Simple Summary
Toxoplasma gondii causes toxoplasmosis, which can develop into severe symptoms in pregnant and immunocompromised people. The T. gondii diagnosis relies on both direct and indirect methods with various specificities and sensitivities. The serodiagnosis has been introduced to T. gondii detection such as latex agglutination test (LAT), indirect immunofluorescent assay (IFAT), and enzyme-linked immunosorbent assays (ELISA). Recombinant antigens are commonly used in serodiagnosis and combinations of recombinant antigens have shown improved serodiagnosis efficacy. In this study, TIM-barrel-based proteins containing T. gondii B- and T-cell epitopes were used to detect T. gondii infection in cats via ELISA assay. Among 10 chimeric protein constructions, V4Z revealed 86% sensitivity and 76% specificity with moderate agreement with the reference IFAT. These findings suggest that TIM-barrel-based multi-epitope proteins are promising candidates for chimeric antigens in serological detection, which could be applied in pathogen detection by incorporating immunodominant epitopes.
Abstract
Toxoplasma gondii, a pathogen of significant concern in animal production, companion animal health, and public health, particularly affects immunocompromised individuals and pregnant women. Current diagnostic techniques employ both direct and indirect methods, with serological assays widely used for detecting T. gondii infections in humans and animals. In this study, the TIM-barrel structure of Br2 β-glucosidase was engineered to create 10 chimeric multi-epitope proteins for T. gondii serological detection. Indirect ELISA screening identified three promising candidate proteins, V4Z, SFF, and S7V-V4Z-SFF, with sensitivities ranging from 71–86% and specificities ranging from 68–76%. Among these, ELISA-V4Z achieved the highest concordance with the reference IFAT method (Kappa = 0.58, 95% CI = 0.32–0.84) and demonstrated a moderate positive predictive value (PPV, 67%) and strong negative predictive value (NPV, 90%). These results suggest that the V4Z chimeric protein demonstrated the strongest performance among the tested candidates for T. gondii detection, exhibiting the highest sensitivity and specificity along with moderate agreement with the reference IFAT. However, its overall diagnostic performance remains limited. These findings highlight the need for further refinement and validation to enhance its diagnostic potential and assess its applicability for broader serological testing.
1. Introduction
Toxoplasma gondii is an obligate intracellular protozoan and one of the most significant zoonotic pathogens, widely distributed among humans and nearly all warm-blooded animals. Its prevalence poses substantial challenges to public health, animal production, and companion animal health globally, particularly due to the role of cats as definitive hosts and the impact on immunosuppressed companion animals [1,2,3]. T. gondii transmission occurs primarily through the ingestion of raw or improperly cooked animal products (meat, milk, eggs) containing tissue cysts or through the consumption of contaminated vegetables, fruits, or water with oocysts shed by infected cats, the definitive hosts [4]. In immunocompetent adults, primary T. gondii infections are often asymptomatic or manifest as mild, flu-like illnesses. However, in immunosuppressed individuals, acute cerebral or systemic diseases can develop. Pregnant women infected with T. gondii may experience miscarriage or stillbirth, and the infection is associated with several neurological disorders in both mothers and newborns. Recent systematic reviews estimate that 25–33% of the global population is seropositive for T. gondii, with notable regional variation: Africa (61.4%), Oceania (38.5%), South America (31.2%), Europe (29.6%), North America (17.5%), and Asia (16.4%). In companion animals, seroprevalence of T. gondii varies considerably, with reported ranges in cats from 11% to over 70%, depending on region and diagnostic method. [5,6,7,8,9]. In Thailand, over three million cats live in close proximity to humans and significantly contribute to the transmission of T. gondii to humans [1,10]. The effective prevention, control, and treatment of toxoplasmosis are hindered by the often asymptomatic or non-specific clinical presentations of the disease, underscoring the need for highly sensitive and specific diagnostic methods [11]. Diagnostic approaches are broadly categorized into direct and indirect methods. Direct methods, including microscopic examination, polymerase chain reaction (PCR), and bioassays, are high specificity but low sensitivity as they require the presence of detectable parasites in the sample. Conversely, indirect methods, particularly serological assays such as the dye test (DT), direct or modified agglutination test (DAT/MAT), indirect hemagglutination test (IHA), latex agglutination test (LAT), indirect immunofluorescent assay (IFAT), and enzyme-linked immunosorbent assays (ELISA), are currently preferred for routine T. gondii diagnosis in both humans and animals due to their practicality and cost-effectiveness [12,13,14]. Among these serological tests, ELISA stands out for its reliability, practicality, and economical advantages, making it widely adopted for detecting T. gondii infections in animals. While commercial ELISA kits are available, there is also a significant emphasis on developing in-house ELISAs utilizing novel antigens and antibodies to enhance diagnostic performance [15,16].
