Balancing Objectivity and Welfare: Physiological and Behavioural Responses of Guide Dogs During an Independent Certification Protocol
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Subjects
2.3. Procedures
- Phase 1—Trainer Test: In the first 45 min session, the guide dog trainer, blindfolded to simulate visual impairment, was guided by the dog along a predefined public route, beginning on the campus (including a brief obedience task) and continuing through urban environments.
- Break: Following Phase 1, a break of at least 30 min was provided in a calm, park-like setting to allow the dog to rest, drink water, and gradually become acquainted with the blind tester.
- Phase 2—Tester Test: In the second 45 min session, the dog was tasked with guiding the unfamiliar blind tester along the return route back to campus. However, a different route as in Phase 1 was used. To ensure the independence of the evaluation, the trainer was instructed to leave the scene entirely—out of sight, hearing, and smell range of the dog—unless the dog refused to proceed with the unfamiliar person. In such cases (observed in one dog, Armani), the trainer followed the dog and tester at close distance to provide support without interfering with the test. This phase also included navigating various forms of public transport (e.g., tram, bus, and subway), further reflecting the realistic challenges of everyday life.
2.4. Data Collection and Analysis
2.4.1. Physiological Measure: Salivary Cortisol
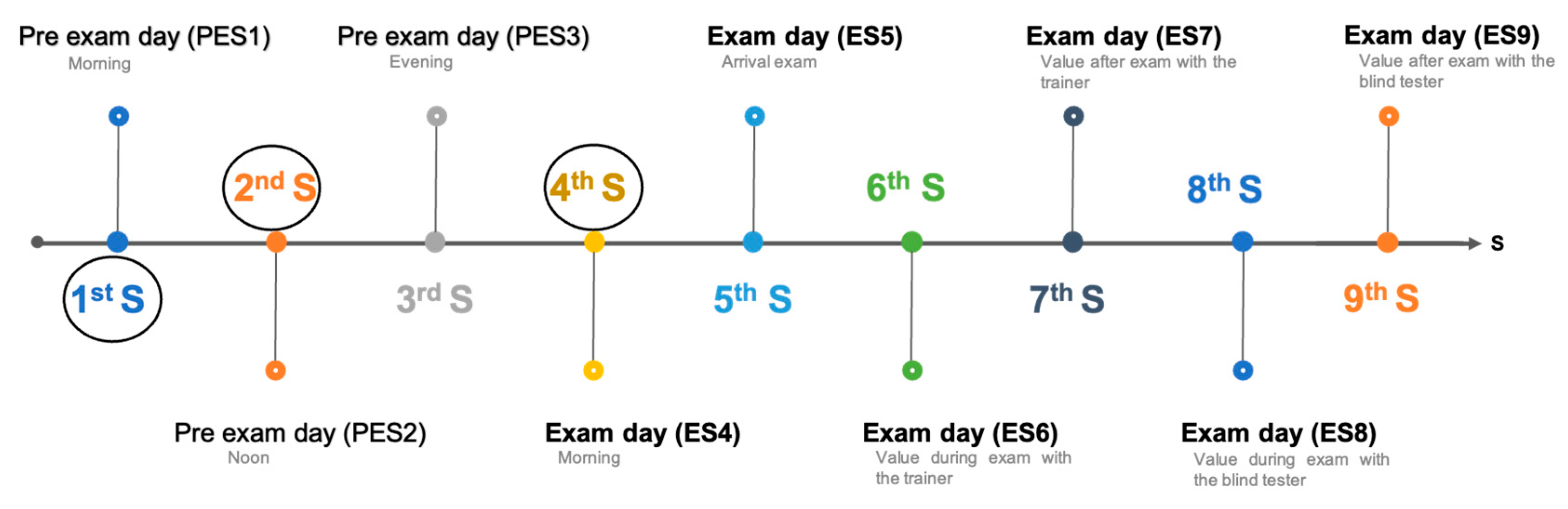
Sampling Schedule
- PES1: Morning, immediately after waking, before feeding or exercise.
- PES2: Noon, approximately six hours after PES1.
- PES3: Evening, approximately six hours after PES2.
- ES4: Morning, immediately after waking, before feeding or exercise.
- ES5: Start of Phase 1—Trainer Test.
- ES6: End of Phase 1—Trainer Test.
