Disjunct Northern Populations as Reservoirs of Evolutionary Diversity: Insights from the Aesculapian Snake (Zamenis longissimus)
Simple Summary
Abstract
1. Introduction

2. Materials and Methods
2.1. Study Areas
2.1.1. Želinský Meander
2.1.2. Central Bohemia
2.2. Monitoring and Survey Data
2.2.1. Želinský Meander
2.2.2. Central Bohemia
2.3. Genetic Analysis
2.3.1. Samples
2.3.2. DNA Extraction and Sequencing
2.3.3. Data Analysis
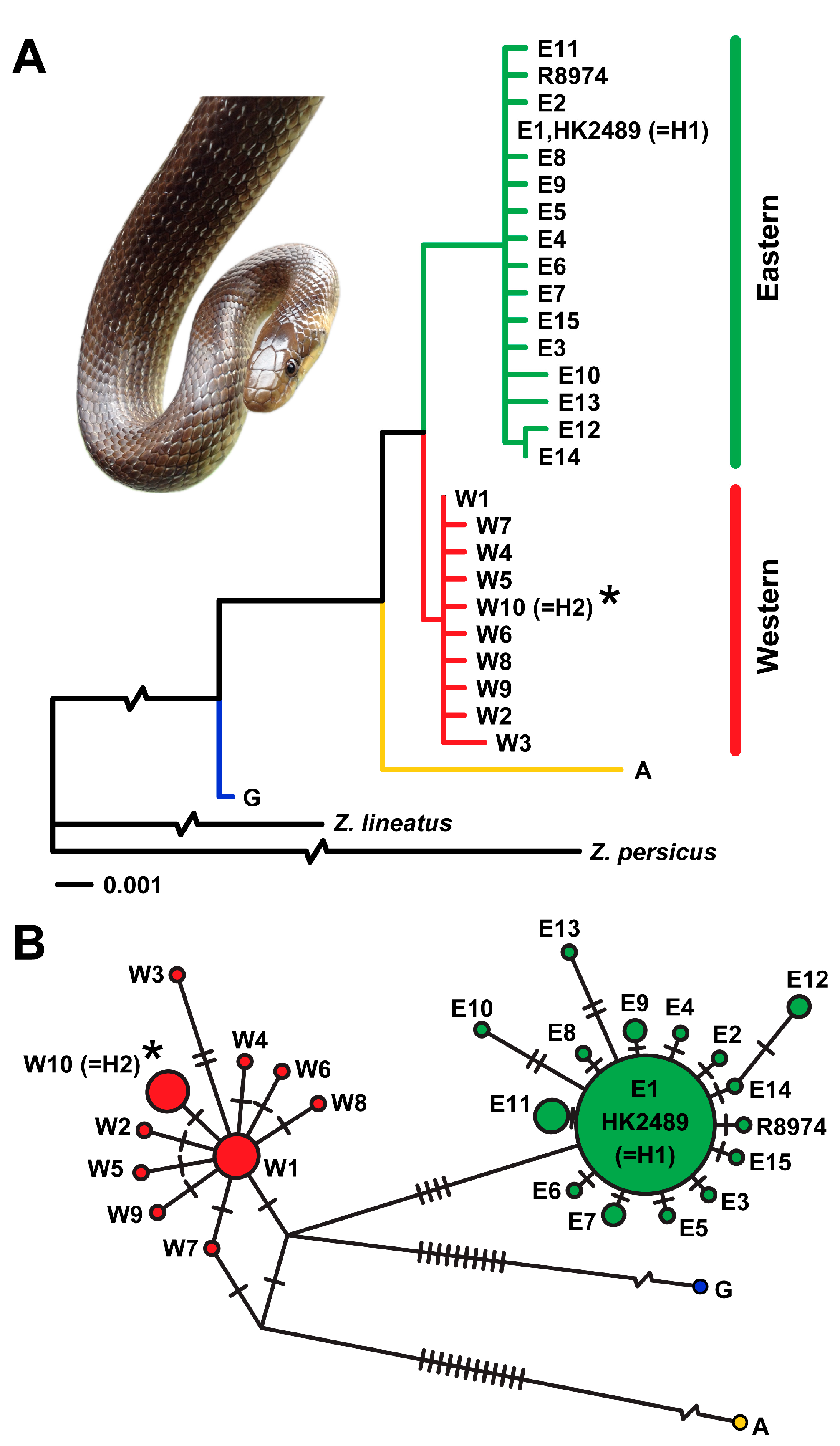
3. Results
3.1. Želinský Meander
3.2. Central Bohemia
4. Discussion
4.1. Želinský Meander
4.2. Central Bohemia
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IUCN | International Union for Conservation of Nature |
| SO | Municipality of Stráž nad Ohří and its surroundings |
| ZM | Želinský meander Nature Monument |
| CB | The middle Povltaví and lower Posázáví in Central Bohemia |
References
- Meek, M.H.; Beever, E.A.; Barbosa, S.; Fitzpatrick, S.W.; Fletcher, N.K.; Mittan-Moreau, C.S.; Reid, N.B.; Campbell-Staton, S.C.; Green, N.F.; Hellmann, J.J. Understanding local adaptation to prepare populations for climate change. BioScience 2023, 73, 36–47. [Google Scholar] [CrossRef]
- Kelly, E.; Phillips, B.L. Targeted gene flow for conservation. Conserv. Biol. 2016, 30, 259–267. [Google Scholar] [CrossRef]
- Rehák, I. Importance of reptiles to humans. In Reptiles—Reptilia, Fauna ČSFR; Baruš, V., Oliva, O., Eds.; Academia: Praha, Czech Republic, 1992; Volume 26, pp. 50–53. (In Czech) [Google Scholar]
- Böhme, W.; Koppetsch, T. Snake names in the Greek-Roman antiquity: Old characterizations, identity in current zoology, and change of their original meaning in post-Linnean herpetology. Salamandra 2021, 57, 475–501. [Google Scholar]
- Salvi, D.; Mendes, J.; Carranza, S.; Harris, D.J. Evolution, biogeography and systematics of the western Palaearctic Zamenis ratsnakes. Zool. Scr. 2018, 47, 441–461. [Google Scholar] [CrossRef]
- The Reptile Database. Available online: http://www.reptile-database.org (accessed on 1 May 2025).
- Musilová, R.; Zavadil, V.; Marková, S.; Kotlík, P. Relics of the Europe’s warm past: Phylogeography of the Aesculapian snake. Mol. Phylogenetics Evol. 2010, 57, 1245–1252. [Google Scholar] [CrossRef]
- Böhme, W. Äskulapnatter (Elaphe longissima LAURENTI, 1768). In Handbuch der Reptilien und Amphibien Europas; Böhme, W., Ed.; Aula Verlag: Wiesbaden, Germany, 1993; Volume 3, pp. 331–372. (In German) [Google Scholar]
- Edgar, P.; Bird, D.R. Action Plan for the Conservation of the Aesculapian Snake (Zamenis longissimus) in Europe. In Convention on the Conservation of European Wildlife and Natural Habitats; Council of Europe: Convention on the Conservation of European Wildlife and Natural Habitats; Standing Committee: Strasbourg, France, 2006; pp. 1–23. [Google Scholar]
- Musilová, R.; Zavadil, V.; Kotlík, P. Isolated populations of Zamenis longissimus (Reptilia: Squamata) above the northern limit of the continuous range in Europe: Origin and conservation status. Acta Soc. Zool. Bohem. 2007, 71, 197–208. [Google Scholar]
- Musilová, R.; Zavadil, V.; Kotlík, P.; Moravec, J. Zamenis longissimus (Laurenti, 1768)—užovka stromová. In Fauna ČR, Plazi—Reptilia; Moravec, J., Ed.; Academia: Praha, Czech Republic, 2015; pp. 304–333. (In Czech) [Google Scholar]
- IUCN Red List of Threatened Species: Zamenis longissimus. Available online: https://www.iucnredlist.org/species/157266/49063773#assessment-information (accessed on 1 May 2025).
