The Influence of Genotype and Seasonality on the Sow Colostrum Quality and Immunoglobulin G Content
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Colostrum Sampling and Statistical Analysis
2.3. Statistical Analysis
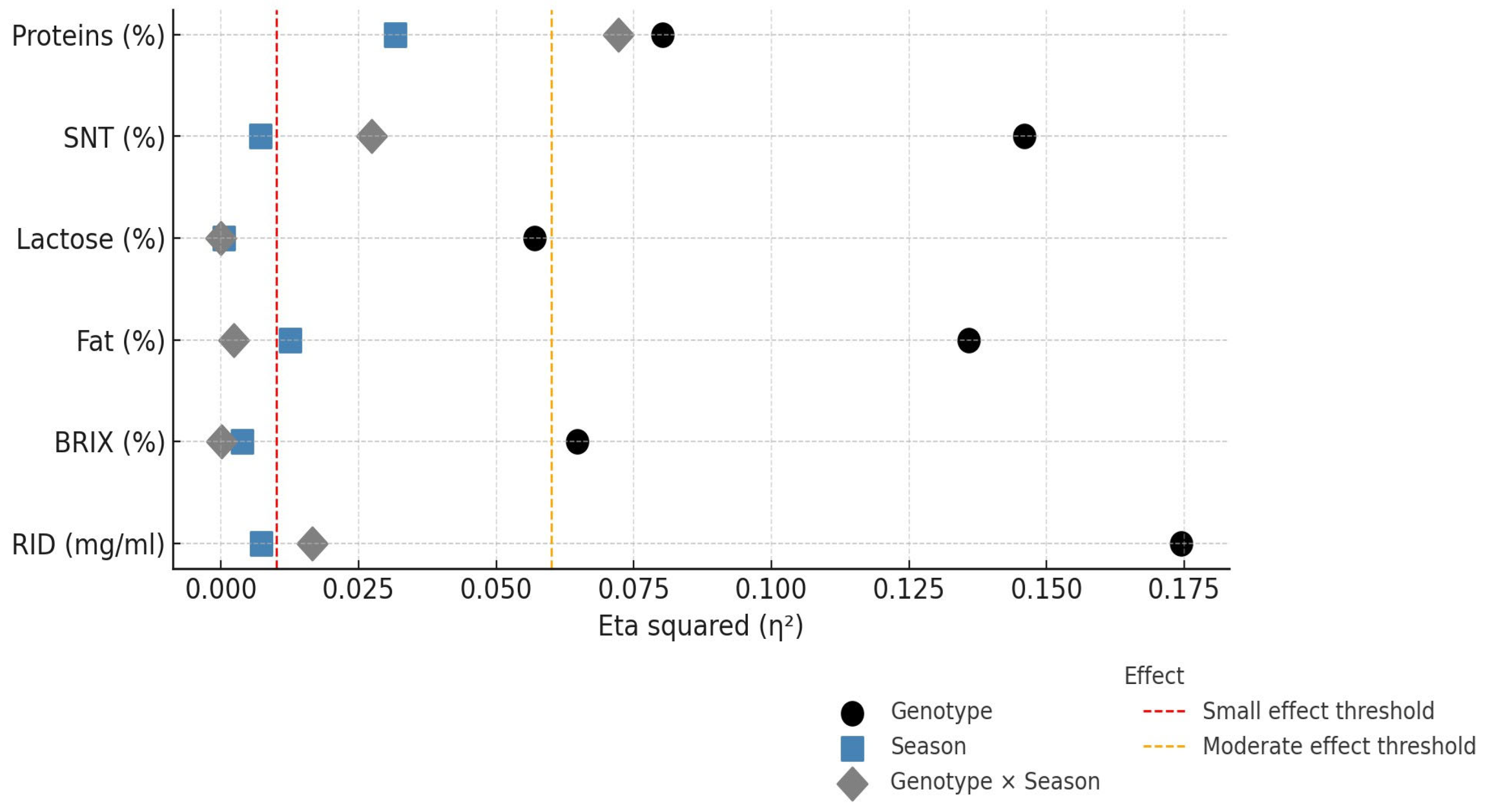
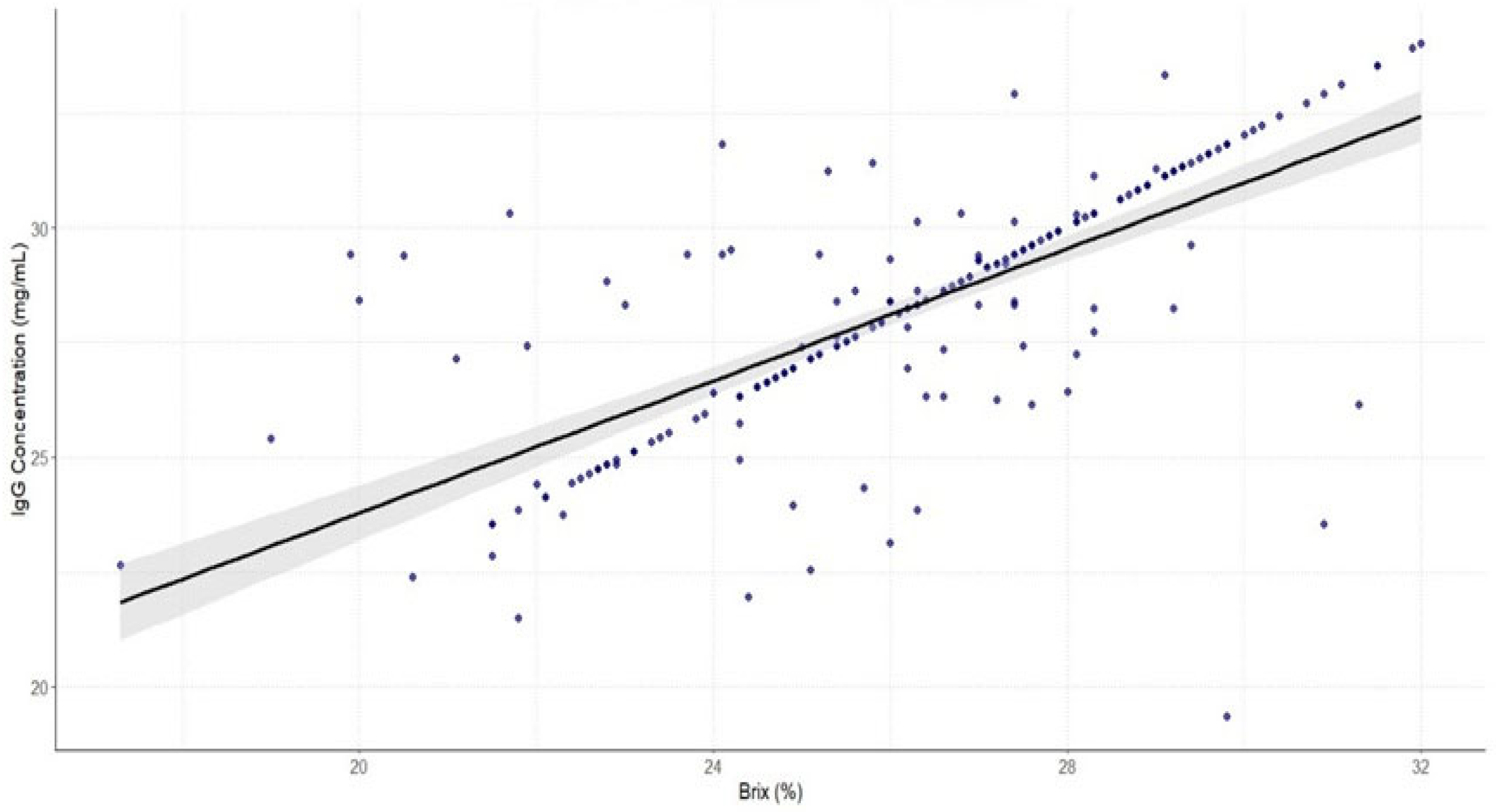
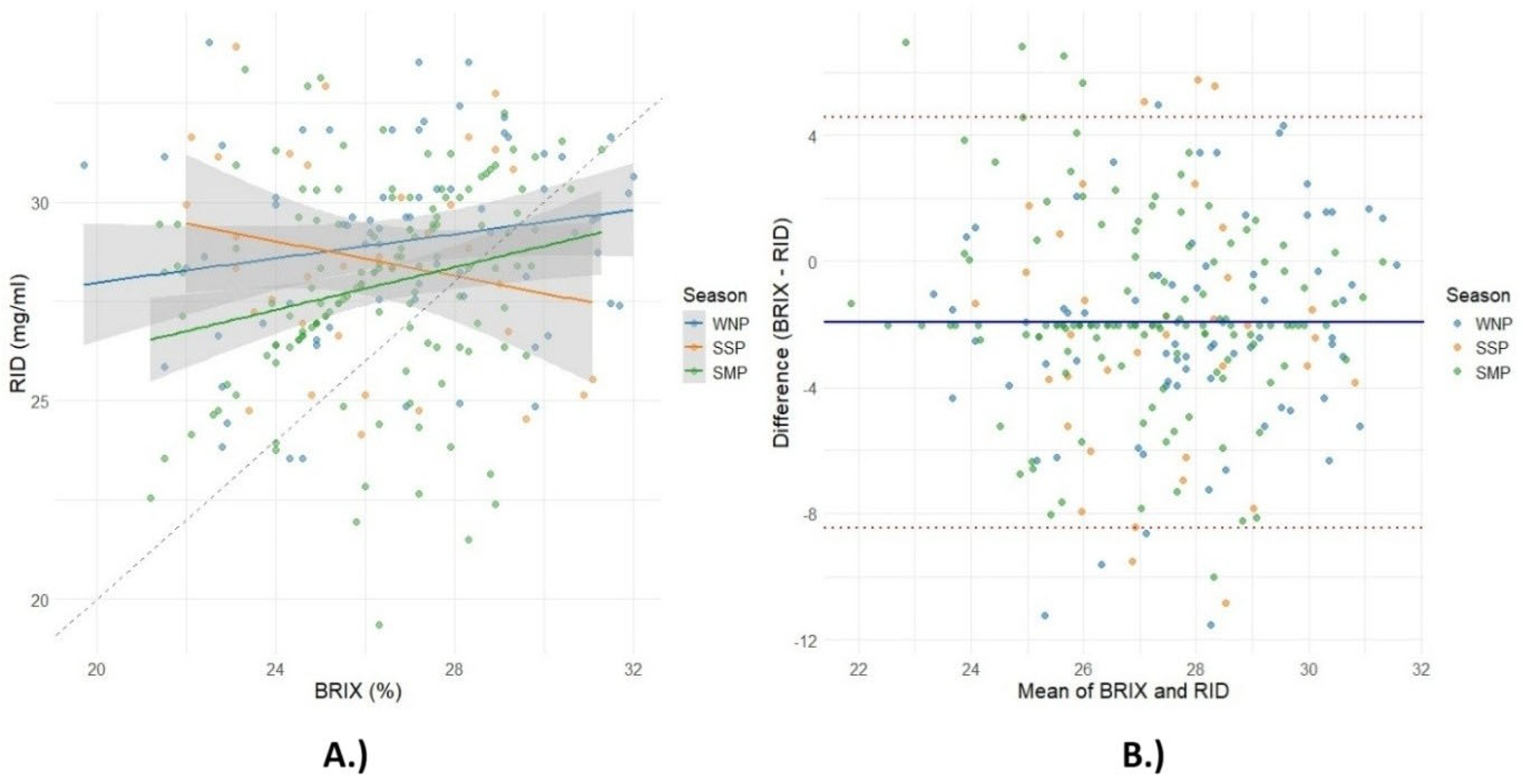
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quesnel, H. Colostrum production by sows: Variability of colostrum yield and immunoglobulin G concentrations. Animal 2011, 5, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Tsukahara, T. Composition and physiological functions of the porcine colostrum. Anim. Sci. J. 2021, 92, e13618. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, C. Management to improve neonate piglet survival. J. Reprod. Infertil. 2013, 68, 203–210. [Google Scholar] [CrossRef]
- Charneca, R.; Nunes, J.T.; Freitas, A.; Le Dividich, J. Effect of litter birth weight standardization before first suckling on colostrum intake, passive immunization, pre-weaning survival, and growth of the piglets. Animal 2021, 15, 100184. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; González-Lozano, M. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Hurley, W.L. Composition of sow colostrum and milk. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 193–230. [Google Scholar]
- Nuntapaitoon, M.; Juthamanee, P.; Theil, P.K.; Tummaruk, P. Impact of sow parity on yield and composition of colostrum and milk in Danish Landrace× Yorkshire crossbred sows. Prev. Vet. Med. 2020, 181, 105085. [Google Scholar] [CrossRef]
- Nuntapaitoon, M.; Suwimonteerabutr, J.; Am-In, N.; Tienthai, P.; Chuesiri, P.; Kedkovid, R.; Tummaruk, P. Impact of parity and housing conditions on concentration of immunoglobulin G in sow colostrum. Trop. Anim. Health Prod. 2019, 51, 1239–1246. [Google Scholar] [CrossRef]