Three main types of antigens, including native, recombinant, and chimeric proteins, have been used for T. gondii diagnosis. Native antigens, typically derived from whole tachyzoites or tachyzoite-based products, require culturing, loss during purification, batch-to-batch variability, and complicating standardization. Recombinant proteins are a viable alternative for producing antigens for serological diagnosis [13,14,17]. Recombinant antigens include dense granule proteins (GRA), cyst matrix antigens (MAG), microneme proteins (MIC), surface antigens (SAG), rhoptry antigens (ROP), and various peptide fragments [12,13,14,16]. Among these, GRAs, particularly GRA7, are extensively studied and have demonstrated sensitivities and specificities ranging from 35.1 to 89.7% and 89.9 to 96.0%, respectively, in feline studies [17,18,19]. Moreover, combinations of recombinant antigens (e.g., SAG2 + GRA2 + GRA6 + GRA7 + GRA15) have shown improved diagnostic performance, achieving sensitivities of 89.2% and specificities of 95.4% compared to single-antigen assays [18]. Chimeric antigens represent a significant advancement in diagnostic approach by incorporating multiple epitopes to enhance sensitivity and specificity. [13,16,20]. Computational methods facilitate the prediction of immunoreactive epitopes based on the physicochemical properties of amino acids, enabling the design of antigens recognized by T-cell and B-cell receptors [21,22,23,24,25]. This approach has been used in various pathogens with high accuracy and no cross-reactivity in Mayaro virus [26], 98.8% sensitivity and 100% specificity in West Nile virus [27], and 100% sensitivity and specificity in Trypanosoma cruzi [28] detections. However, only a few studies have investigated the use of chimeric antigens for detecting T. gondii in animals such as horses, pigs, sheep, and goats, with reported sensitivities ranging from 28.4% to 100% and specificities from 95.1% to 100% [29,30,31]. Notably, only a SAG1-GRA8 chimeric antigen has been used to detect T. gondii in cats [32]. In addition, most of the current studies on T. gondii epitopes have focused on characterized proteins, which may lose some potential proteins, especially uncharacterized proteins [33]. A classical (β/α)8-TIM barrel structure contains the central eight β-sheet surrounded by the eight α-helices. The β-strand core interconnects with βα-loops to form a catalytic face and αβ-loops to form a stable face of protein structure. The outer α-helical surfaces interact with other proteins, peptides, or substrates, which may show potential for multi-epitope replacement and be used in serological diagnosis [34].
This study aims to engineer a Br2 β-glucosidase, derived from the bovine rumen metagenome [35] and characterized by a classical (β/α)8-TIM barrel structure [36]. The outer α-helices were replaced by a novel group of B-cell and T-cell epitopes, identified from T. gondii membrane proteins through a genome-wide screening by Li et al. [33], to construct the multi-epitope proteins for serological detection of T. gondii infections in cat.
2. Materials and Methods
2.1. Cat Serum Preparation and ETHICAL Approval
Serum samples from 39 mixed-breed cats (17 females and 22 males), aged 1 to 7 years (mean ± standard deviation: 2.13 ± 1.34 years), were obtained from a previous study [19]. These animals were sampled from the Rabies Control Division, Buddhist temples, and animal clinics within the Bangkok metropolitan area, Thailand. Blood samples (1–2 mL) were kept in sterile plain tubes, centrifuged to separate serum, and stored at −70 °C until used. Ethical approval for animal use in this research was obtained from the Kasetsart University Institutional Animal Care and Use Committee (Approval No.: ACKU-60-VTN-007), Kasetsart University, Bangkok, Thailand.
2.2. Constructions of Chimeric Multi-Epitopes
Chimeric multi-epitope constructs were engineered by modifying the recombinant plasmid pET15b-Br2 [35] using the Q5® Site-Directed Mutagenesis Kit (New England Biolabs, Inc., Massachusetts, USA) according to the manufacturer’s instructions. Large-scale screening of T. gondii membrane proteins from the UniProt database [37] was performed to identify B-cell and T-cell epitopes by Li, et al. [33]. Based on sequence similarities, S7VVK4 (S7V), V4ZJG5 (V4Z), A0A125YIN1 (A125Y), S8F3F6-1 (SFF), and S8EYA7 (S8E) were selected to replace outer α-helices of TIM-barrel structure. The helix 3 (H3) was replaced with S7V, while helix 4 (H4) was replaced with A125Y or SFF, and helix 6 (H6) was replaced with S8E or V4Z. Primer pairs were designed using NEBaseChanger software to facilitate precise epitope insertions (Table S1).
The construction process involved a two-step PCR protocol. Initially, the target helix was deleted from the plasmid pET15b-Br2, followed by simultaneous phosphorylation, ligation, and template removal using a Kinase, Ligase, and DpnI (KLD) enzyme mixture, which yielded a deleted plasmid. Subsequently, specific primers were used to amplify and insert the selected epitopes into the deleted plasmid, followed by KLD reactions. The resulting plasmids were then transformed into E. coli NEB5α competent cells. Positive clones underwent plasmid extraction, DNA sequencing (ATGC Co., Ltd., Pathum Thani, Thailand), and sequence alignment using Clustal Omega (https://www.ebi.ac.uk/jdispatcher/msa/clustalo (accessed on 19 November 2023)) [38] and Jalview software version 2 [39] to confirm successful mutations and insertion accuracy. The confirmed constructs were further modified to produce multi-epitope combinations, resulting in a set of ten chimeric constructs, which included single, double, and triple epitope insertions.