- Peters, G. Die Reptilien aus dem fossilen Tierbautensystem von Pisede bei Malchen. Wissensch. Zeitschr. Humboldt-Uni Math. Nat. R. 1977, 26, 307–326. [Google Scholar]
- Szyndlar, Z. Fossil snakes from Poland. Acta Zool. Cracov. 1984, 28, 1–156. [Google Scholar]
- Böhme, G. Zur Verbreitungsgeschichte der Herpetofaunen des jüngeren Quartärs im nördlichen Deutschland. Rana Sonderh. 1999, 3, 5–11. [Google Scholar]
- Allentoft, M.E.; Rasmussen, A.R.; Kristensen, H.V. Centuries-old DNA from an extinct population of Aesculapian snake (Zamenis longissimus) offers new phylogeographic insight. Diversity 2018, 10, 14. [Google Scholar] [CrossRef]
- Alkins, D. Habitat Selection of a Non-Native Snake: Implications for Future Management of Zamenis longissimus in Colwyn Bay, North Wales. Ph.D. Thesis, Bangor University, Bangor, UK, 2021. [Google Scholar]
- Clemens, D.J.; Allain, S.J. First record of the Aesculapian snake (Zamenis longissimus) in South Wales. Herpetol. Bull. 2020, 152, 30–31. [Google Scholar] [CrossRef]
- Major, T. The Ecology, Biogeography, and Taxonomy of Isolated Snake Populations. Ph.D. Thesis, Bangor University, Bangor, UK, 2024. [Google Scholar]
- Gomille, A. Die Äskulapnatter, Elaphe longissimi. In Verbreitung und Lebensweise in Mitteleuropa; Chimaira: Frankfurt am Main, Germany, 2002; 158p. (In German) [Google Scholar]
- Hartman, L. Die Äskulapnatter im Taunus. Jahrbücher Nassau. Ver. Naturkunde 2021, 142, 57–77. [Google Scholar]
- Šolcová-Danihelková, M. Occurrence of the Aesculapian Snake (Elaphe longissima Laur.) in the Karlovy Vary Region. Sborník Biol. Geol. Věd Pedagog. Fak. 1966, 2, 183–187. (In Czech) [Google Scholar]
- Zavadil, V.; Musilová, R.; Jeřábková, L.; Moravec, J. Užovka stromová—Výskyt v České republice. In Fauna ČR, Plazi—Reptilia; Moravec, J., Ed.; Academia: Praha, Czech Republic, 2015; pp. 333–335. (In Czech) [Google Scholar]
- Horníková, M.; Lanier, H.C.; Marková, S.; Escalante, M.A.; Searle, J.B.; Kotlík, P. Genetic admixture drives climate adaptation in the bank vole. Commun. Biol. 2024, 7, 863. [Google Scholar] [CrossRef]
- Management Plan for the Želinský Meander Natural Monument for the Period 2013–2022. Available online: https://drusop.nature.cz/ost/archiv/plany_pece/ug_file.php?FULLTEXT_UPLOAD=&RECORD_ID=25557 (accessed on 1 May 2025). (In Czech).
- Quitt, E. Method of climatic regionalization of the western part of the Czechoslovakia. Sborník Českosl. Spol. Zem. 1968, 73, 118–129. (In Czech) [Google Scholar]
- Quitt, E. 1971: Climatic regions of Czechoslovakia. Stud. Geogr. 1971, 16, 1–73. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zavadil, V.; Tejrovský, V.; Matějů, J. Summary of the current knowledge on the distribution of the Aesculapian snake (Zamenis longissimus) in the Ústí nad Labem region. Sborník Muz. Karlov. Kraj. 2016, 24, 225–238. (In Czech) [Google Scholar]
- Fritz, U.; Guicking, D.; Kami, H.; Arakelyan, M.; Auer, M.; Ayaz, D.; Fernández, C.A.; Bakiev, A.; Celani, A.; Džukić, G.; et al. Mitochondrial phylogeography of European pond turtles (Emys orbicularis, Emys trinacris)—An update. Amphibia-Reptil. 2007, 28, 418–426. [Google Scholar] [CrossRef]
- Krása, A. New locality of the Aesculapian snake in Povltaví. Herpetol. Inf. 2018, 15–16, 19. (In Czech) [Google Scholar]
- Vaissi, S.; Kürşat Şahin, M.; Kurnaz, M. Niche dynamics and climate change sensitivity in western Palearctic Zamenis ratsnakes (Reptilia: Colubridae). Amphibia-Reptil. 2024, 46, 33–50. [Google Scholar] [CrossRef]
- Zahradníček, P.; Brázdil, R.; Štěpánek, P.; Trnka, M. Reflections of global warming in trends of temperature characteristics in the Czech Republic, 1961–2019. Int. J. Clim. 2020, 41, 1211–1229. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehák, I.; Musilová, R.; Marková, S.; Fischer, D.; Kotlík, P. Disjunct Northern Populations as Reservoirs of Evolutionary Diversity: Insights from the Aesculapian Snake (Zamenis longissimus). Animals 2025, 15, 1894. https://doi.org/10.3390/ani15131894
Rehák I, Musilová R, Marková S, Fischer D, Kotlík P. Disjunct Northern Populations as Reservoirs of Evolutionary Diversity: Insights from the Aesculapian Snake (Zamenis longissimus). Animals. 2025; 15(13):1894. https://doi.org/10.3390/ani15131894
Chicago/Turabian StyleRehák, Ivan, Radka Musilová, Silvia Marková, David Fischer, and Petr Kotlík. 2025. "Disjunct Northern Populations as Reservoirs of Evolutionary Diversity: Insights from the Aesculapian Snake (Zamenis longissimus)" Animals 15, no. 13: 1894. https://doi.org/10.3390/ani15131894
APA StyleRehák, I., Musilová, R., Marková, S., Fischer, D., & Kotlík, P. (2025). Disjunct Northern Populations as Reservoirs of Evolutionary Diversity: Insights from the Aesculapian Snake (Zamenis longissimus). Animals, 15(13), 1894. https://doi.org/10.3390/ani15131894