- Amavizca-Nazar, A.; Montalvo-Corral, M.; González-Rios, H.; Pinelli-Saavedra, A. Hot environment on reproductive performance, immunoglobulins, vitamin E, and vitamin A status in sows and their progeny under commercial husbandry. J. Anim. Sci. Technol. 2019, 61, 340. [Google Scholar] [CrossRef]
- Amatucci, L.; Luise, D.; Correa, F.; Bosi, P.; Trevisi, P. Importance of breed, parity and sow colostrum components on litter performance and health. Animals 2022, 12, 1230. [Google Scholar] [CrossRef]
- Picone, G.; Zappaterra, M.; Luise, D.; Trimigno, A.; Capozzi, F.; Motta, V.; Trevisi, P. Metabolomics characterization of colostrum in three sow breeds and its influences on piglets’ survival and litter growth rates. J. Anim. Sci. Biotechnol. 2018, 9, 23. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Theil, P.K. Colostrum and milk production. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 151–190. [Google Scholar]
- Maciag, S.S.; Bellaver, F.V.; Bombassaro, G.; Haach, V.; Morés, M.A.Z.; Baron, L.F.; Bastos, A.P. On the influence of the source of porcine colostrum in the development of early immune ontogeny in piglets. Sci. Rep. 2022, 12, 15630. [Google Scholar] [CrossRef] [PubMed]
- Vallet, J.L.; Miles, J.R.; Rempel, L.A. A simple novel measure of passive transfer of maternal immunoglobulin is predictive of preweaning mortality in piglets. Vet. J. 2013, 195, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, T.A.; Behling-Kelly, E.L.; Mann, S. Comparison of radial immunodiffusion, turbidimetric immunoassay, and Brix refractometry for determining bovine colostrum quality. JDS Commun. 2024, 5, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Duffy, C.; Gordon, A.; Morrison, S.; Argűello, A.; Welsh, M.; Earley, B. Comparison of single radial immunodiffusion and ELISA for the quantification of immunoglobulin G in bovine colostrum, milk and calf sera. J. Appl. Anim. Res. 2018, 46, 758–765. [Google Scholar] [CrossRef]
