Evaluation of Change in Center of Pressure During Perturbation of Balance Including Blindfolding in Healthy Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants and Inclusion Criteria
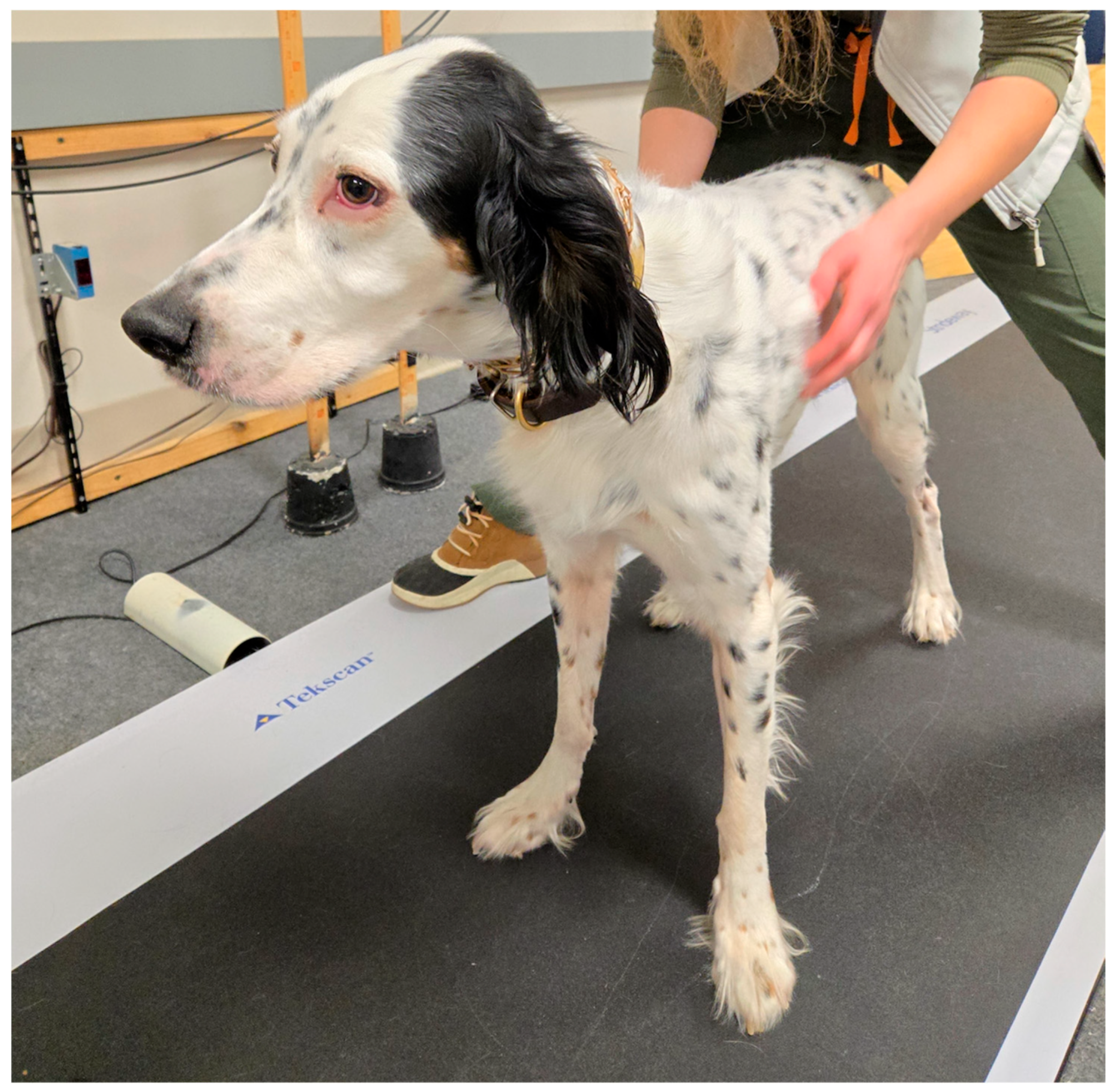
2.3. Procedure and Equipment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COF | Center of Force |
| COP | Center of Pressure |
| COM | Center of Mass |
| COG | Center of Gravity |
| BOS | Base of Support |
| RL | Right–Left |
| CC | Cranio-Caudal |
| SD | Standard Deviation |
References
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to Differentiate Balance Deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.E.; McGowan, C.M.; Hyytiäinen, H.K. Posture and Postural Dysfunction in Dogs: Implications for Veterinary Physiotherapy. Vet. J. 2024, 305, 106107. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Wollacott, M. Motor Control. In Translating Research into Clinical Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Pai, Y.-C.; Rogers, M.W.; Patton, J.; Cain, T.D.; Hanke, T.A. Static Versus Dynamic Predictions of Protective Stepping Following Waist–Pull Perturbations in Young and Older Adults. J. Biomech. 1998, 31, 1111–1118. [Google Scholar] [CrossRef]
- Bieber, B.; Reicher, B.; Tichy, A.; Bockstahler, B. Changes in Ground Reaction Forces and Center of Pressure Parameters of Paws When Wearing Dog Boots in Dogs. Front. Vet. Sci. 2022, 9, 906277. [Google Scholar] [CrossRef] [PubMed]
- Mondino, A.; Wagner, G.; Russell, K.; Lobaton, E.; Griffith, E.; Gruen, M.; Lascelles, B.D.X.; Olby, N.J. Static Posturography as a Novel Measure of the Effects of Aging on Postural Control in Dogs. PLoS ONE 2022, 17, e0268390. [Google Scholar] [CrossRef]
- Brantberg, I.; Grooten, W.J.A.; Essner, A. The Effect of Therapeutic Exercise on Body Weight Distribution, Balance, and Stifle Function in Dogs Following Stifle Injury. Animals 2024, 14, 92. [Google Scholar] [CrossRef]
- Aghapour, M.; Affenzeller, N.; Lutonsky, C.; Peham, C.; Tichy, A.; Bockstahler, B. A Validation Study to Analyze the Reliability of Center of Pressure Data in Static Posturography in Dogs. Front. Vet. Sci. 2024, 11, 1353824. [Google Scholar] [CrossRef]
- Lutonsky, C.; Peham, C.; Mucha, M.; Reicher, B.; Gaspar, R.; Tichy, A.; Bockstahler, B. External Mechanical Perturbations Challenge Postural Stability in Dogs. Front. Vet. Sci. 2023, 10, 1249951. [Google Scholar] [CrossRef]
