Toxic Effects of Acute Water Selenium Exposure on Litopenaeus vannamei: Survival, Physiological Responses, Transcriptome, and Intestinal Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Experimental Animal Management
2.2. Acute Toxicity Assay
2.3. Sample Collection and Processing
2.4. Histological Analysis
2.5. Biochemical Analysis
2.6. Transcriptome Analyze
2.7. qRT-PCR Verification of DEGs
2.8. Intestinal Microbiota
2.9. Statistical Analyses
3. Results
3.1. Se Toxicity Thresholds
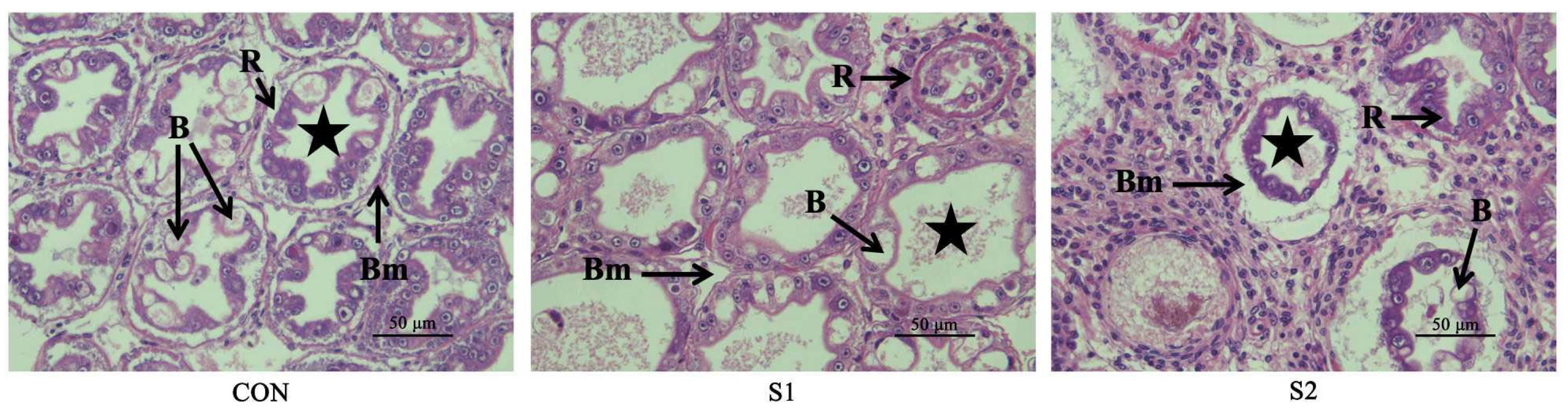
3.2. Hepatopancreatic Histopathology
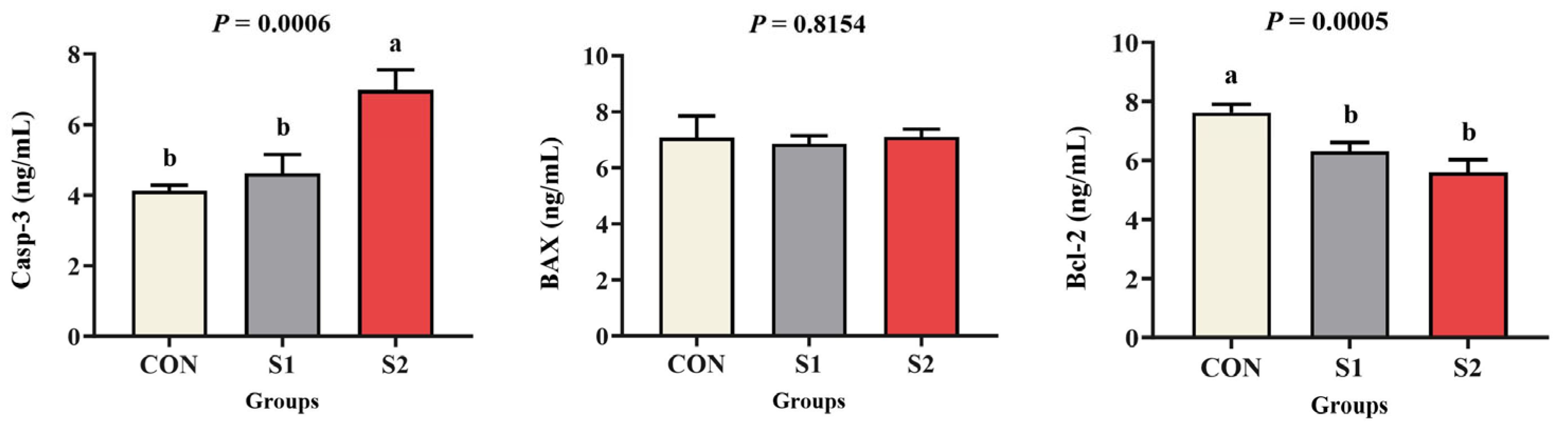