- Niero, G.; Thomas, S.A.; Mouratidou, K.; Visentin, G.; De Marchi, M.; Penasa, M.; Cassandro, M. Lactoferrin concentration in bovine milk: Validation of radial immunodiffusion technique, sources of variation, and association to udder health status. Ital. J. Anim. Sci. 2023, 22, 230–238. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, L.; Ma, N.; Su, Q.; Wan, Y.; Zhang, Y.; Qian, W. Real-time monitoring of immunoglobulin G levels in milk using an ordered porous layer interferometric optical sensor. Talanta 2022, 237, 122958. [Google Scholar] [CrossRef]
- Stastna, M.; Šlais, K. Preparative separation of immunoglobulins from bovine colostrum by continuous divergent-flow electrophoresis. J. Sep. Sci. 2023, 46, 2200679. [Google Scholar] [CrossRef]
- Balzani, A.; Cordell, H.J.; Edwards, S.A. Evaluation of an on-farm method to assess colostrum IgG content in sows. Animal 2016, 10, 643–648. [Google Scholar] [CrossRef]
- Sievert, M.; Schuler, G.; Büttner, K.; Wehrend, A. Comparison of different methods to determine the absorption of colostral IgG in newborn foals. J. Equine Vet. Sci. 2022, 114, 104008. [Google Scholar] [CrossRef]
- Souza, A.P.; Bombassaro, G.E.; Fonseca, F.N.; Lopes, L.D.S.; Maciag, S.S.; Volpato, F.B.; Bastos, A.P. A comparative evaluation of methods for estimating the colostrum quality in sows. Arq. Bras. Med. Vet. Zootec. 2021, 73, 1047–1057. [Google Scholar] [CrossRef]
- Schoos, A.; De Spiegelaere, W.; Cools, A.; Pardon, B.; Van Audenhove, E.; Bernaerdt, E.; Maes, D. Evaluation of the agreement between Brix refractometry and serum immunoglobulin concentration in neonatal piglets. Animal 2021, 15, 100041. [Google Scholar] [CrossRef] [PubMed]