2.3. Productions of Chimeric Multi-Epitope Proteins
The confirmed chimeric plasmids were transformed into E. coli BL21(pLysE) cells for expression. A single colony of each chimeric clone was inoculated into Luria–Bertani (LB) broth and grown overnight at 37 °C with shaking at 220 rpm. The culture was diluted 1:100 into fresh LB broth and grown until the OD600nm reached 0.4–0.6. The protein expression was induced with 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and incubated for an additional 3 h. Cells were harvested, lysed by sonication, and centrifuged to collect the supernatant containing the expressed chimeric protein. Proteins were purified using Ni2+-NTA affinity chromatography (GE Healthcare, Uppsala, Sweden) and eluted in imidazole buffer. Purity and concentration of the purified proteins were verified by 10% SDS-PAGE and Bradford assay.
2.4. Indirect ELISA Condition and Candidate Chimeric Multi-Epitopes Testing
Indirect ELISA was performed to identify the optimal concentration and efficacy of each chimeric multi-epitope protein for detecting T. gondii infection in cat sera. Microplate wells were coated with 0.5, 0.75, or 1.5 µg/mL of each protein at 37 °C for 2 h. Plates were blocked with 7% skim milk in phosphate-buffered saline (PBS, pH 7.4) for 1 h at 37 °C. Diluted cat sera (1:100) from three true positive and three true negative samples [18] were added and incubated at 25 °C with shaking. The plates were washed with PBS containing 0.1% Tween 20, then incubated with a 1:8000 dilution of peroxidase-conjugated AffiniPure Goat Anti-Cat IgG (H + L) (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 30 min at 25 °C. After further washing, TMB substrate (Avantor, Inc., Radnor, PA, USA) was added and incubated in the dark for 30 min. Absorbance at 655 nm was measured using an iMark™ Microplate Absorbance Reader with Microplate Manager® 6 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to determine the optimal concentration and distinguish between positive and negative samples.
2.5. Evaluation of Chimeric-Multi-Epitopes and Statistical Analysis
To evaluate the diagnostic efficacy of selected chimeric multi-epitope proteins, ELISA was performed on a panel of 39 cat sera previously categorized by Suwan, et al. [19] as either positive or negative for T. gondii. The panel included 13 confirmed positive samples, 5 false positives, 20 confirmed negatives, and 1 false negative. The ELISA protocol described above was used, with a protein concentration of 0.5 µg/mL. The statistical analyses were conducted using version 4.4.1 of the R programming language [40]. A 95% confidence interval (CI) was applied, and p-Value < 0.05 was considered significant. To indicate the precision and reliability of the assay, the intra-assay coefficient of variation (CV) was calculated. The ELISA results were analyzed using the ‘epiR’ package in R to determine the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each candidate chimeric protein, by comparing with the IFAT results serving as the reference standard. The diagnostic performance of each ELISA assay across various cutoff points was evaluated using receiver operating characteristic (ROC) curve analysis with the ‘ROCR’ package in the R program. The accuracy of area under the curve (AUC) values was determined using the same package and evaluated following the guidelines established by Swets [41] and Reynoso-Palomar et al. [42]. AUC values were interpreted as follows: non-informative (AUC < 0.5), low accuracy (0.5 ≤ AUC < 0.7), moderate accuracy (0.7 ≤ AUC < 0.9), and high accuracy (0.9 ≤ AUC ≤ 1.0). Additionally, McNemar’s test, implemented using the ‘mcnemar.test’ function in R, was utilized to compare the prevalence between the assays [43]. Moreover, the agreement between ELISA and IFAT results was evaluated using Cohen’s Kappa coefficient with the ‘fmsb’ package in R, and the Kappa values were interpreted according to the guidelines established by Landis and Koch [44]. Furthermore, to evaluate the agreement between ELISA and IFAT results while accounting for prevalence and bias, the prevalence-adjusted bias-adjusted Kappa (PABAK) was evaluated using the ‘epi.kappa’ function, thereby enhancing the accuracy of agreement estimates.
3. Results
3.1. Chimeric Multi-Epitopes
Ten chimeric multi-epitope proteins were successfully constructed, encompassing various epitope configurations, including single (A125Y, S7V, S8E, SFF, and V4Z), double (S7V-V4Z, S7V-S8E), and triple insertions (S7V-V4Z-SFF, S7V-S8E-SFF, and S7V-S8E-A125Y). Each protein was engineered by substituting specific α-helix regions of Br2 β-glucosidase with selected T. gondii B-cell and T-cell epitopes (Figure 1). Specifically, helix 3 of Br2 was replaced with S7V (a guanylate cyclase, predicted T-cell HLA-I epitope), helix 4 with A125Y (uncharacterized protein, T-cell HLA-II epitope) or SFF (chloride transporter, B-cell epitope), and helix 6 with V4Z (surface antigen, T-cell HLA-I epitope) or S8E (uncharacterized protein, T-cell HLA-II epitope).