- ES7: Start of Phase 2—Tester Test.
- ES8: End of Phase 2—Tester Test.
- ES9: Approximately 30 min after ES8.
Analysis
2.4.2. Behavioural Measures
Sampling
Behavioural Variables
Statistical Analysis
3. Results
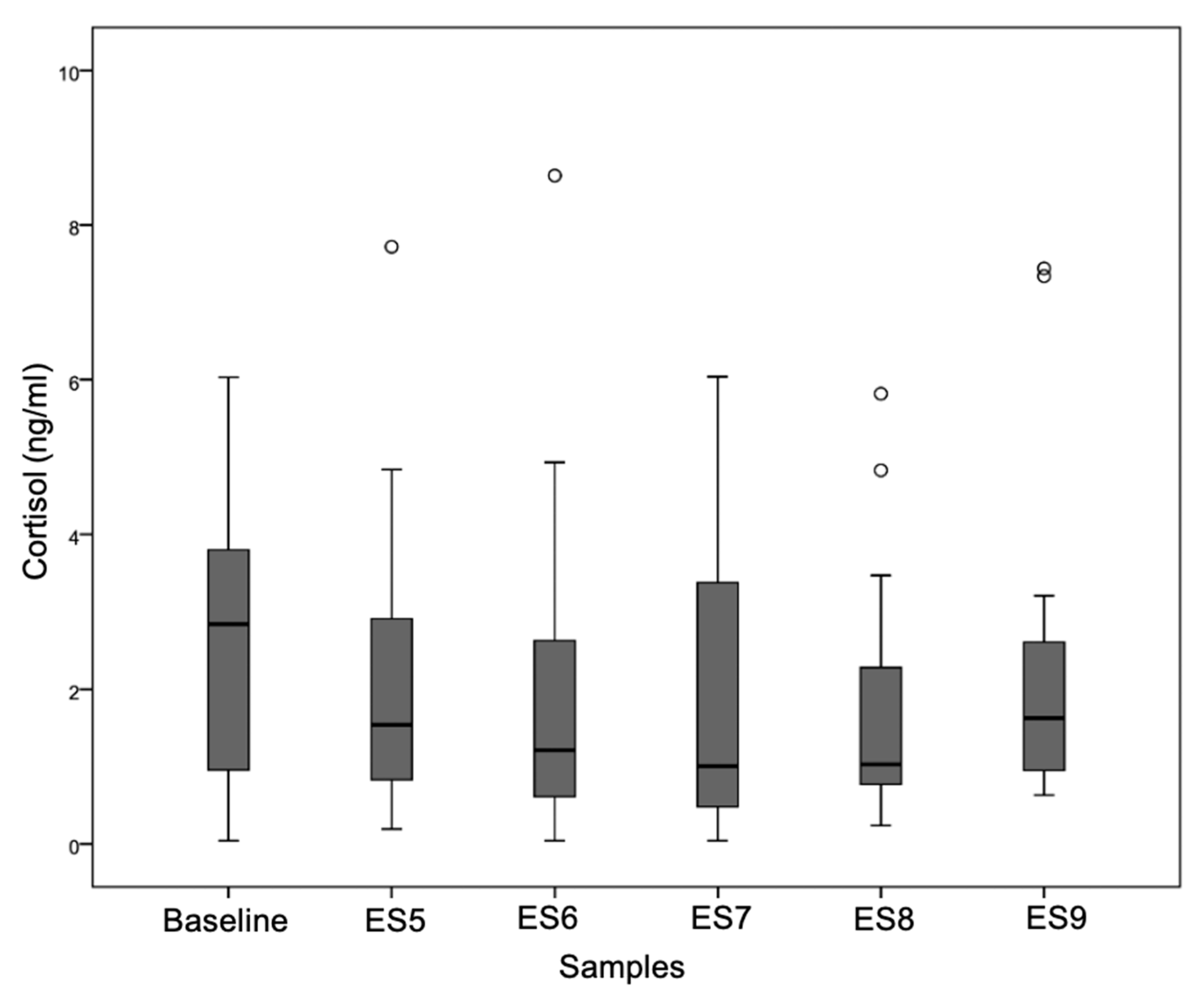
3.1. Physiological Parameter: Salivary Cortisol
3.1.1. Preliminary Analyses
Descriptive Statistics
Normality Testing
3.1.2. Inferential Analyses
Repeated Measures ANOVA
Pairwise Comparisons Between Time Points
Cortisol Profile
3.2. Behavioural Parameters
3.2.1. Reliability Analysis
3.2.2. Behavioural Data Analysis: Early Response (First 5 min)
3.2.3. Behavioural Data Analysis: Extended Response (First 15 min)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Audrestch, H.M.; Whelan, C.T.; Grice, D.; Asher, L.; England, G.C.W.; Freeman, S.L. Recognizing the Value of Assistance Dogs in Society. Disabil. Health J. 2015, 8, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Whitmarsh, L. The Benefits of Guide Dog Ownership. Vis. Impair. Res. 2005, 7, 27–42. [Google Scholar] [CrossRef]
- Lane, D.R.; McNicholas, J.; Collis, G.M. Dogs for the Disabled: Benefits to Recipients and Welfare of the Dog. Appl. Anim. Behav. Sci. 1998, 59, 49–60. [Google Scholar] [CrossRef]
- Glenk, L.M.; Weissenbacher, K.; Přibylová, L.; Stetina, B.U.; Demirel, S. Perceptions on Health Benefits of Guide Dog Ownership in an Austrian Population of Blind People with and without a Guide Dog. Animals 2019, 9, 428. [Google Scholar] [CrossRef]
- Lloyd, J.; Budge, C.; Stafford, K. Handlers’ Expectations and Perceived Compatibility Regarding the Partnership with Their First Guide Dogs. Animals 2021, 11, 2765. [Google Scholar] [CrossRef]
- Lloyd, J.; Budge, C.; La Grow, S.; Stafford, K. An Investigation of the Complexities of Successful and Unsuccessful Guide Dog Matching and Partnerships. Front. Vet. Sci. 2016, 3, 114. [Google Scholar] [CrossRef]
- Bremhorst, A.; Mongillo, P.; Howell, T.; Marinelli, L. Spotlight on Assistance Dogs—Legislation, Welfare and Research. Animals 2018, 8, 129. [Google Scholar] [CrossRef]
- Cobb, M.L.; Iskandarani, K.; Chinchilli, V.M.; Dreschel, N.A. A Systematic Review and Meta-Analysis of Salivary Cortisol Measurement in Domestic Canines. Domest. Anim. Endocrinol. 2016, 57, 31–42. [Google Scholar] [CrossRef]
- Ferrans, M.; Salomons, H.; Moore, K.; White, P.; Hare, B.; Gruen, M.E. Salivary Cortisol Is an Unreliable Correlate of Serum Cortisol in Adult Pet Dogs and Assistance Dog Puppies. Sci. Rep. 2025, 15, 15986. [Google Scholar] [CrossRef]
- Quervel-Chaumette, M.; Faerber, V.; Faragó, T.; Marshall-Pescini, S.; Range, F. Investigating Empathy-Like Responding to Conspecifics’ Distress in Pet Dogs. PLoS ONE 2016, 11, e0152920. [Google Scholar] [CrossRef]