- Breuer, R.M.; Wiley, C.; Dohlman, T.; Smith, J.S.; McKeen, L.; Kreuder, A.J. Comparison of turbidometric immunoassay and brix refractometry to radial immunodiffusion for assessment of colostral immunoglobulin concentration in beef cattle. J. Vet. Intern. Med. 2023, 37, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Turini, L.; Bonelli, F.; Nocera, I.; Meucci, V.; Conte, G.; Sgorbini, M. Evaluation of different methods to estimate the transfer of immunity in donkey foals fed with colostrum of good IgG quality: A preliminary study. Animals 2021, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- de Lima, T.C.; de Sobral, G.G.; de França Queiroz, A.E.S.; Chinelate, G.C.B.; Porto, T.S.; Oliveira, J.T.C.; Carneiro, G.F. Characterization of lyophilized equine colostrum. J. Equine Vet. Sci. 2024, 132, 104975. [Google Scholar] [CrossRef]
- Shao, X.; Cheng, M.; Zhang, X.; Wang, C.; Jiang, H. Analysis on the effect of the various factors on immunoglobulin G in goat colostrum. In Proceedings of the 2020 International Conference on Environmental Engineering and Energy Science and Technology (IAECST 2020), Guangzhou, China, 18–20 December 2020; EDP Sciences: Les Ulis, France, 2021; p. 2042. [Google Scholar]
- Zamuner, F.; Cameron, A.W.N.; Carpenter, E.K.; Arcos-Gómez, G.; Leury, B.J.; DiGiacomo, K. Postponing first colostrum collection: Impact on immunoglobulin G in goat colostrum. Animal 2024, 18, 101277. [Google Scholar] [CrossRef]
- McCue, P.M. Colostrum banking. In Equine Reproductive Procedures; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 299–301. [Google Scholar]
- Quigley, J.D.; Lago, A.; Chapman, C.; Erickson, P.; Polo, J. Evaluation of the Brix refractometer to estimate immunoglobulin G concentration in bovine colostrum. J. Dairy Sci. 2013, 96, 1148–1155. [Google Scholar] [CrossRef]