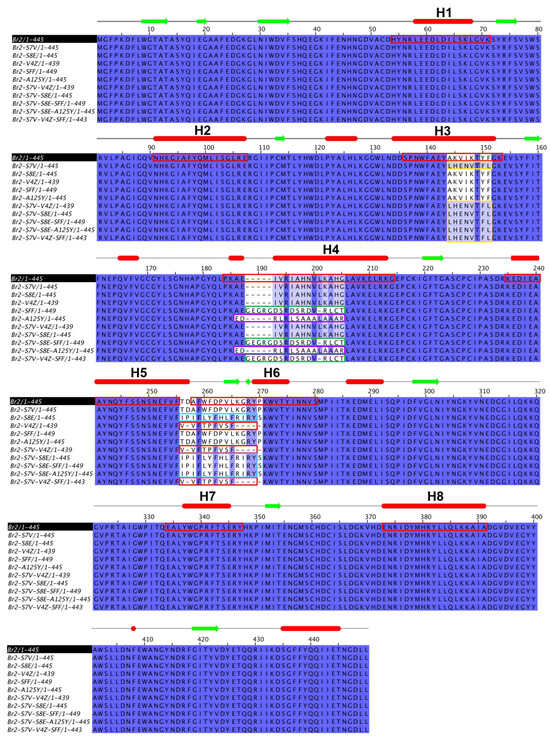
Figure 1.
The scheme of multiple sequence alignment between Br2 wildtype and 10 chimeric multi-epitope proteins. The secondary structure was shown the prediction for wild type (α-helices in red bars and β-sheet in green arrows). The α-helix residues of Br2 were shown in red boxes. The inserted residues of S7V, SFF, A125Y, V4Z, and S8E were shown in yellow, green, magenta, pink, and cyan boxes, respectively.
Structural modeling revealed that these substitutions produced various structural shifts, especially in the outer loop regions (Figure 2). The V4Z insertion largely retained the TIM-barrel structure, while SFF insertion showed slight deviations in inner loop regions. Triple epitope insertions, such as S7V-V4Z-SFF, induced more pronounced structural changes. These modifications are expected to influence the immunogenic properties of the proteins, potentially impacting their antigen–antibody binding capacity.
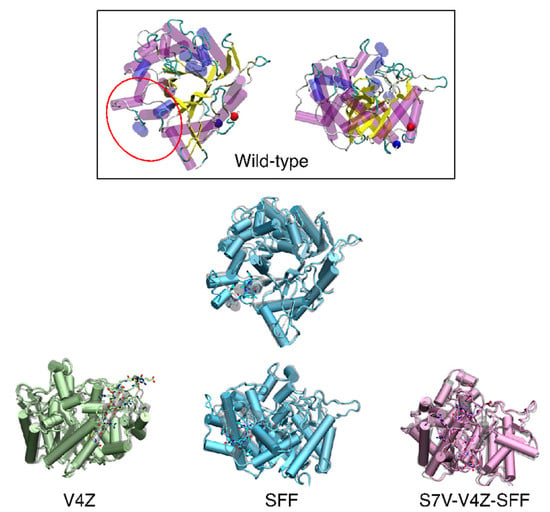
Figure 2.
Superimposition between wild-type (grey) and the promising mutations, V4Z (green), SFF (light blue), and S7V-V4Z-SFF (pink). The mutation loop showed in licorice.
3.2. Serological Test
The primary indirect ELISA testing of positive and negative cat sera from Suwan et al. [19] revealed that a protein concentration of 0.5 µg/mL provided optimal differentiation between positive and negative samples, as evidenced by OD655 nm values in Table S2. In contrast, concentrations of 0.75 and 1.5 µg/mL resulted in excessively high OD655 nm readings, impairing the effective distinction between positive and negative samples. Among the 10 chimeric proteins at 0.5 µg/mL concentration, S7V-V4Z-SFF, SFF, and V4Z exhibited potential for detecting antibodies against T. gondii in cat sera.
The chimeric proteins S7V-V4Z-SFF, SFF, and V4Z, at a concentration of 0.5 µg/mL, were subsequently evaluated using 39 cat serum samples. ELISA-SFF displayed the highest OD655 nm for positive (0.751) and negative (0.198) controls, followed by ELISA-V4Z (0.549 and 0.154) and ELISA-S7V-V4Z-SFF (0.523 and 0.116). The average intra-assay CV was 6.02%, 5.24%, and 5.49% for ELISA-S7V-V4Z-SFF, ELISA-SFF, and ELISA-V4Z, respectively, as presented in Table S3. CV < 10% is generally considered as high repeatability [45].
3.3. Evaluation of Indirect ELISA Assays
The performance of each indirect ELISA assay at various cutoff points was assessed using the ROC curve to determine the optimal cutoff, ensuring distinction between positive and negative samples while maintaining appropriate test sensitivity and specificity. The optimal cutoff was determined by analyzing the OD of 39 cat serum samples tested with ELISA-S7V-V4Z-SFF, ELISA-SFF, and ELISA-V4Z, compared to the IFAT results from Suwan et al. [19]. The ROC-based model identified optimal cutoff values of 0.136, 0.243, and 0.199 for ELISA-S7V-V4Z-SFF, ELISA-SFF, and ELISA-V4Z, respectively, as illustrated in Figure 3.