- Lutonsky, C.; Peham, C.; Affenzeller, N.; Aghapour, M.; Wegscheider, J.; Tichy, A.; Bockstahler, B. Impact of Aging and Visual Input on Postural Stability in Dogs: Insights from Center-of-Pressure Analysis. Sensors 2025, 25, 1300. [Google Scholar] [CrossRef]
- Rodriguez, O.; Regueiro-Purriños, M.; Figueirinhas, P.; Gonzalo-Orgen, J.M.; Prada, I.; Vilar, J.M.; Millán, L.; Rodríguez-Altónage, J. Dynamic and Postural Changes in Forelimb Amputee Dogs: A Pilot Study. Animals 2024, 14, 1960. [Google Scholar] [CrossRef]
- Charalambous, D.; Strasser, T.; Tichy, A.; Bockstahler, B. Ground Reaction Forces and Center of Pressure within the Paws When Stepping over Obstacles in Dogs. Animals 2022, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.F.; Lins, D.; Toledo, T.; Álvarez, C.B.G. Postural Stability Measures in Healthy Miniature Dachshunds Obtained Using a Pressure Mat and a Force Platform: A Validity and Reliability Study. BMC Vet. Res. 2023, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.; Shaheen, A.F.; Álvarez, C.B.G. Biomechanical Comparison of Standing Posture and during Trot between German Shepherd and Labrador Retriever Dogs. PLoS ONE 2020, 15, e0239832. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.M.; Manera, M.E.; Rubio, M.; Sopena, J.; Santana, A.; Vilar, J.M. Posturography and Dynamic Pedobarography in Lame Dogs with Elbow Dysplasia and Cranial Cruciate Ligament Rupture. BMC Vet. Res. 2018, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.E.; Carrillo, J.M.; Batista, M.; Rubio, M.; Sopena, J.; Santana, A.; Vilar, J.M. Static Posturography: A New Perspective in the Assessment of Lameness in a Canine Model. PLoS ONE 2017, 12, e0170692. [Google Scholar] [CrossRef]
- López, S.; Vilar, J.M.; Rubio, M.; Sopena, J.J.; Damiá, E.; Chicharro, D.; Santana, A.; Carrillo, J.M. Center of Pressure Limb Path Differences for the Detection of Lameness in Dogs: A Preliminary Study. BMC Vet. Res. 2019, 15, 138. [Google Scholar] [CrossRef]
- Reicher, B.; Tichy, A.; Bockstahler, B. Center of Pressure in the Paws of Clinically Sound Dogs in Comparison with Orthopedically Diseased Dogs. Animals 2020, 10, 1366. [Google Scholar] [CrossRef]
- Virag, Y.; Gumpenberger, M.; Tichy, A.; Lutonsky, C.; Peham, C.; Bockstahler, B. Center of Pressure and Ground Reaction Forces in Labrador and Golden Retrievers with and Without Hip Dysplasia at 4, 8, and 12 Months of Age. Front. Vet. Sci. 2022, 9, 1087693. [Google Scholar] [CrossRef]
- Blau, S.R.; Davis, L.M.; Gorney, A.M.; Dohse, C.S.; Williams, K.D.; Lim, J.H.; Pfitzner, W.G.; Laber, E.; Sawicki, G.S.; Olby, N.J. Quantifying Center of Pressure Variability in Chondrodystrophoid Dogs. Vet. J. 2017, 226, 26–31. [Google Scholar] [CrossRef]
- Ruhe, A.; Fejer, R.; Walker, B. Center of Pressure Excursion as a Measure of Balance Performance in Patients with Non-Specific Low Back Pain Compared to Healthy Controls: A Systematic Review of the Literature. Eur. Spine J. 2011, 20, 358–368. [Google Scholar] [CrossRef]
- Nardone, A.; Grasso, M.; Schieppati, M. Balance Control in Peripheral Neuropathy: Are Patients Equally Unstable Under Static and Dynamic Conditions? Gait Posture 2006, 23, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.E.; Carpenter, M.G.; van der Kooij, H.; Bloem, B.R. The Clinical Utility of Posturography. Clin. Neurophysiol. 2008, 119, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Centomo, H.; Termoz, N.; Savoie, S.; Béliveau, L.; Prince, F. Postural Control Following a Self-Initiated Reaching Task in Type 2 Diabetic Patients and Age-Matched Controls. Gait Posture 2007, 25, 509–514. [Google Scholar] [CrossRef]
- Beebe, J.A.; Kronman, C.; Mahmud, F.; Basch, M.; Hogan, M.; Li, E.; Ploski, C.; Simons, L.E. Gait Variability and Relationships with Fear, Avoidance, and Pain in Adolescents with Chronic Pain. Phys. Ther. 2021, 101, pzab012. [Google Scholar] [CrossRef]
- Knarr, B.A.; Reisman, D.S.; Binder-Macleod, S.A.; Higginson, J.S. Understanding Compensatory Strategies for Muscle Weakness during Gait by Simulating Activation Deficits Seen Post-Stroke. Gait Posture 2013, 38, 270–275. [Google Scholar] [CrossRef]
- Horak, F.B. Postural Compensation for Vestibular Loss and Implications for Rehabilitation. Restor. Neurol. Neurosci. 2010, 28, 57–68. [Google Scholar] [CrossRef]
- Lewis, M.J.; Williams, K.D.; Langley, T.; Jarvis, L.M.; Sawicki, G.S.; Olby, N.J. Development of a Novel Gait Analysis Tool Measuring Center of Pressure for Evaluation of Canine Chronic Thoracolumbar Spinal Cord Injury. J. Neurotrauma 2019, 36, 3018–3025. [Google Scholar] [CrossRef] [PubMed]
- Gerards, M.H.G.; McCrum, C.; Mansfield, A.; Meijer, K. Perturbation-based Balance Training for Falls Reduction among Older Adults: Current Evidence and Implications for Clinical Practice. Geriatr. Gerontol. Int. 2017, 17, 2294–2303. [Google Scholar] [CrossRef]
- McCrum, C.; Bhatt, T.S.; Gerards, M.H.G.; Karamanidis, K.; Rogers, M.W.; Lord, S.R.; Okubo, Y. Perturbation-Based Balance Training: Principles, Mechanisms and Implementation in Clinical Practice. Front. Sports Act. Living 2022, 4, 1015394. [Google Scholar] [CrossRef]
- Termoz, N.; Martin, L.; Prince, F. Assessment of Postural Response After a Self-Initiated Perturbation. Mot. Control 2004, 8, 51–63. [Google Scholar] [CrossRef]
- Fujimoto, M.; Bair, W.N.; Rogers, M.W. Center of Pressure Control for Balance Maintenance During Lateral Waist-Pull Perturbations in Older Adults. J. Biomech. 2015, 48, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, A.N.; Bodkin, S.G.; Saliba, S.A.; Hart, J.M. Measures of Agility and Single-Legged Balance as Clinical Assessments in Patients with Anterior Cruciate Ligament Reconstruction and Healthy Individuals. J. Athl. Train. 2019, 54, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Mademli, L.; Mavridi, D.; Bohm, S.; Patikas, D.A.; Santuz, A.; Arampatzis, A. Standing on Unstable Surface Challenges Postural Control of Tracking Tasks and Modulates Neuromuscular Adjustments Specific to Task Complexity. Sci. Rep. 2021, 11, 6122. [Google Scholar] [CrossRef]
- Jöbges, M.; Heuschkel, G.; Pretzel, C.; Illhardt, C.; Renner, C.; Hummelsheim, H. Repetitive Training of Compensatory Steps: A Therapeutic Approach for Postural Instability in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1682–1687. [Google Scholar] [CrossRef]
- Wolszky, V.; Zablotski, Y.; Fischer, A.; Lauer, S. Balance Assessment on a Modified Posturomed Platform in Healthy Dogs. Vet. Sci. 2024, 11, 498. [Google Scholar] [CrossRef]
- Clayton, H.M.; Nauwelaerts, S. Effect of Blindfolding on Centre of Pressure Variables in Healthy Horses During Quiet Standing. Vet. J. 2014, 199, 365–369. [Google Scholar] [CrossRef]
- Helmich, I.; Gemmerich, R. Neuronal Control of Posture in Blind Individuals. Brain Topogr. 2024, 37, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Reynard, F.; Terrier, P. Role of Visual Input in the Control of Dynamic Balance: Variability and Instability of Gait in Treadmill Walking While Blindfolded. Exp. Brain Res. 2015, 233, 1031–1040. [Google Scholar] [CrossRef]
- Tekscan. Sway Analysis Module. Available online: https://www.tekscan.com/products-solutions/software/sway-analysis-module-sam (accessed on 17 February 2025).
- Scanaill, C.N.; Garattini, C.; Greene, B.R.; McGrath, M.J. Technology Innovation Enabling Falls Risk Assessment in a Community Setting. Ageing Int. 2011, 32, 217–231. [Google Scholar] [CrossRef]
- Shibata, D.; Yoshida, Y. Self-Mobilization Exercise Program Improved Postural Stability in the Anterior-Posterior Direction with Eyes Closed. Symmetry 2023, 15, 1321. [Google Scholar] [CrossRef]