- Hasan, S.; Orro, T.; Valros, A.; Junnikkala, S.; Peltoniemi, O.; Oliviero, C. Factors affecting sow colostrum yield and composition, and their impact on piglet growth and health. Livest. Sci. 2019, 227, 60–67. [Google Scholar] [CrossRef]
- Gamsjäger, L.; Elsohaby, I.; Pearson, J.M.; Levy, M.; Pajor, E.A.; Haines, D.M.; Windeyer, M.C. Assessment of Brix refractometry to estimate immunoglobulin G concentration in beef cow colostrum. J. Vet. Intern. Med. 2020, 34, 1662–1673. [Google Scholar] [CrossRef]
- Oliviero, C.; Junnikkala, S.; Peltoniemi, O. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 2019, 54, 12–21. [Google Scholar] [CrossRef]
- Farmer, C.; Edwards, S.A. Improving the performance of neonatal piglets. Animal 2022, 16, 100350. [Google Scholar] [CrossRef]
- Yarberry, W.; Yarberry, W. CRAN Recipes: DPLYR, Stringr, Lubridate, and RegEx in R; Apress: Berkeley, CA, USA, 2021; Available online: https://link.springer.com/book/10.1007/978-1-4842-6876-6 (accessed on 16 June 2025).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. Version 2. 2016. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 16 June 2025).
- Husson, F.; Josse, J.; Le, S.; Mazet, J.; Husson, M.F. Package ‘Factominer’. Version 2.11. 2016. Available online: https://cran.r-project.org/web/packages/FactoMineR/index.html (accessed on 16 June 2025).
- Kassambara, A.; Mundt, F. Package ‘Factoextra’. Extract and Visualize the Results of Multivariate Data Analyses. R Package Version. Available online: https://rpkgs.datanovia.com/factoextra/ (accessed on 16 June 2025).
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Heiberger, R. Package ‘Car’. 2012. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 16 June 2025).
- R Development Core Team. R. A Language and Environment for Statistical Computing, Version 4.0.2. Computer Software : Vienna, Austria. 2020. Available online: https://www.R-project.org (accessed on 8 April 2025).