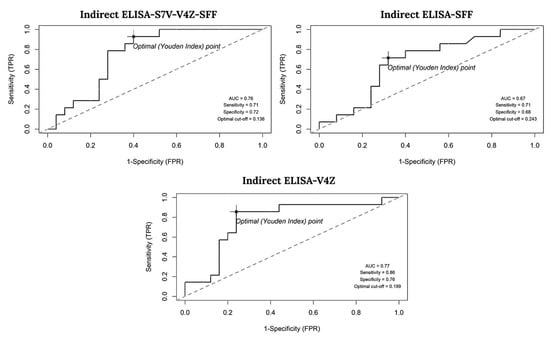
Figure 3.
Receiver operating characteristic (ROC) curve illustrating the area under the curve (AUC) and the optimal cutoff point that achieves the highest sensitivity and specificity.
Sensitivity and Specificity: Among the assays, ELISA-V4Z achieved the highest sensitivity (86%, 95% CI: 57–98%) and specificity (76%, 95% CI: 55–91%). In contrast, ELISA-S7V-V4Z-SFF showed balanced performance, with sensitivity and specificity of 71% (95% CI: 42–92%) and 72% (95% CI: 51–88%), respectively. ELISA-SFF also exhibited moderate sensitivity (71%) but a lower specificity of 68% (95% CI: 39–79%).
Predictive Values: ELISA-V4Z demonstrated the highest positive predictive value (PPV) at 67% (95% CI: 41–87%) among the assays and achieved the strongest negative predictive value (NPV) of 90% (95% CI: 70–99%), as indicated in Table 1.

Table 1.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each indirect ELISA when using IFAT as a reference test.
Prevalence Comparison: Among the three indirect ELISA assays, both indirect ELISA-SFF and ELISA-V4Z exhibited the highest prevalence at 46.1% (95% CI = 31.6–61.4%). Meanwhile, the indirect ELISA-S7V-V4Z-SFF demonstrated a slightly lower prevalence at 43.6% (95% CI = 29.3–59.0%). In contrast, the prevalence detected using IFAT was the lowest at 35.9% (95% CI = 22.7–51.6%). However, McNemar’s test indicated no statistically significant difference between IFAT and any of the indirect ELISA assays, nor among the indirect ELISA assays themselves (Table 2).

Table 2.
The T. gondii indirect ELISA results against 39 cat sera compared with IFAT and another ELISA assay.
Analysis of Agreement: The concordance assessment between the indirect ELISA and IFAT methods demonstrated that ELISA-V4Z exhibited the highest agreement with IFAT (Kappa = 0.58, 95% CI: 0.32–0.84), indicating moderate concordance. This was followed by ELISA-S7V-V4Z-SFF, which showed a Kappa value of 0.41 (95% CI: 0.12–0.71), also indicating moderate concordance. In contrast, ELISA-SFF achieved a Kappa value of 0.37 (95% CI: 0.07–0.67), reflecting fair concordance. The PABAK values corresponded with the Kappa estimates across all three assessments, affirming consistency in agreement measures (Table 3).

Table 3.
The analysis of agreement of the indirect ELISA using IFAT as a reference test.
4. Discussion
In this study, we engineered 10 chimeric multi-epitope proteins based on the TIM-barrel structure of Br2 β-glucosidase by substituting selected α-helices with B-cell and T-cell epitopes from T. gondii. These substitutions were confirmed by sequence alignment, and the resulting proteins were modeled to assess potential structural impacts. The multi-epitope proteins V4Z, S7V-V4Z-SFF, and SFF exhibited promising sensitivity and specificity in distinguishing between T. gondii-positive and -negative samples in ELISA assays. The V4Z revealed the highest sensitivity, specificity, positive predictive value, and negative predictive value due to its detectable antigenicity. Structural analysis revealed that these engineered proteins retained the TIM-barrel’s core stability, a β-sheet core encased in α-helices containing B- and T-cell epitopes, with targeted mutations enhancing epitope facet binds to other proteins, peptides, or substrates.
Serological methods, particularly ELISA, have long been used to diagnose toxoplasmosis in humans and animals, with significant advancements in antigen development over recent years. ELISAs are practical and cost-effective, making them suitable for routine T. gondii diagnosis. Commercially available ELISAs and in-house adaptations often utilize recombinant or chimeric antigens, which are increasingly favored due to their high specificity and sensitivity compared to a single antigen [13,15,16]. Advances in epitope mapping techniques such as immunoproteomics [46], protein microarrays [1], and two-dimensional gel electrophoresis [47] have expanded the potential for identifying and incorporating multiple antigenic regions within chimeric proteins. Computational approaches allow for the precise prediction of immunoreactive B- and T-cell epitopes facilitating the design of antigens with enhanced diagnostic properties [33]. However, the effectiveness of these in silico predictions requires rigorousness in vitro validation. Chimeric antigens, which combine multiple epitopes, have emerged as promising tools for T. gondii detection in various species, offering enhanced accuracy in livestock diagnosis [29,30,31].