- Dickerson, V.M.; Coleman, K.D.; Ogawa, M.; Saba, C.F.; Cornell, K.K.; Radlinsky, M.G.; Schmiedt, C.W. Outcomes of Dogs Underoing Limb Amputation, Owner Satisfaction with Limb Amputation Procedures, and Owner Perceptions Regarding Postsurgical Adaptation: 64 Cases (2005–2012). JAVMA 2015, 247, 786–792. [Google Scholar] [CrossRef]
- Van Der Kooij, H.; Van Asseldonk, E.; Van Der Helm, F.C.T. Comparison of Different Methods to Identify and Quantify Balance Control. J. Neurosci. Methods 2005, 145, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Goetschius, J.; Feger, M.A.; Hertel, J.; Hart, J.M. Validating Center-of-Pressure Balance Measurements Using the Matscan® Pressure Mat. J. Sport. Rehabil. 2018, 27. [Google Scholar] [CrossRef]
- Brenton-Rule, A.; Mattock, J.; Carroll, M.; Dalbeth, N.; Bassett, S.; Menz, H.B.; Rome, K. Reliability of the TekScan MatScan® System for the Measurement of Postural Stability in Older People with Rheumatoid Arthritis. J. Foot Ankle Res. 2012, 5, 21. [Google Scholar] [CrossRef]
- Cornilleau-Pérès, V.; Shabana, N.; Droulez, J.; Goh, J.C.H.; Lee, G.S.M.; Chew, P.T.K. Measurement of the Visual Contribution to Postural Steadiness from the COP Movement: Methodology and Reliability. Gait Posture 2005, 22, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Costa, M.; Roupa, I.; Pequito, M.; Prazeres, J.; Gaivão, M.; Abrantes, J.; Clayton, H.M. The Use of Pressure Plates for Static Center of Pressure Analysis in Horses. J. Equine Vet. Sci. 2015, 35, 315–320. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the p Value is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Clark, R.A.; Bell, S.W.; Feller, J.A.; Whitehead, T.S.; Webster, K.E. Standing Balance and Inter-Limb Balance Asymmetry at One Year Post Primary Anterior Cruciate Ligament Reconstruction: Sex Differences in a Cohort Study of 414 Patients. Gait Posture 2017, 52, 318–324. [Google Scholar] [CrossRef]
- Carrick, F.R.; Pagnacco, G.; Hunfalvay, M.; Azzolino, S.; Oggero, E. Head Position and Posturography: A Novel Biomarker to Identify Concussion Sufferers. Brain Sci. 2020, 10, 1003. [Google Scholar] [CrossRef]
- Szczygieł, E.; Fudacz, N.; Golec, J.; Golec, E. The Impact of the Position of the Head on the Functioning of the Human Body: A Systematic Review. Int. J. Occup. Med. Environ. Health 2020, 33, 559–568. [Google Scholar] [CrossRef]
- Hansson, E.E.; Beckman, A.; Håkansson, A. Effect of Vision, Proprioception, and the Position of the Vestibular Organ on Postural Sway. Acta Otolaryngol. 2010, 130, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.G.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S.N. Forces and Their Measurement. In Research Methods in Biomechanics; Human Kinetics: Champaign, IL, USA, 2014; pp. 79–108. [Google Scholar]