3.3. Apoptotic Response
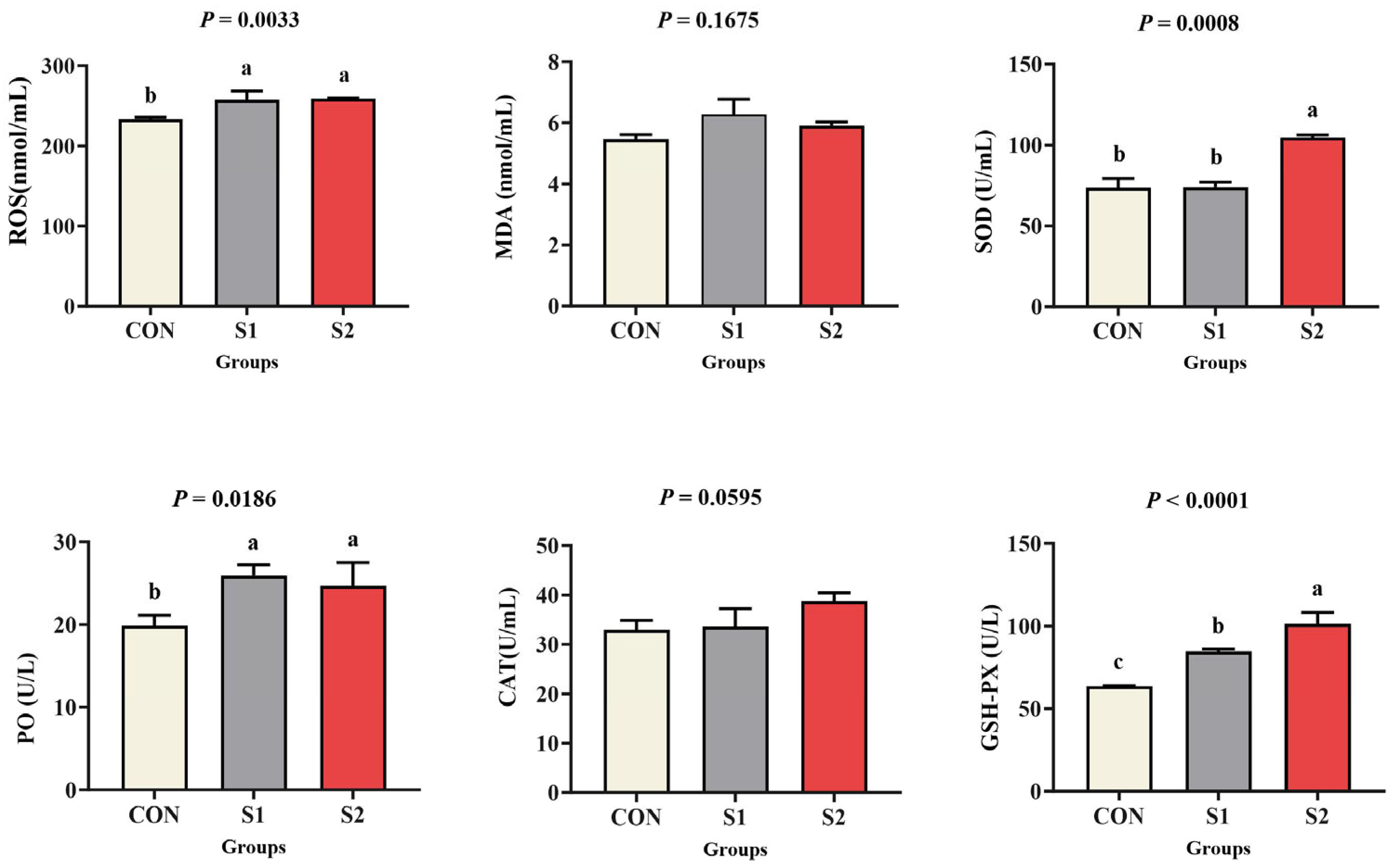
3.4. Oxidative Stress and Antioxidant Capacity
3.5. Immune Response
3.6. Transcriptome Analysis
3.6.1. Sequencing Data Evaluation Statistics
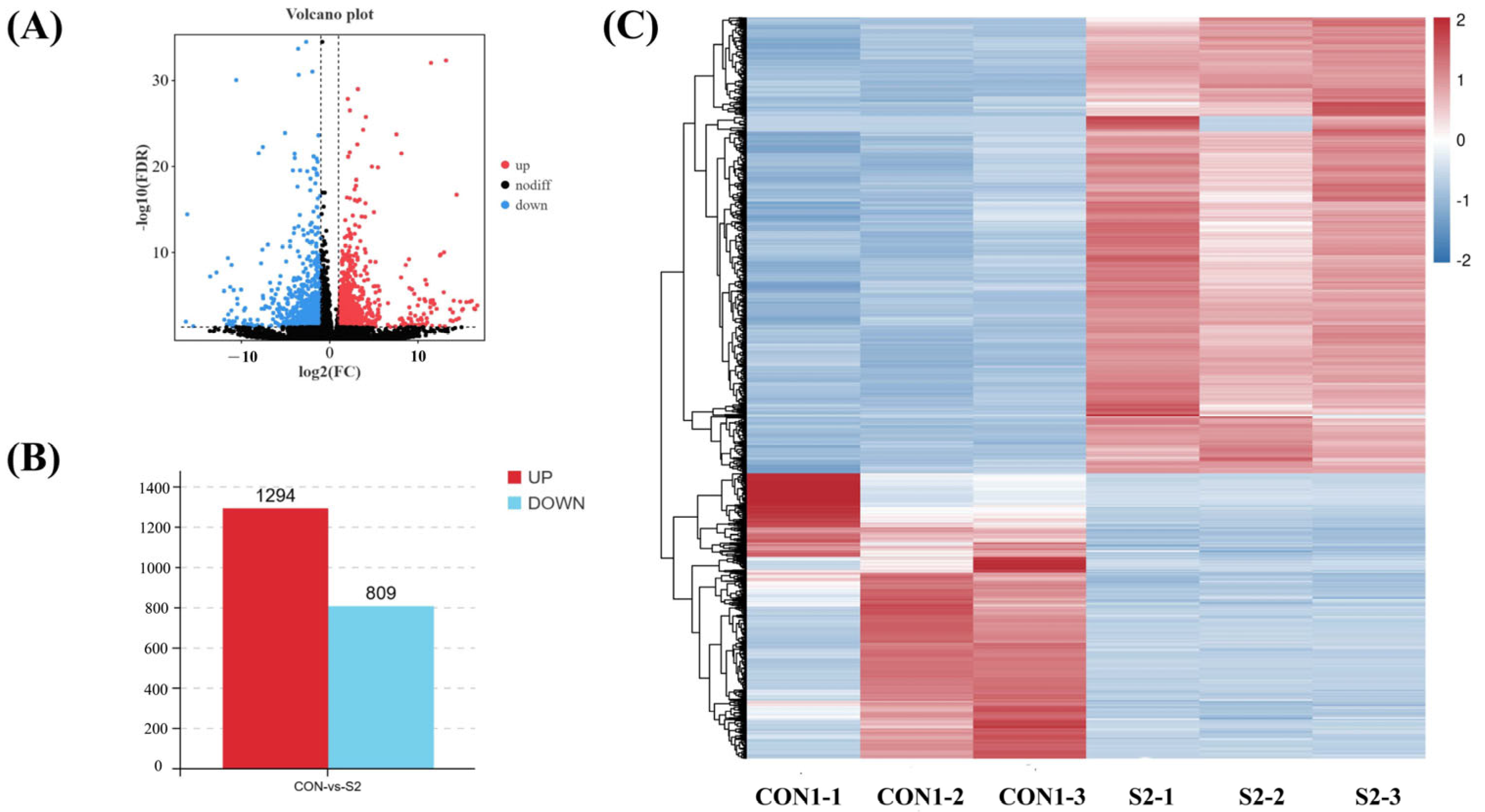
3.6.2. Differential Gene Expression