The diagnostic performance of our chimeric proteins did not reach the high efficacy reported for tetravalent and trivalent chimeric proteins [30,31]. The moderate sensitivity and specificity achieved in this study suggest that further optimization, such as including additional immunodominant epitopes, could enhance their diagnostic potential. Variability in immune responses among different host species also underscores the need for tailored antigen designs, suggesting that further testing across multiple animal models could support species-specific ELISA optimization [29,30]. Moreover, the relatively small sample size may limit the statistical power and generalizability of the present findings; therefore, the diagnostic performance values should be interpreted with caution and regarded as preliminary until validated in larger and more diverse feline populations. Additionally, potential false-positive outcomes may result from cross-reactivity with antibodies against other pathogens, particularly those sharing similar epitopes [48,49], while false-negative results may occur due to immune suppression in the host, leading to weak or undetectable antibody responses [50]. These limitations could affect diagnostic accuracy and should be carefully considered in interpreting the results. Furthermore, the lack of confirmatory diagnostic methods, such as PCR, constrains the validation of ELISA results and contributes to uncertainty in interpreting serological data. Although intra-assay consistency was monitored, the reproducibility of the ELISA across different runs and personnel was not systematically assessed, potentially impacting its reliability under varied laboratory conditions. These limitations collectively highlight the importance of future investigations employing confirmatory diagnostics and standardized reproducibility evaluations to strengthen diagnostic confidence. Accordingly, we recommend that future studies incorporate broader pathogen panels and account for the health status of cat populations to enhance assay validation and refinement.
5. Conclusions
In this study, we successfully designed and evaluated a TIM-barrel-based multi-epitope protein by incorporating selected T. gondii B- and T-cell epitopes for serological diagnostics in cats. Our findings, particularly with the V4Z chimeric protein, demonstrate the potential of this approach to enhance diagnostic accuracy for T. gondii detection, exhibiting favorable sensitivity, specificity, and moderate agreement with the reference IFAT. While these initial results are encouraging and suggest that TIM-barrel-based multi-epitope proteins represent a valuable new strategy, further comprehensive studies are essential to validate these findings across diverse feline populations, confirm assay reproducibility, and fully assess its applicability for broader serological testing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15131893/s1, Table S1: The set of deletions and insertions primer used in this study; Table S2: The absorbance at 655 nm of in cat serum for T. gondii detection via indirect ELISA; Table S3: The absorbance at 655 nm of T. gondii indirect ELISA from each protein; Table S4: The percentage of 11 sequences alignments.
Author Contributions
Conceptualization, E.S. and S.J.; Data curation, E.S., J.M., W.R. and P.T.; formal analysis, E.S., W.R. and P.T.; Investigation, E.S., J.M., W.R. and P.T.; methodology, E.S., W.R., P.T. and S.J.; project administration, B.M., R.R., M.S., S.U., S.J. and E.S.; resources, B.M., R.R., M.S., S.U., S.J. and E.S.; software, E.S. and W.R.; supervision, B.M., R.R., M.S., S.U., E.S. and S.J.; validation, E.S., W.R. and P.T.; writing—original draft, E.S.; writing—review and editing, E.S., P.T., W.R. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financially supported by Kasetsart University Research and Development Institute, KURDI (project number YF(KU)1.65).
Institutional Review Board Statement
This study was approved by the Kasetsart University Institutional Animal Care and Committee Use (approval number: ACKU-60-VTN-007), Kasetsart University, Bangkok, Thailand.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their sincere gratitude to Prachumporn T. Kongsaeree for her generous laboratory material support and insightful suggestions. We would also like to thank Phiraya Pitchayatanakorn for her invaluable technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Doskaya, M.; Liang, L.; Jain, A.; Can, H.; Iz, S.G.; Felgner, P.L.; Doskaya, A.D.; Davies, D.H.; Guruz, A.Y. Author Correction: Discovery of new Toxoplasma gondii antigenic proteins using a high throughput protein microarray approach screening sera of murine model infected orally with oocysts and tissue cysts. Parasites Vectors 2024, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. The history of Toxoplasma gondii—The first 100 years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef]
- Liu, Q.; Singla, L.D.; Zhou, H. Vaccines against Toxoplasma gondii: Status, challenges and future directions. Hum. Vaccin. Immunother. 2012, 8, 1305–1308. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Marra, C.M.; Zhu, X.Q. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin. Microbiol. Rev. 2021, 34, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, S.; Rostami, A.; Nourollahpour Shiadeh, M.; Behniafar, H.; Paktinat, S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 133–140. [Google Scholar] [CrossRef]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global status of Toxoplasma gondii infection: Systematic review and prevalence snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar]
- Moncada, P.A.; Montoya, J.G. Toxoplasmosis in the fetus and newborn: An update on prevalence, diagnosis and treatment. Expert. Rev. Anti-Infect. Ther. 2012, 10, 815–828. [Google Scholar] [CrossRef]
- Calero-Bernal, R.; Gennari, S.M. Clinical Toxoplasmosis in Dogs and Cats: An Update. Front. Veter Sci. 2019, 6, 54. [Google Scholar] [CrossRef]
- Faculty of Veterinary Medicine, K.U. Rabies One Data. Available online: http://rabiesonedata.ku.ac.th/ (accessed on 14 July 2024).