- Besancon, M.F.; Conzemius, M.G.; Derrick, T.R.; Ritter, M.J. Comparison of Vertical Forces in Normal Greyhounds Between Force Platform and Pressure Walkway Measurement Systems. Vet. Comp. Orthop. Traumatol. 2003, 16, 153–157. [Google Scholar] [CrossRef]
| Standing | Perturbation | Head Turn | Blindfold | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Mean (SD) | Median | Mean Difference (SD) | Median Difference | p-Value | d | Mean Difference (SD) | Median Difference | p-Value | d | Mean Difference (SD) | Median Difference | p-Value | d |
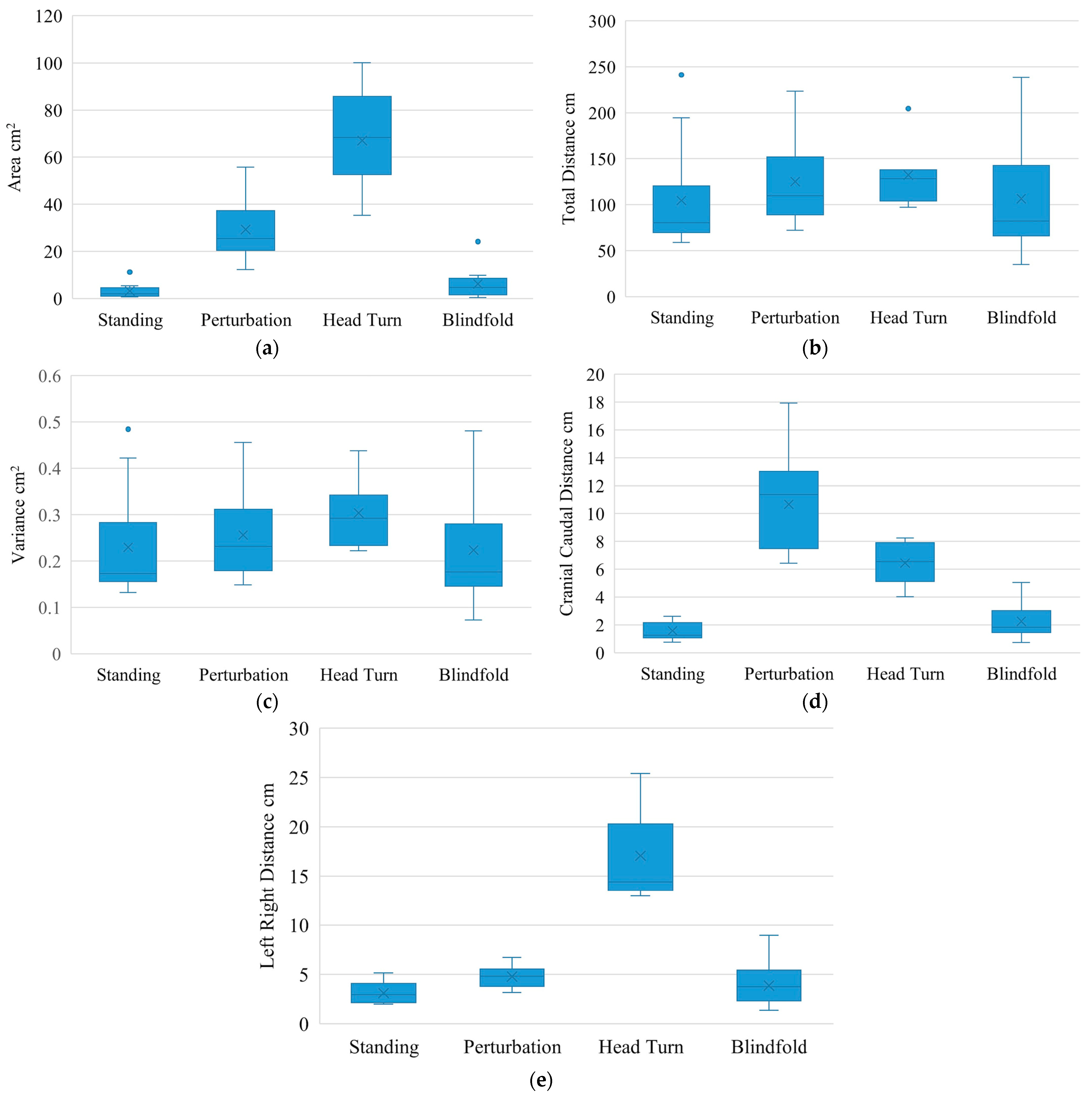
| Area (cm2) | 3.2 (2.9) | 2.0 | 26.1 (12.9) | 22.6 | 0.0002 * | 2.0 | 64.1 (24.0) | 64.5 | 0.0156 | 2.7 | 3.0 (4.2) | 2.2 | 0.0266 | 0.7 |
| Distance (cm) | 104.5 (54.9) | 80.5 | 20.7 (24.9) | 26.2 | 0.0171 | 0.8 | 48.6 (23.6) | 46.9 | 0.0156 | 2.1 | 2.0 (22.6) | 1.7 | 0.6848 | 0.1 |
| Variance (cm2) | 0.2 (0.1) | 0.2 | 0.0 (0.1) | 0.0 | 0.0803 | 0.5 | 0.1 (0.1) | 0.1 | 0.0156 | 2.0 | 0.0 (0.0) | 0.0 | 0.8926 | −0.1 |
| Cranial Caudal Distance (cm) | 1.6 (0.7) | 1.3 | 9.1 (3.6) | 10.2 | 0.0002 * | 2.5 | 5.1 (1.8) | 5.8 | 0.0156 | 2.8 | 0.7 (0.8) | 0.6 | 0.0105 | 0.8 |
| Left Right Distance (cm) | 3.1 (1.1) | 2.9 | 1.7 (1.0) | 1.3 | 0.0005 * | 1.6 | 14.3 (5.1) | 12.4 | 0.0156 | 2.8 | 0.7 (1.5) | 0.3 | 0.1272 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, H.; Evans, R.B.; Balogh, M.; Gordon-Evans, W.J. Evaluation of Change in Center of Pressure During Perturbation of Balance Including Blindfolding in Healthy Dogs. Animals 2025, 15, 1790. https://doi.org/10.3390/ani15121790
Hall H, Evans RB, Balogh M, Gordon-Evans WJ. Evaluation of Change in Center of Pressure During Perturbation of Balance Including Blindfolding in Healthy Dogs. Animals. 2025; 15(12):1790. https://doi.org/10.3390/ani15121790
Chicago/Turabian StyleHall, Hayley, Richard B. Evans, Makayla Balogh, and Wanda J. Gordon-Evans. 2025. "Evaluation of Change in Center of Pressure During Perturbation of Balance Including Blindfolding in Healthy Dogs" Animals 15, no. 12: 1790. https://doi.org/10.3390/ani15121790
APA StyleHall, H., Evans, R. B., Balogh, M., & Gordon-Evans, W. J. (2025). Evaluation of Change in Center of Pressure During Perturbation of Balance Including Blindfolding in Healthy Dogs. Animals, 15(12), 1790. https://doi.org/10.3390/ani15121790