- Pastore, C.; Pirrone, F.; Balzarotti, F.; Faustini, M.; Pierantoni, L.; Albertini, M. Evaluation of Physiological and Behavioral Stress-Dependent Parameters in Agility Dogs. J. Vet. Behav. 2011, 6, 188–194. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; Van Hooff, A.R.A.M.; De Vries, H.W. Manifestations of Chronic and Acute Stress in Dogs. Appl. Anim. Behav. Sci. 1997, 52, 307–319. [Google Scholar] [CrossRef]
- Mostl, E.; Palme, R. Measurement of Cortisol Metabolites in Faeces of Sheep as a Parameter of Cortisol Concentration in Blood. J. Mamm. Biol. 1997, 62, 192–197. [Google Scholar]
- Chacón Pérez, G.; García-Belenguer Laita, S.; Illera del Portal, J.C.; Palacio Liesa, J. Validation of an EIA Technique for the Determination of Salivary Cortisol in Cattle. Span. J. Agric. Res. 2004, 2, 45–51. [Google Scholar] [CrossRef]
- Heintz, M.R.; Santymire, R.M.; Parr, L.A.; Lonsdorf, E.V. Lonsdorf Validation of a Cortisol Enzyme Immunoassay and Characterization of Salivary Cortisol Circadian Rhythm in Chimpanzees (Pan Troglodytes). Am. J. Primatol. 2011, 73, 903–908. [Google Scholar] [CrossRef]
- Thomsson, O.; Ström-Holst, B.; Sjunnesson, Y.; Bergqvist, A.S. Validation of an Enzyme-Linked Immunosorbent Assay Developed for Measuring Cortisol Concentration in Human Saliva and Serum for Its Applicability to Analyze Cortisol in Pig Saliva. Acta Vet. Scand. 2014, 56, 55. [Google Scholar] [CrossRef]
- Kobelt, A.J.; Hemsworth, P.H.; Barnett, J.L.; Butler, K.L. Sources of Sampling Variation in Saliva Cortisol in Dogs. Res. Vet. Sci. 2003, 75, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Salivary Cortisol and Behavior in Therapy Dogs during Animal-Assisted Interventions: A Pilot Study. J. Vet. Behav. Clin. Appl. Res. 2014, 9, 98–106. [Google Scholar] [CrossRef]
- Vincent, I.C.; Michell, A.R. Comparison of Cortisol Concentrations in Saliva and Plasma of Dogs. Res. Vet. Sci. 1992, 53, 342–345. [Google Scholar] [CrossRef]
- Stellato, A.C.; Flint, H.E.; Widowski, T.M.; Serpell, J.A.; Niel, L. Assessment of Fear-Related Behaviours Displayed by Companion Dogs (Canis Familiaris) in Response to Social and Non-Social Stimuli. Appl. Anim. Behav. Sci. 2017, 188, 84–90. [Google Scholar] [CrossRef]
- Part, C.E.; Kiddie, J.L.; Hayes, W.A.; Mills, D.S.; Neville, R.F.; Morton, D.B.; Collins, L.M. Physiological, Physical and Behavioural Changes in Dogs (Canis Familiaris) When Kennelled: Testing the Validity of Stress Parameters. Physiol. Behav. 2014, 133, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, N.; Guo, K.; Wilkinson, A.; Resende, B.; Mills, D.S. Mouth-Licking by Dogs as a Response to Emotional Stimuli. Behav. Process. 2018, 146, 42–45. [Google Scholar] [CrossRef]
- Bremhorst, A.; Sutter, N.A.; Würbel, H.; Mills, D.S.; Riemer, S. Differences in Facial Expressions during Positive Anticipation and Frustration in Dogs Awaiting a Reward. Sci. Rep. 2019, 9, 19312. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; Bernadina, W.; Van Hooff, J.A.; De Vries, H.W.; Mol, J.A. Chronic Stress in Dogs Subjected to Social and Spatial Restriction. II. Hormonal and Immunological Responses. Physiol. Behav. 1999, 66, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Haverbeke, A.; Diederich, C.; Depiereux, E.; Giffroy, J.M. Cortisol and Behavioral Responses of Working Dogs to Environmental Challenges. Physiol. Behav. 2008, 93, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Z.; Dóka, A.; Miklósi, Á. Affiliative and Disciplinary Behavior of Human Handlers during Play with Their Dog Affects Cortisol Concentrations in Opposite Directions. Horm. Behav. 2008, 54, 107–114. [Google Scholar] [CrossRef]
- Schöberl, I.; Wedl, M.; Bauer, B.; Day, J.; Möstl, E.; Kotrschal, K. Effects of Owner—Dog Relationship and Owner Personality on Cortisol Modulation in Human—Dog Dyads. Anthrozoos 2012, 25, 199–214. [Google Scholar] [CrossRef]
- De la Fuente-Moreno, E.; Paredes-Ramos, P.; Carrasco-García, A.; Hernandez-Cruz, B.; Alvarado, M.; Edwards, C. Salivary Cortisol in Guide Dogs. Animals 2023, 13, 1981. [Google Scholar] [CrossRef]
- Cimarelli, G.; Turcsán, B.; Range, F.; Virányi, Z. The Other End of the Leash: An Experimental Test to Analyze How Owners Interact with Their Pet Dogs. J. Vis. Exp. 2017, 2017, 56233. [Google Scholar] [CrossRef]
- Thoits, P.A. Mechanisms Linking Social Ties and Support to Physical and Mental Health. J. Health Soc. Behav. 2011, 52, 145–161. [Google Scholar] [CrossRef]
- Sümegi, Z.; Oláh, K.; Topál, J. Emotional Contagion in Dogs as Measured by Change in Cognitive Task Performance. Appl. Anim. Behav. Sci. 2014, 160, 106–115. [Google Scholar] [CrossRef]
- Huber, A.; Barber, A.L.A.; Faragó, T.; Müller, C.A.; Huber, L. Investigating Emotional Contagion in Dogs (Canis familiaris) to Emotional Sounds of Humans and Conspecifics. Anim. Cogn. 2017, 20, 703–715. [Google Scholar] [CrossRef]
- Albuquerque, N.; Resende, B. Dogs Functionally Respond to and Use Emotional Information from Human Expressions. Evol. Hum. Sci. 2022, 5, e2. [Google Scholar] [CrossRef]
- Roy, T.; Saroka, K.S.; Hossack, V.L.; Dotta, B.T. The Effects of Exam-Induced Stress on EEG Profiles and Memory Scores. Behav. Sci. 2023, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Kubo, T.; Yamakawa, T.; Fujiwara, K.; Nomoto, K.; Ikeda, K.; Mogi, K.; Nagasawa, M.; Kikusui, T. Emotional Contagion from Humans to Dogs Is Facilitated by Duration of Ownership. Front. Psychol. 2019, 10, 1678. [Google Scholar] [CrossRef]
- Rivera, M.M.; Meyers-Manor, J.E. Beware of Strangers: Dogs’ Empathetic Response to Unknown Humans. Animals 2024, 14, 2130. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Lourenco, A.; Lima, M.; Serpell, J.; Silva, K. Evaluation of Mediating and Moderating Effects on the Relationship between Owners’ and Dogs’ Anxiety: A Tool to Understand a Complex Problem. J. Vet. Behav. 2021, 44, 55–61. [Google Scholar] [CrossRef]
- Lit, L.; Boehm, D.; Marzke, S.; Schweitzer, J.; Oberbauer, A.M. Certification Testing as an Acute Naturalistic Stressor for Disaster Dog Handlers. Stress 2010, 13, 392–401. [Google Scholar] [CrossRef]
- Zubedat, S.; Aga-Mizrachi, S.; Cymerblit-Sabba, A.; Shwartz, J.; Leon, J.F.; Rozen, S.; Varkovitzky, I.; Eshed, Y.; Grinstein, D.; Avital, A. Human–Animal Interface: The Effects of Handler’s Stress on the Performance of Canines in an Explosive Detection Task. Appl. Anim. Behav. Sci. 2014, 158, 69–75. [Google Scholar] [CrossRef]
- Sundman, A.S.; Van Poucke, E.; Svensson Holm, A.C.; Faresjö, Å.; Theodorsson, E.; Jensen, P.; Roth, L.S.V. Long-Term Stress Levels Are Synchronized in Dogs and Their Owners. Sci. Rep. 2019, 9, 7391. [Google Scholar] [CrossRef]
- Wanser, S.H.; Udell, M.A.R. Does Attachment Security to a Human Handler Influence the Behavior of Dogs Who Engage in Animal Assisted Activities? Appl. Anim. Behav. Sci. 2019, 210, 88–94. [Google Scholar] [CrossRef]
- Scandurra, A.; Alterisio, A.; Di Cosmo, A.; D’Aniello, B. Behavioral and Perceptual Differences between Sexes in Dogs: An Overview. Animals 2018, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Bidoli, E.M.Y.; Erhard, M.H.; Döring, D. Heart Rate and Heart Rate Variability in School Dogs. Appl. Anim. Behav. Sci. 2022, 248, 105574. [Google Scholar] [CrossRef]
- Kooriyama, T.; Ogata, N. Salivary Stress Markers in Dogs: Potential Markers of Acute Stress. Res. Vet. Sci. 2021, 141, 48–55. [Google Scholar] [CrossRef] [PubMed]
| Name | Breed | Age Months | Sex | Dog Cortisol Data | Dog Behavioural Data | Trainer Sex | Tester Sex |
|---|---|---|---|---|---|---|---|
| Elisa -Pixie | Collie (long hair) | 30 | f | ok | ok | f | f |
| Tasha | Labrador Retriever | 23 | f | ok | ok | m | m |
| Mira | Labrador Retriever | 26 | f | ok | ok | m | f |
| Darla | Labrador Retriever | 25 | f | ok | ok | m | f |
| Sam | Labrador-Hovawarth mixed breed | 45 | m | ok | ok | m | m |
| Hadis | Curly Coated Retriever | 32 | m | ok | ok | f | m |
| Harmony | Curly Coated Retriever | 21 | f | ok | ok | f | m |
| Darell | Labrador Retriever | 26 | m | ok | ok | m | m |
| Maya | Labrador Retriever | 18 | f | ✕ | ok | m | m |
| Pablo | Labrador Retriever | 24 | m | ok | ok | m | m |
| Coco | Flat Coated Retriever | 22 | f | ok | ok | f | f |
| Armani | Poodle | 23 | m | ok | ok | f | f |
| Colleen | Collie (long hair) | 29 | f | ok | ✕ | f | ✕ |