- Terkawi, M.A.; Kameyama, K.; Rasul, N.H.; Xuan, X.; Nishikawa, Y. Development of an immunochromatographic assay based on dense granule protein 7 for serological detection of Toxoplasma gondii infection. Clin. Vaccine Immunol. 2013, 20, 596–601. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.D.; Huang, S.Y.; Zhu, X.Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 2015, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Uddin, A.; Hossain, D.; Ahsan, M.I.; Atikuzzaman, M.; Karim, M.R. Review on diagnosis and molecular characterization of Toxoplasma gondii in humans and animals. Trop. Biomed. 2021, 38, 511–539. [Google Scholar] [CrossRef] [PubMed]
- Ybanez, R.H.D.; Ybanez, A.P.; Nishikawa, Y. Review on the Current Trends of Toxoplasmosis Serodiagnosis in Humans. Front. Cell Infect. Microbiol. 2020, 10, 204. [Google Scholar] [CrossRef]
- Huertas-Lopez, A.; Cantos-Barreda, A.; Sanchez-Sanchez, R.; Martinez-Carrasco, C.; Ibanez-Lopez, F.J.; Martinez-Subiela, S.; Ceron, J.J.; Alvarez-Garcia, G. A systematic review and meta-analysis of the validation of serological methods for detecting anti-Toxoplasma gondii antibodies in humans and animals. Veter Parasitol. 2024, 328, 110173. [Google Scholar] [CrossRef]
- Liyanage, K.; Wiethoelter, A.; Hufschmid, J.; Jabbar, A. Descriptive Comparison of ELISAs for the Detection of Toxoplasma gondii Antibodies in Animals: A Systematic Review. Pathogens 2021, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Z.; Li, J.; Li, N.; Wei, F.; Liu, Q. Evaluation of an indirect ELISA using recombinant granule antigen Gra7 for serodiagnosis of Toxoplasma gondii infection in cats. J. Parasitol. 2015, 101, 37–40. [Google Scholar] [CrossRef]
- Abdelbaset, A.E.; Alhasan, H.; Salman, D.; Karram, M.H.; Ellah Rushdi, M.A.; Xuenan, X.; Igarashi, M. Evaluation of recombinant antigens in combination and single formula for diagnosis of feline toxoplasmosis. Exp. Parasitol. 2017, 172, 1–4. [Google Scholar] [CrossRef]
- Suwan, E.; Chalermwong, P.; Rucksaken, R.; Sussadee, M.; Kaewmongkol, S.; Udonsom, R.; Jittapalapong, S.; Mangkit, B. Development and evaluation of indirect enzyme-linked immunosorbent assay using recombinant dense granule antigen 7 protein for the detection of Toxoplasma gondii infection in cats in Thailand. Veter World 2022, 15, 602–610. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, M.; Wang, Y.; Qu, L.; Gong, R.; Si, J. Evaluation of a recombinant multiepitope peptide for serodiagnosis of Toxoplasma gondii infection. Clin. Vaccine Immunol. 2012, 19, 338–342. [Google Scholar] [CrossRef]
- Andreatta, M.; Nielsen, M. Gapped sequence alignment using artificial neural networks: Application to the MHC class I system. Bioinformatics 2016, 32, 511–517. [Google Scholar] [CrossRef]
- Bui, H.H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform. 2006, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Lund, O. Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform. 2007, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Napoleao-Pego, P.; Carneiro, F.R.G.; Durans, A.M.; Gomes, L.R.; Morel, C.M.; Provance, D.W., Jr.; De-Simone, S.G. Performance assessment of a multi-epitope chimeric antigen for the serological diagnosis of acute Mayaro fever. Sci. Rep. 2021, 11, 15374. [Google Scholar] [CrossRef]
- Johnston, R.A.; Habarugira, G.; Harrison, J.J.; Isberg, S.R.; Moran, J.; Morgan, M.S.; Davis, S.S.; Melville, L.; Howard, C.B.; Henry, C.S.; et al. Application of chimeric antigens to paper-based diagnostics for detection of West Nile virus infections of Crocodylus porosus—A novel animal test case. Sens. Actuators B Chem. 2025, 422, 136611. [Google Scholar] [CrossRef]
- Freitas, N.E.M.; Campos, D.A.A.; Ferreira, R.Q.V.; Jesus, F.S.S.; Silva, A.A.O.; Mota, C.O.D.; Marchini, F.K.; Celedon, P.A.F.; Zanchin, N.I.T.; Santos, F.L.N. Comparison of Four Chimeric Antigens and Commercial Serological Assays for the Diagnosis of Trypanosoma cruzi Infection. Am. J. Trop. Med. Hyg. 2025, 112, 89–95. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gasior, L.; Kur, J. Serodiagnosis of Toxoplasma gondii infection in farm animals (horses, swine, and sheep) by enzyme-linked immunosorbent assay using chimeric antigens. Parasitol. Int. 2015, 64, 288–294. [Google Scholar] [CrossRef]