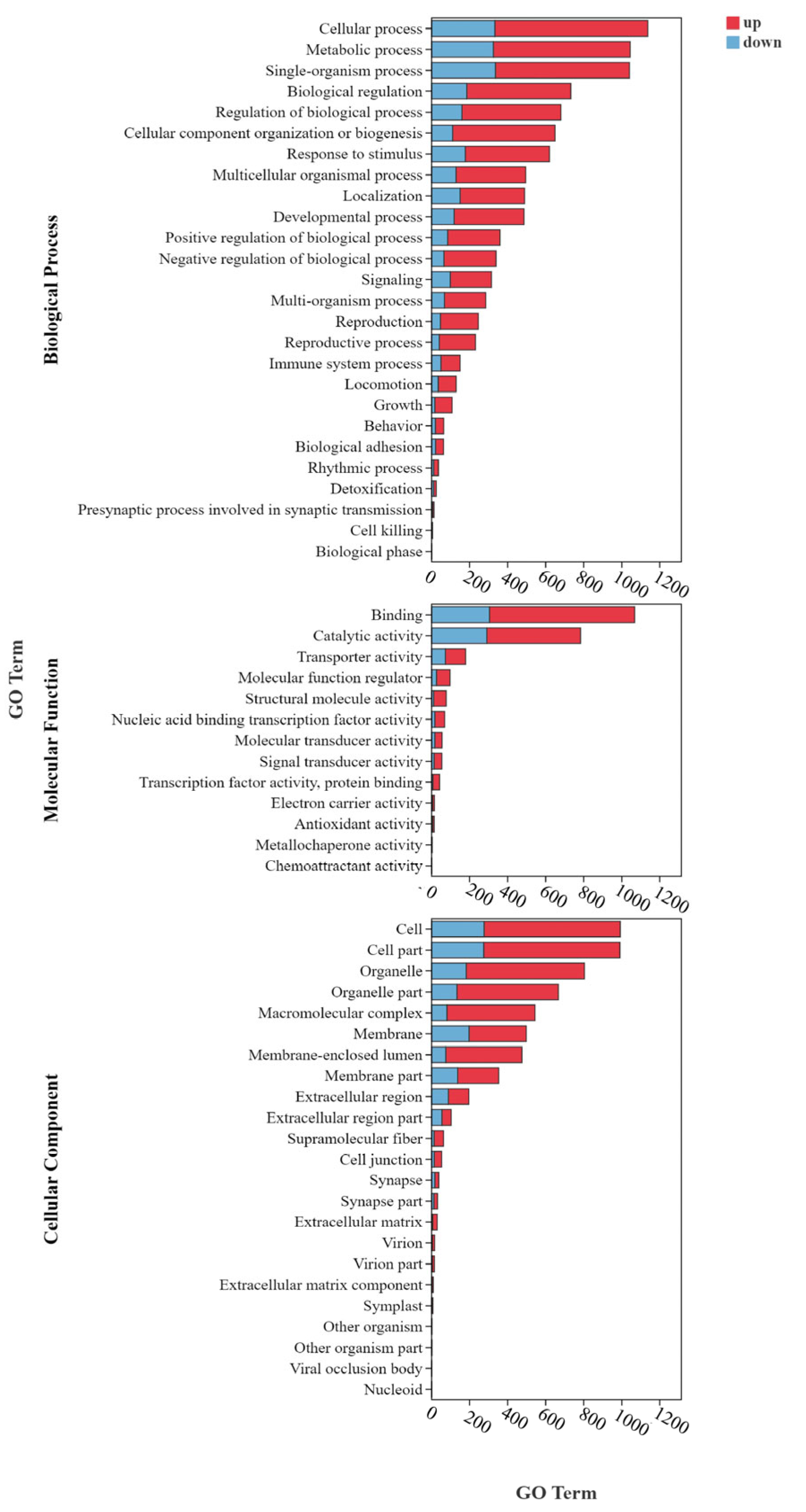
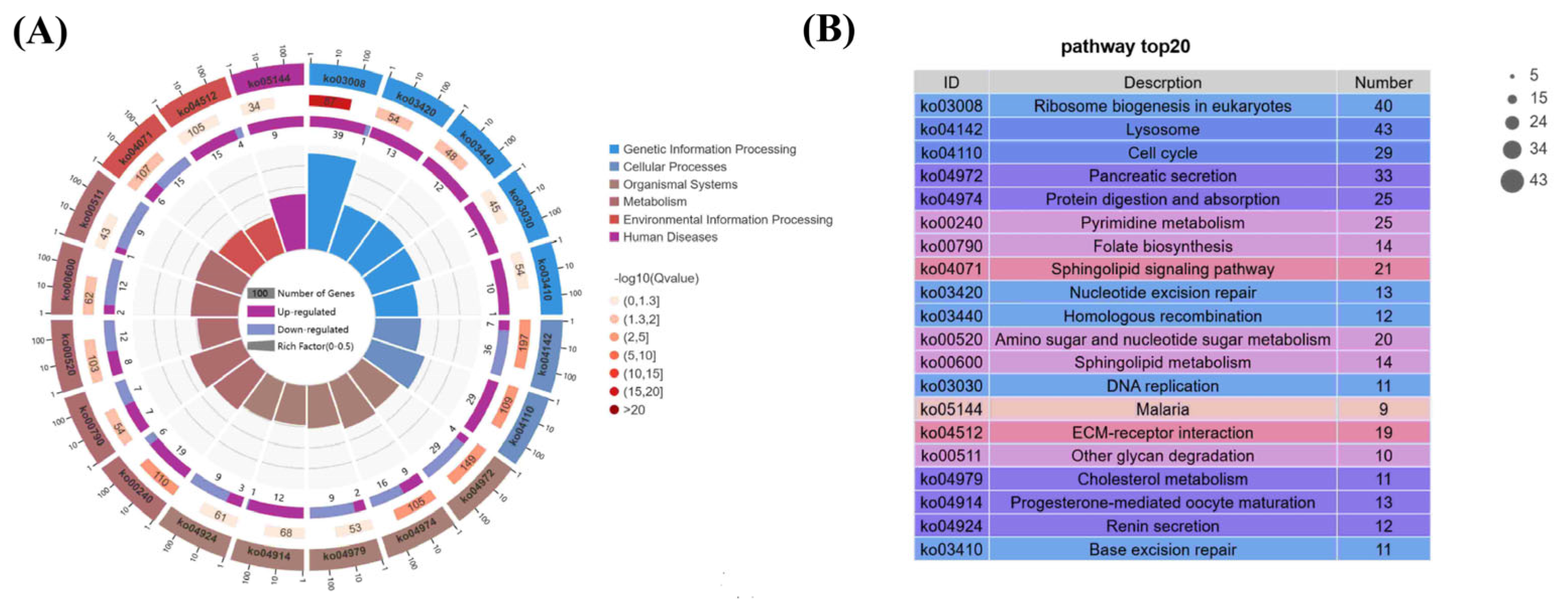
3.6.3. GO and KEGG Enrichment Analysis of DEGs
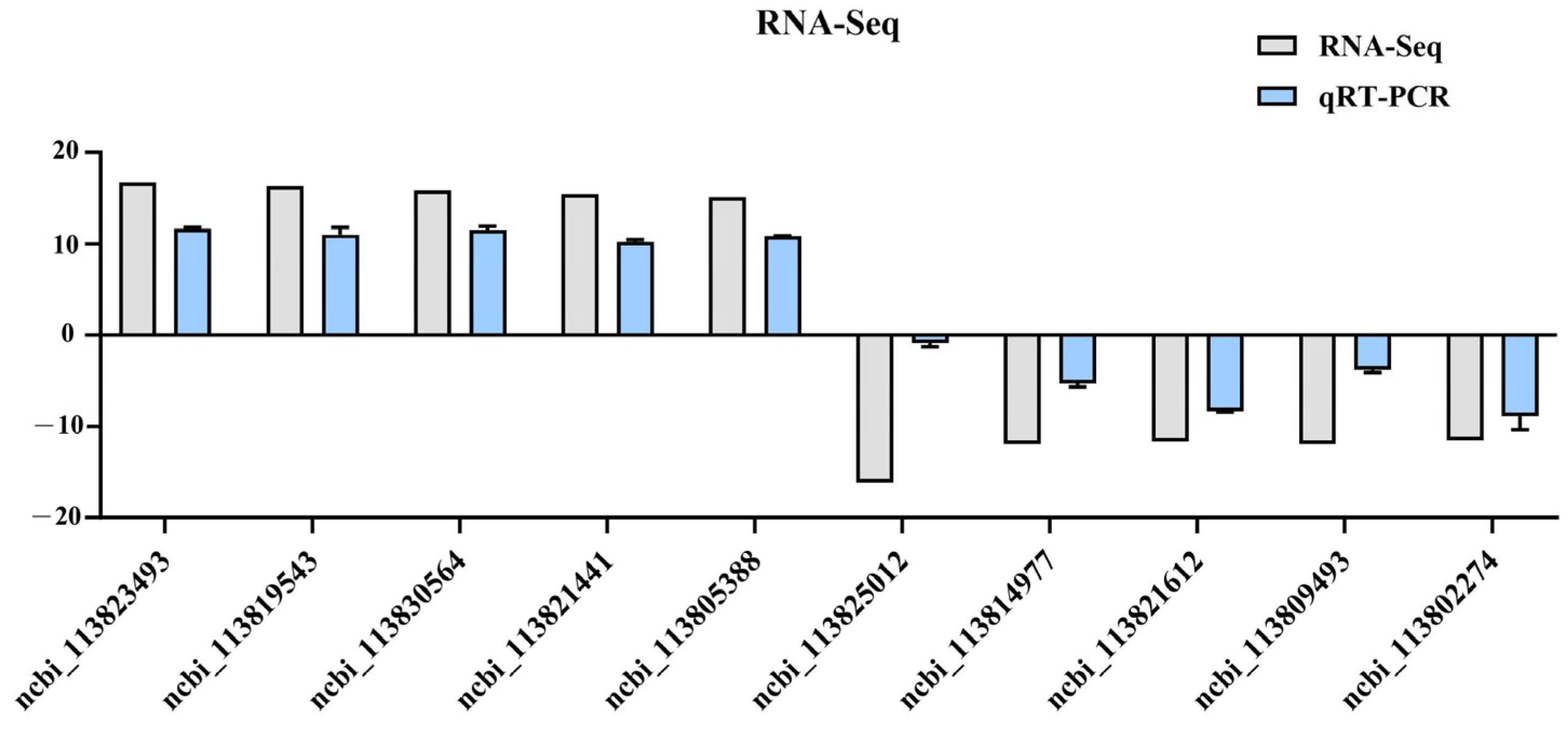
3.7. qPCR Validation of RNA-Seq Results
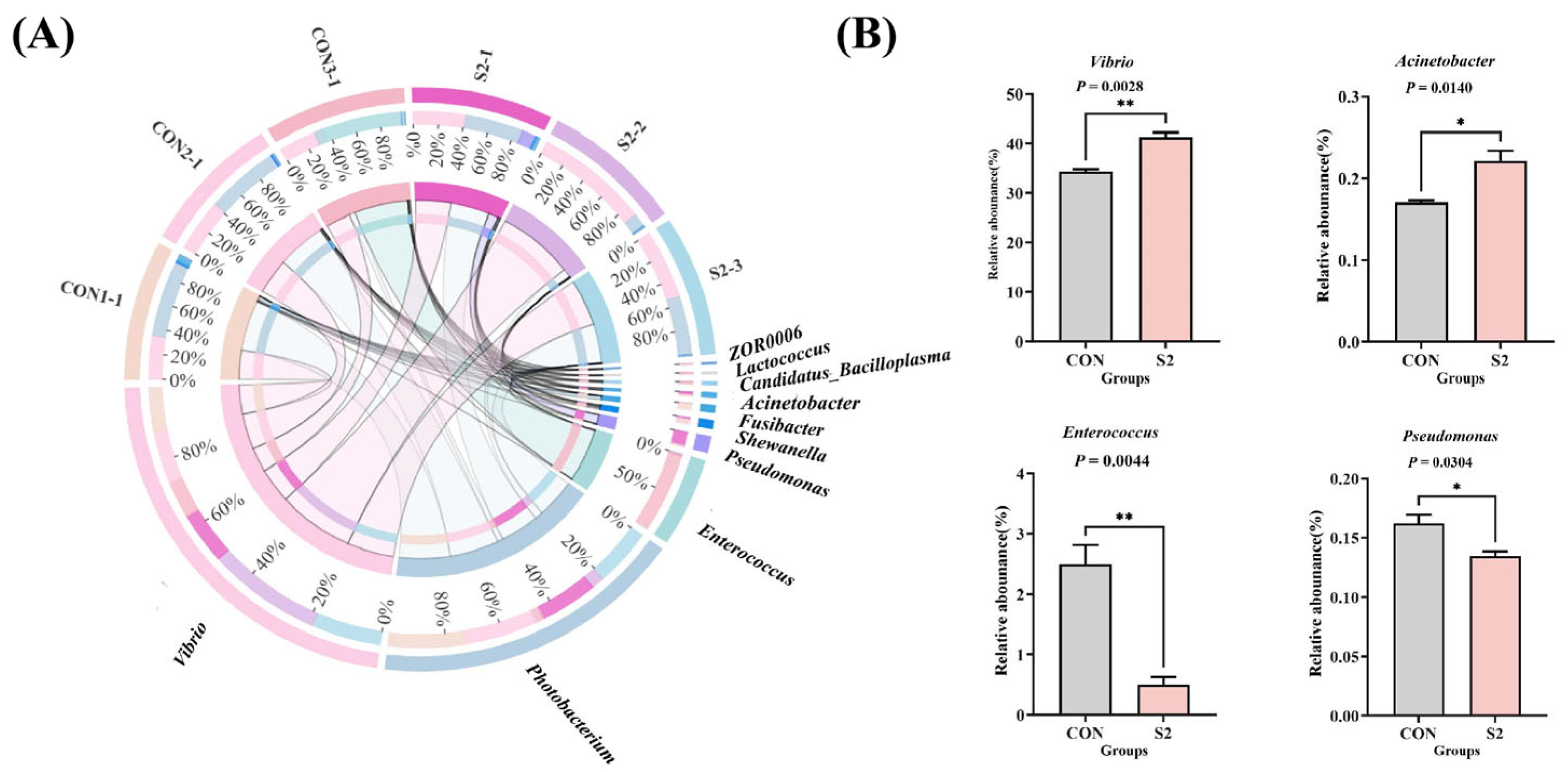
3.8. Intestinal Microbiota Analysis