- Virdis, S.; Luise, D.; Correa, F.; Laghi, L.; Arrigoni, N.; Amarie, R.E.; Trevisi, P. Productive and metabolomic consequences of arginine supplementation in sows during different gestation periods in two different seasons. J. Anim. Sci. Biotechnol. 2024, 15, 1–121. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ajuwon, K.M. Metabolomics of heat stress response in pig adipose tissue reveals alteration of phospholipid and fatty acid composition during heat stress. J. Anim. Sci. 2018, 96, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yan, H.; Lu, H.; Donkin, S.S.; Ajuwon, K.M. Heat stress in pigs is accompanied by adipose tissue–specific responses that favor increased triglyceride storage. J. Anim. Sci. 2016, 94, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.U.; Li, Z.Y.; Jia, A.F.; Su, H.G.; Hu, C.H.; Zhang, M.H.; Feng, J.H. Effects of high ambient temperature on lipid metabolism in finishing pigs. J. Integr. Agric. 2016, 15, 391–396. [Google Scholar]
- Dado-Senn, B.; Skibiel, A.L.; Fabris, T.F.; Dahl, G.E.; Laporta, J. Dry period heat stress induces microstructural changes in the lactating mammary gland. PLoS ONE 2019, 14, e0222120. [Google Scholar] [CrossRef]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Z.; Zheng, C.; Hu, Y.; Wang, L. Nutritional regulation for meat quality and nutrient metabolism of pigs exposed to high temperature environment. J. Nutr. Sci. 2015, 5, 1. [Google Scholar] [CrossRef]
- Farmer, C.; Quesnel, H. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 2009, 87, 56–64. [Google Scholar] [CrossRef]
- Declerck, I.; Dewulf, J.; Piepers, S.; Decaluwé, R.; Maes, D. Sow and litter factors influencing colostrum yield and nutritional composition. J. Anim. Sci. 2015, 93, 1309–1317. [Google Scholar] [CrossRef]
- Quesnel, H.; Resmond, R.; Merlot, E.; Père, M.C.; Gondret, F.; Louveau, I. Physiological traits of newborn piglets associated with colostrum intake, neonatal survival and preweaning growth. Animal 2023, 17, 100843. [Google Scholar] [CrossRef]
- Hasan, S.; Saha, S.; Junnikkala, S.; Orro, T.; Peltoniemi, O.; Oliviero, C. Late gestation diet supplementation of resin acid-enriched composition increases sow colostrum immunoglobulin G content, piglet colostrum intake and improve sow gut microbiota. Animal 2019, 13, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, P.; Luise, D.; Won, S.; Salcedo, J.; Bertocchi, M.; Barile, D.; Bosi, P. Variations in porcine colostrum oligosaccharide composition between breeds and in association with sow maternal performance. J. Anim. Sci. Biotechnol. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, S.; Deng, Z.; Zhou, Q.; Cheng, L.; Kim, S.W.; Guan, W. Regulation of amino acid transporters in the mammary gland from late pregnancy to peak lactation in the sow. J. Anim. Sci. Biotechnol. 2018, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Renaudeau, D.; Noblet, J. Effects of exposure to high ambient temperature and dietary protein level on sow milk production and performance of piglets. J. Anim. Sci. 2001, 79, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Basiricò, L.; Morera, P. Impact of hot environment on colostrum and milk composition. Cell. Mol. Biol. 2013, 58, 9–25. [Google Scholar]
- Inoue, T.; Kitano, K.; Inoue, K. Possible factors influencing the immunoglobulin G concentration in swine colostrum. Am. J. Vet. Res. 1980, 41, 1134–1136. [Google Scholar] [CrossRef]
- Juthamanee, P.; Suwimonteerabutr, J.; Tummaruk, P. The influence of parity, body condition, litter size and carbetocin administration on colostrum production and immunoglobulin levels in highly productive sows within a tropical environment. Trop. Anim. Health Prod. 2024, 56, 74. [Google Scholar] [CrossRef]
- Patra, M.K.; De, U.K.; Kent, Y.; Rungsung, S.; Krishnaswamy, N.; Deka, B.C. Influence of seasonal variation on post-farrowing dysgalactia syndrome (PFDS) and serum biochemistry profiles in the periparturient sow. Trop. Anim. Health Prod. 2021, 53, 346. [Google Scholar] [CrossRef]
- Szabó, C.; Ortega, A.D.S.V.; Lugata, J.K.; Czeglédi, L.; Csernus, B.; Gulyás, G.; Horváth, M. Factors Affecting the Ig Content of Sow’s Colostrum: A Systematic Review and Meta-Analysis. Agriculture 2025, 15, 641. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Papatsiros, V.; Argyris, G.; Papakonstantinou, G.; Meletis, E.; Tsekouras, N.; Kantas, D.; Kostoulas, P. Evaluation of an on-farm method to assess colostrum IgG content in hyperprolific sows. Anim. Reprod. Sci. 2022, 239, 106958. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Ford, H.R.; Bionaz, M. Impact of maternal diet on the antioxidant status, immune function, and whole-blood selenium levels of lamb offspring. Ital. J. Anim. Sci. 2025, 24, 738–752. [Google Scholar] [CrossRef]
- Röder, M.; Borchardt, S.; Heuwieser, W.; Rauch, E.; Sargent, R.; Sutter, F. Evaluation of laboratory and on-farm tests to estimate colostrum quality for dairy cows. J. Anim. Sci. 2023, 106, 9164–9173. [Google Scholar] [CrossRef] [PubMed]
- Elsohaby, I.; McClure, J.T.; Dow, N.; Keefe, G.P. Effect of heat-treatment on accuracy of infrared spectroscopy and digital and optical brix refractometers for measuring immunoglobulin G concentration in bovine colostrum. J. Vet. Intern. Med. 2018, 32, 491–496. [Google Scholar] [CrossRef]
- Stojić, M.; Fratrić, N.; Kovačić, M.; Ilić, V.; Gvozdić, D.; Savić, O.; Đoković, R. Brix refractometry of colostrum from primiparous dairy cows and new-born calf blood serum in the evaluation of failure of passive transfer. Acta Vet. 2017, 67, 508–524. [Google Scholar] [CrossRef]