- Ferra, B.T.; Chyb, M.; Solowinska, K.; Holec-Gasior, L.; Skwarecka, M.; Baranowicz, K.; Gatkowska, J. The Development of Toxoplasma gondii Recombinant Trivalent Chimeric Proteins as an Alternative to Toxoplasma Lysate Antigen (TLA) in Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Immunoglobulin G (IgG) in Small Ruminants. Int. J. Mol. Sci. 2024, 25, 4384. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Ferra, B.; Grazlewska, W. Toxoplasma gondii Tetravalent Chimeric Proteins as Novel Antigens for Detection of Specific Immunoglobulin G in Sera of Small Ruminants. Animals 2019, 9, 1146. [Google Scholar] [CrossRef]
- Huertas-López, A.; Rojo, M.C.; Sukhumavasi, W.; Martínez-Subiela, S.; Álvarez-García, G.; López-Ureña, N.M.; Cerón, J.J.; Martínez-Carrasco, C. Comparative performance of five recombinant and chimeric antigens in a time-resolved fluorescence immunoassay for detection of Toxoplasma gondii infection in cats. Veter Parasitol. 2022, 304, 109703. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, N.; Li, Z.L.; Dibo, N.; Ma, Z.R.; Lu, B.; Huang, Y.H.; Chang, Y.F.; Chen, H.Z.; Wu, X. Epitope vaccine design for Toxoplasma gondii based on a genome-wide database of membrane proteins. Parasites Vectors 2022, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.; Hocker, B. Catalytic versatility, stability, and evolution of the (betaalpha)8-barrel enzyme fold. Chem. Rev. 2005, 105, 4038–4055. [Google Scholar] [CrossRef]
- Suwan, E.; Arthornthurasuk, S.; Kongsaeree, P.T. A metagenomic approach to discover a novel β-glucosidase from bovine rumens. Pure Appl. Chem. 2017, 89, 941–950. [Google Scholar] [CrossRef]
- Kaenying, W.; Tagami, T.; Suwan, E.; Pitsanuwong, C.; Chomngam, S.; Okuyama, M.; Kongsaeree, P.; Kimura, A.; Kongsaeree, P.T. Structural and mutational analysis of glycoside hydrolase family 1 Br2 beta-glucosidase derived from bovine rumen metagenome. Heliyon 2023, 9, e21923. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Reynoso-Palomar, A.; Moreno-Galvez, D.; Villa-Mancera, A. Prevalence of Toxoplasma gondii parasite in captive Mexican jaguars determined by recombinant surface antigens (SAG1) and dense granular antigens (GRA1 and GRA7) in ELISA-based serodiagnosis. Exp. Parasitol. 2020, 208, 107791. [Google Scholar] [CrossRef]
- Pembury Smith, M.Q.R.; Ruxton, G.D. Effective use of the McNemar test. Behav. Ecol. Sociobiol. 2020, 74, 133. [Google Scholar] [CrossRef]
- Landis, J.; Koch, G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Jalilibal, Z.; Amiri, A.; Castagliola, P.; Khoo, M.B.C. Monitoring the coefficient of variation: A literature review. Comput. Ind. Eng. 2021, 161, 107600. [Google Scholar] [CrossRef]
- Sun, X.M.; Ji, Y.S.; Elashram, S.A.; Lu, Z.M.; Liu, X.Y.; Suo, X.; Chen, Q.J.; Wang, H. Identification of antigenic proteins of Toxoplasma gondii RH strain recognized by human immunoglobulin G using immunoproteomics. J. Proteom. 2012, 77, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.N.; Yeo, S.J.; Park, H. Identification of novel biomarkers for anti-Toxoplasma gondii IgM detection and the potential application in rapid diagnostic fluorescent tests. Front. Microbiol. 2024, 15, 1385582. [Google Scholar] [CrossRef]
- Hebbar, B.K.; Roy, M.; Mitra, P.; Chavhan, K.; Chaudhari, S.; Shinde, S.; Deshmukh, A.S. Seroprevalence, risk factors, and serological cross-reactivity for diagnosis of Toxoplasma gondii and Neospora caninum infections in goats in India. Microb. Pathog. 2022, 173, 105780. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Urena, N.M.; Calero-Bernal, R.; Vazquez-Calvo, A.; Sanchez-Sanchez, R.; Ortega-Mora, L.M.; Alvarez-Garcia, G. A comparative study of serological tests used in the diagnosis of Toxoplasma gondii infection in small ruminants evidenced the importance of cross-reactions for harmonizing diagnostic performance. Res. Veter Sci. 2023, 165, 105052. [Google Scholar] [CrossRef]
- Rajput, R.; Denniston, A.K.; Murray, P.I. False Negative Toxoplasma Serology in an Immunocompromised Patient with PCR Positive Ocular Toxoplasmosis. Ocul. Immunol. Inflamm. 2018, 26, 1200–1202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).