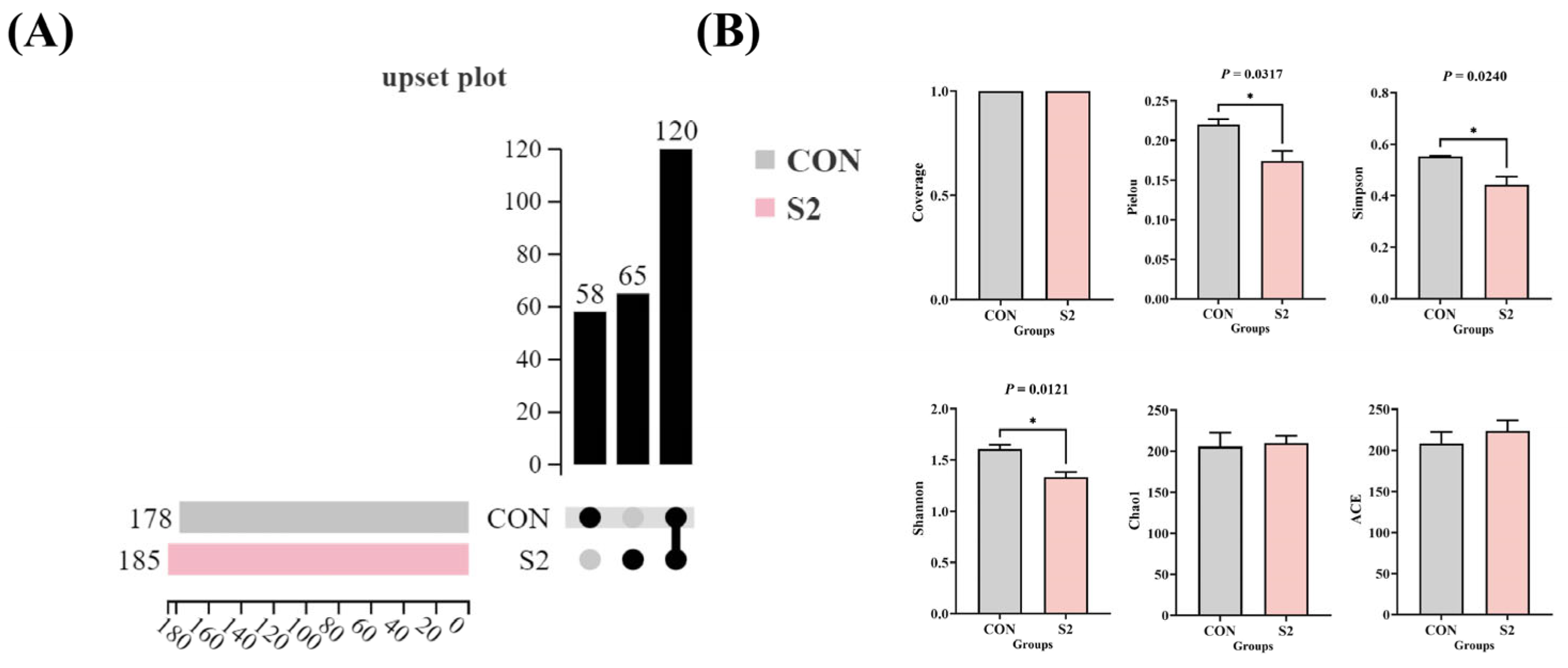
3.8.1. Diversity of Intestinal Microbiota
3.8.2. Composition of the Intestinal Microbiota
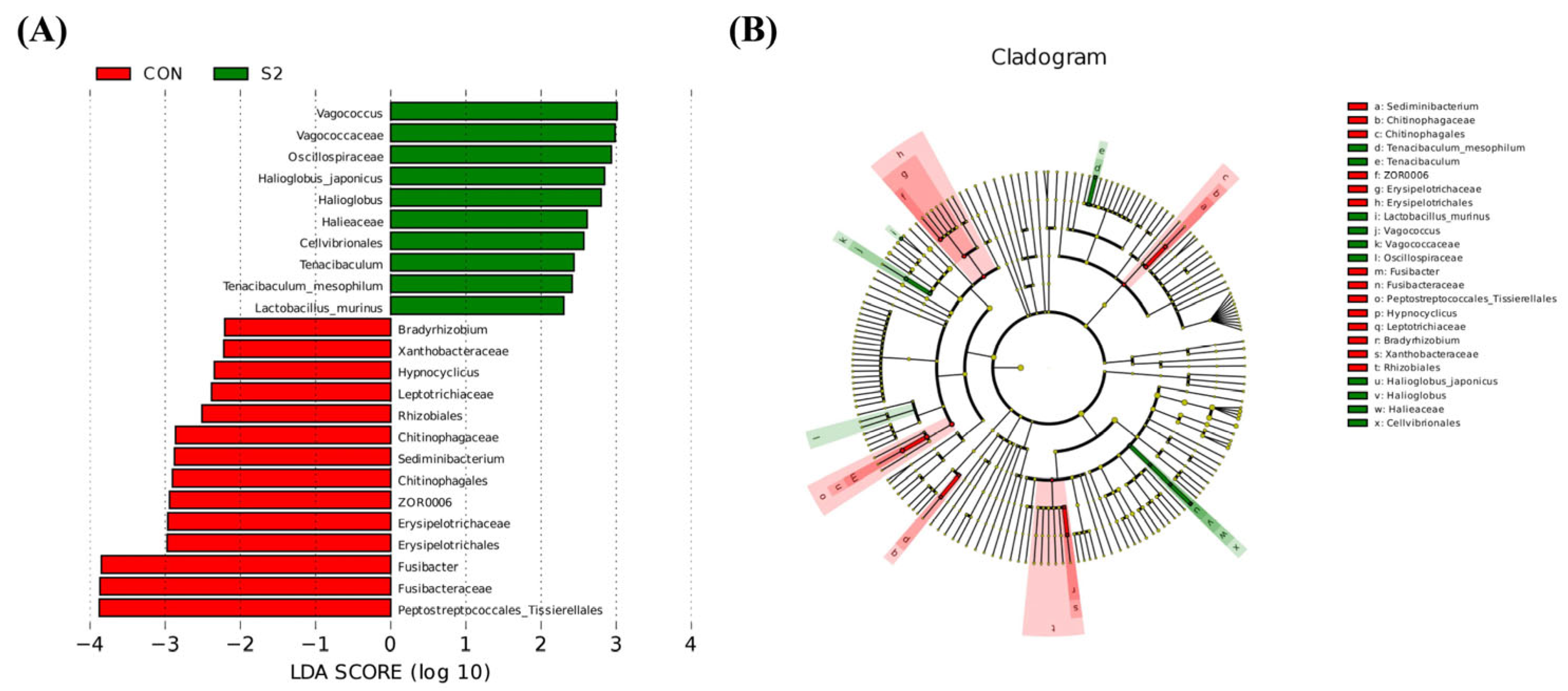
3.8.3. Analysis of Intestinal Microbiota Differences
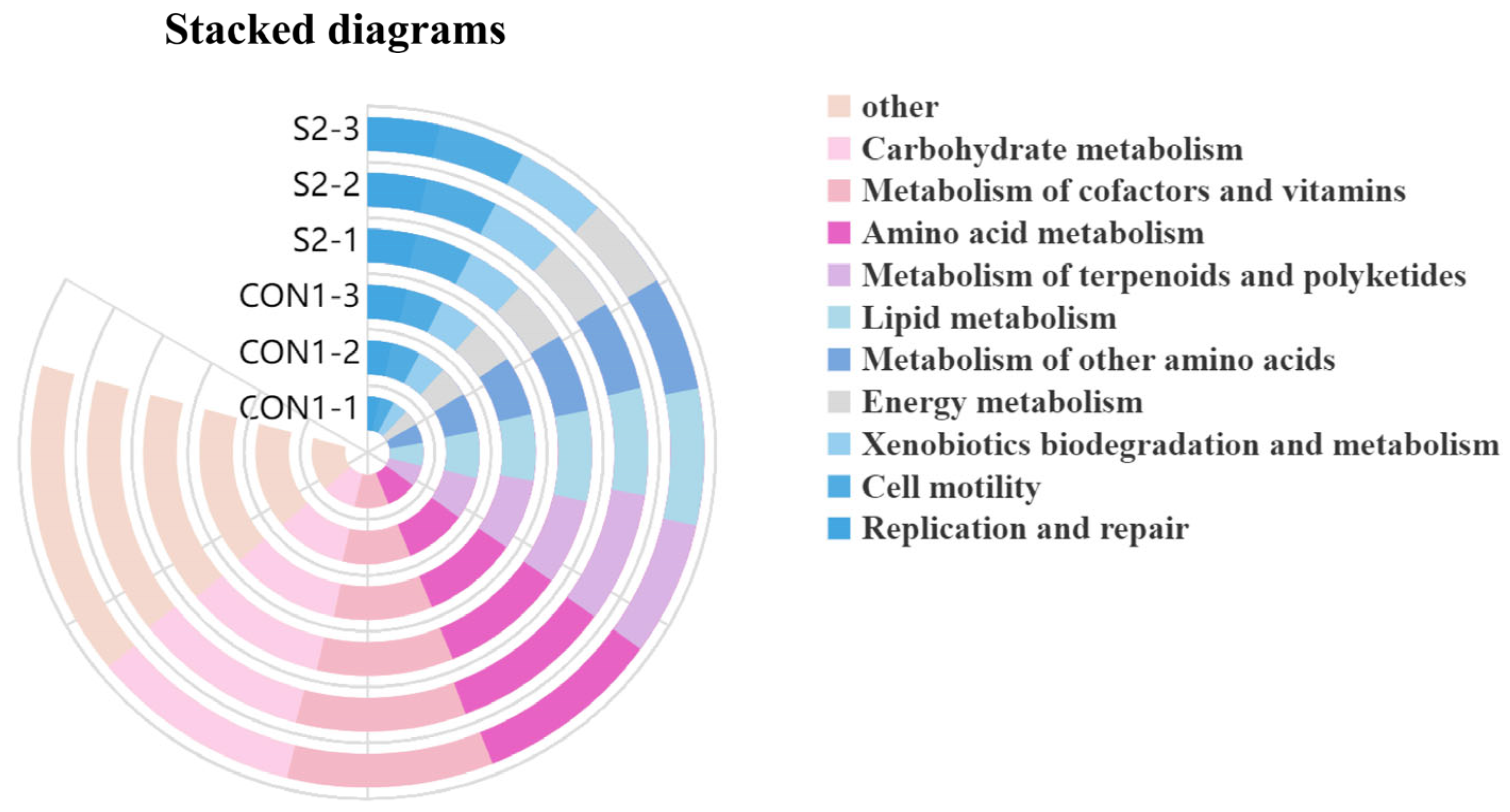
3.8.4. Analysis of Intestinal Microbial Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagarjuna, A.; Karthikeyan, P.; Mohan, D.; Marigoudar, S.R. Effect of selenium on Penaeus monodon and Perna viridis: Enzyme activities and histopathological responses. Chemosphere 2018, 199, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. Hazardous substances and their removal in recirculating aquaculture systems: A review. Aquaculture 2023, 569, 739399. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Let. Appl. NanoBioSci. 2020, 10, 2148–2166. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Perrone, D.; Monteiro, M.; Nunes, J.C. The Chemistry of Selenium. In Selenium: Chemistry, Analysis, Function and Effects; Royal Society of Chemistry: Washington, DC, USA, 2015; pp. 3–15. [Google Scholar] [CrossRef]
- Khalil, H.S.; Maulu, S.; Verdegem, M.; Abdel-Tawwab, M. Embracing nanotechnology for selenium application in aquafeeds. Rev. