), 1897. https://doi.org/10.3390/ani15131897