- Acar, T.O. Comparing Measurement Reliability Estimation Techniques: Correlation Coefficient vs. Bland–Altman Plot. Meas-Interdiscip. Res. Perspect. 2024, 1, 12. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Waters, R.; Nazemipour, M.; Bland, M.; Altman, D.G. Bland-Altman methods for comparing methods of measurement and response to criticisms. Glob. Epidemiol. 2021, 3, 100045. [Google Scholar] [CrossRef]

| Trait | Fat, % | SEM | Proteins, % | SEM | Lactose, % | SEM | SNT, % | SEM |
|---|---|---|---|---|---|---|---|---|
| GT1 | ||||||||
| SSP | 5.49 a | 0.13 | 14.72 bc | 0.19 | 2.57 | 0.04 | 18.77 | 0.19 |
| SMP | 5.05 b | 0.21 | 15.50 b | 0.31 | 2.67 | 0.05 | 20.52 | 0.86 |
| WNP | 5.62 a | 0.12 | 16.20 a | 0.25 | 2.56 | 0.04 | 18.52 | 0.20 |
| GT2 | ||||||||
| SSP | 4.64 a | 0.14 | 16.55 a | 0.22 | 2.43 | 0.05 | 19.43 | 0.18 |
| SMP | 3.87 b | 0.15 | 15.53 b | 0.32 | 2.27 | 0.09 | 19.38 | 0.29 |
| WNP | 4.96 a | 0.19 | 16.25 a | 0.26 | 2.42 | 0.06 | 20.18 | 0.27 |
| Genotype | 0.0001 | 0.001 | 0.0001 | 0.24 | ||||
| Season | 0.0001 | 0.05 | 0.88 | 0.11 | ||||
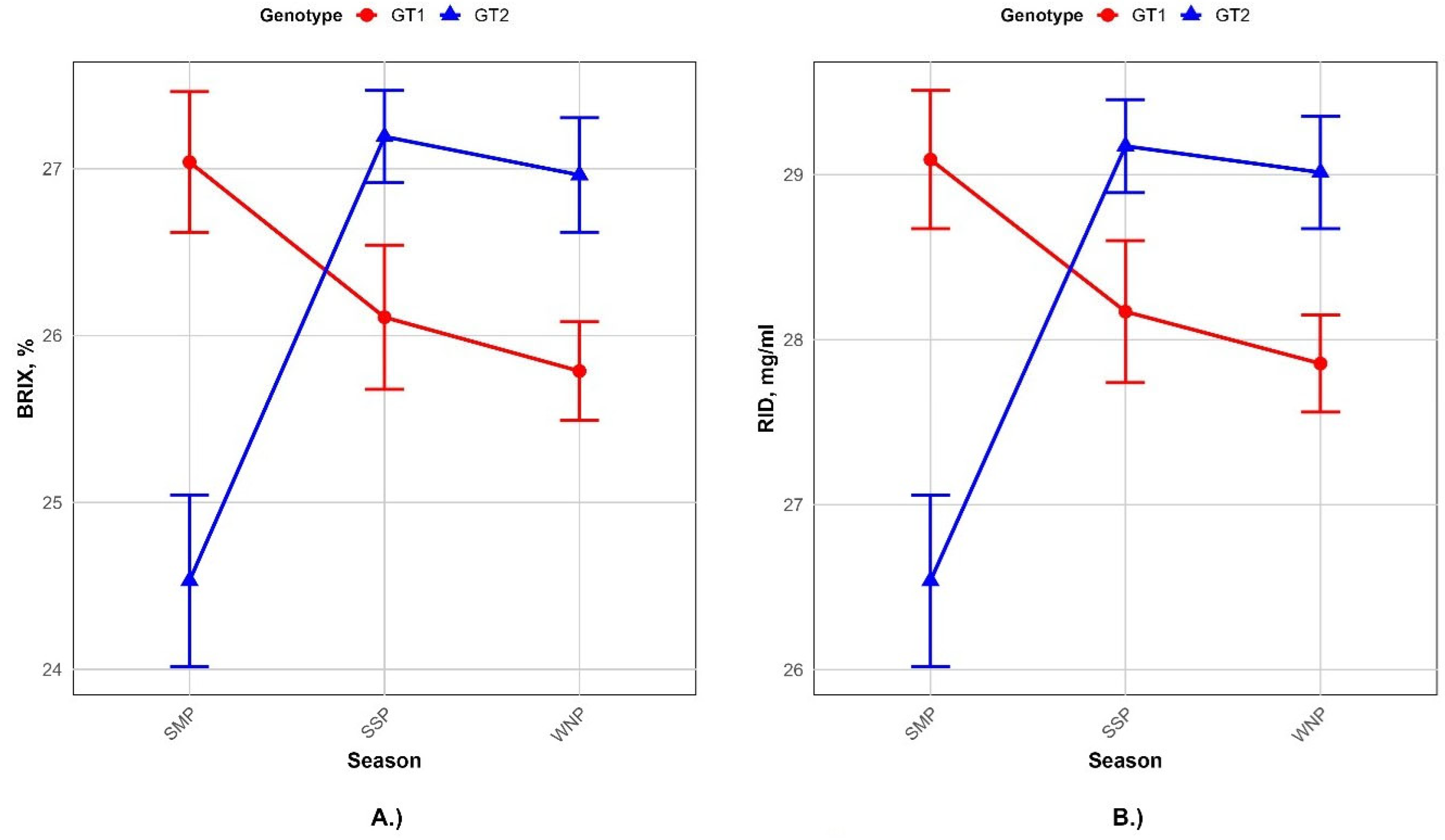
| Trait | RID, mg/mL | SEM | BRIX, % | SEM |
|---|---|---|---|---|
| GT1 | ||||
| SSP | 28.17 b | 0.45 | 26.11 b | 0.43 |
| SMP | 29.09 a | 0.40 | 27.04 a | 0.42 |
| WNP | 27.85 b | 0.27 | 25.84 b | 0.29 |
| GT2 | ||||
| SSP | 29.17 a | 0.27 | 27.24 a | 0.28 |
| SMP | 26.53 b | 0.51 | 24.43 b | 0.22 |
| WNP | 29.04 a | 0.99 | 26.96 a | 0.34 |
| Genotype | 0.70 | 0.81 | ||
| Season | 0.05 | 0.05 | ||
| Repeatability | 0.0151 | 0.0189 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdanović, K.; Čuljak, V.; Margeta, V.; Kušec, I.D.; Antunović, B.; Galović, D.; Kušec, G. The Influence of Genotype and Seasonality on the Sow Colostrum Quality and Immunoglobulin G Content. Animals 2025, 15, 1802. https://doi.org/10.3390/ani15121802
Gvozdanović K, Čuljak V, Margeta V, Kušec ID, Antunović B, Galović D, Kušec G. The Influence of Genotype and Seasonality on the Sow Colostrum Quality and Immunoglobulin G Content. Animals. 2025; 15(12):1802. https://doi.org/10.3390/ani15121802
Chicago/Turabian StyleGvozdanović, Kristina, Vice Čuljak, Vladimir Margeta, Ivona Djurkin Kušec, Boris Antunović, Dalida Galović, and Goran Kušec. 2025. "The Influence of Genotype and Seasonality on the Sow Colostrum Quality and Immunoglobulin G Content" Animals 15, no. 12: 1802. https://doi.org/10.3390/ani15121802
APA StyleGvozdanović, K., Čuljak, V., Margeta, V., Kušec, I. D., Antunović, B., Galović, D., & Kušec, G. (2025). The Influence of Genotype and Seasonality on the Sow Colostrum Quality and Immunoglobulin G Content. Animals, 15(12), 1802. https://doi.org/10.3390/ani15121802