| Sultan | Golden Retriever | 24 | m | ok | ✕ | m | ✕ |
| Category | Behaviour | Definition |
|---|---|---|
| Stress-related behaviours (dog) | Lip licking * | Tongue is moving from the front part of the mouth sideways to the upper lip |
| Smacking * | Dog opens and closes mouth immediately | |
| Yawning * | Dog opens the mouth widely to the fullest extent while moving the ears sideways | |
| Shaking * | Moving mostly the whole part of the body from one side to the other starting from the head caudally | |
| Scratching * | Scratching a body part with one of the hind limbs | |
| Stretching | Putting the front of the body down while lengthening the front limbs and/or the hind limbs while holding one limb after each other in the air | |
| Task-related performance behaviours (dog) | Refuse signal | Handler gives a cue and dog does not respond to the cue |
| Turn around | Dog turns head more than 90 degrees to the left or right side and looks back over shoulder | |
| Handler behaviours (human) | Treat | Handler is delivering food reward to the dog |
| Praise | Handler is praising dog verbally |
| Behaviour | ICC | 95% CI | F-Test | p-Value |
|---|---|---|---|---|
| Lip licking | 0.992 | 0.963–0.998 | F(7,8) = 240 | <0.001 |
| Shaking | 1.00 | Not computable | ||
| Smacking | 0.832 | 0.413–0.963 | F(7,8) = 10.9 | 0.002 |
| Scratching | 1.00 | Not computable | ||
| Yawning | 1.00 | Not computable | ||
| Turn around | 0.823 | 0.389–0.961 | F(7,8) = 10.3 | 0.002 |
| Refuse signal | 0.936 | 0.741–0.987 | F(7,8) = 30.4 | <0.001 |
| Praise | 0.991 | 0.962–0.998 | F(7,8) = 231 | <0.001 |
| Treat | 0.963 | 0.841–0.992 | F(7,8) = 52.6 | <0.001 |
| Parameter | B | SE | Df | t | p |
|---|---|---|---|---|---|
| |||||
| Phase 2 (Tester) | −0.191515 | 0.20 | 20.00 | −0.944 | 0.356 |
| Sex (Male) | 0.260870 | 0.23 | 20.00 | 1.118 | 0.277 |
| Age (Months) | −0.007416 | 0.02 | 20.00 | −0.433 | 0.670 |
| |||||
| Turn around | |||||
| Phase 2 (Tester) | 0.955402 | 0.37 | 10.999975 | 2.592 | 0.025 |
| Sex (Male) | 0.956772 | 0.44 | 8.999980 | 2.191 | 0.056 |
| Age (Months) | 0.002562 | 0.03 | 8.999980 | 0.080 | 0.938 |
| Refuse signal | |||||
| Phase 2 (Tester) | 0.59747 | 0.41 | 10.98185 | 1.451 | 0.175 |
| Sex (Male) | −0.01532 | 0.47 | 8.98190 | −0.032 | 0.975 |
| Age (Months) | 0.01973 | 0.03 | 8.98191 | 0.567 | 0.585 |
| |||||
| Praise | |||||
| Phase 2 (Tester) | 3.00000 | 2.00 | 11.00000 | 1.497 | 0.163 |
| Sex (Male) | −3.03543 | 2.69 | 9.00000 | −1.130 | 0.288 |
| Age (Months) | −0.04782 | 0.20 | 9.00000 | −0.242 | 0.814 |
| Treat | |||||
| Phase 2 (Tester) | 0.10033 | 0.27 | 20.00000 | 0.373 | 0.713 |
| Sex (Male) | −0.27617 | 0.31 | 20.00000 | −0.893 | 0.383 |
| Age (Months) | −0.00382 | 0.02 | 20.00000 | −0.168 | 0.868 |
| Parameter | B | SE | Df | t | p |
|---|---|---|---|---|---|
| |||||
| Test Phase (2) | −0.40065 | 0.19 | 11.00 | −2.120 | 0.058 |
| Sex (Male) | 0.38739 | 0.31 | 9.00 | 1.259 | 0.240 |
| Age (Months) | −0.02424 | 0.02 | 9.00 | −1.072 | 0.312 |
| |||||
| Turn around | |||||
| Test Phase (2) | 9.4167 | 3.14 | 20.00 | 3.003 | 0.007 |
| Sex (Male) | 8.8142 | 3.61 | 20.00 | 2.443 | 0.024 |
| Age (Months) | −0.1955 | 0.27 | 20.00 | −0.738 | 0.469 |
| Refuse signal | |||||
| Test Phase (2) | 6.83333 | 3.92 | 20.00 | 1.742 | 0.097 |
| Sex (Male) | 0.70524 | 4.51 | 20.00 | 0.156 | 0.877 |
| Age (Months) | 0.06807 | 0.33 | 20.00 | 0.205 | 0.839 |
| |||||
| Praise | |||||
| Test Phase (2) | 14.3333 | 4.73 | 11.0000 | 3.028 | 0.012 |
| Sex (Male) | −9.5996 | 8.09 | 9.0000 | −1.186 | 0.266 |
| Age (Months) | −0.1023 | 0.59 | 9.0000 | −0.172 | 0.867 |
| Treat | |||||
| Test Phase (2) | −0.529676 | 0.25 | 10.999961 | −2.127 | 0.057 |
| Sex (Male) | −0.383759 | 0.32 | 8.999995 | −1.183 | 0.267 |
| Age (Months) | 0.004237 | 0.02 | 8.999995 | 0.178 | 0.863 |
| Category | Variable | Early Period: 5 min | Extended Period: 15 min |
|---|---|---|---|
| Physiological analysis | Cortisol | Phase 1 = Phase 2 | |
| Behavioural analysis | |||
| Stress-related behaviours | SSScore | Phase 1 = Phase 2 | Phase 1 = Phase 2 |
| Task-related performance behaviours | Turn around | Phase 1 < Phase 2 * | Phase 1 < Phase 2 * |
| Refuse signal | Phase 1 = Phase 2 | Phase 1 = Phase 2 | |
| Handler behaviours | Praise | Phase 1 = Phase 2 | Phase 1 < Phase 2 * |
| Treat | Phase 1 = Phase 2 | Phase 1 = Phase 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faerber-Morak, V.; Glenk, L.-M.; Weissenbacher, K.; Bremhorst, A. Balancing Objectivity and Welfare: Physiological and Behavioural Responses of Guide Dogs During an Independent Certification Protocol. Animals 2025, 15, 1896. https://doi.org/10.3390/ani15131896
Faerber-Morak V, Glenk L-M, Weissenbacher K, Bremhorst A. Balancing Objectivity and Welfare: Physiological and Behavioural Responses of Guide Dogs During an Independent Certification Protocol. Animals. 2025; 15(13):1896. https://doi.org/10.3390/ani15131896
Chicago/Turabian StyleFaerber-Morak, Viola, Lisa-Maria Glenk, Karl Weissenbacher, and Annika Bremhorst. 2025. "Balancing Objectivity and Welfare: Physiological and Behavioural Responses of Guide Dogs During an Independent Certification Protocol" Animals 15, no. 13: 1896. https://doi.org/10.3390/ani15131896
APA StyleFaerber-Morak, V., Glenk, L.-M., Weissenbacher, K., & Bremhorst, A. (2025). Balancing Objectivity and Welfare: Physiological and Behavioural Responses of Guide Dogs During an Independent Certification Protocol. Animals, 15(13), 1896. https://doi.org/10.3390/ani15131896






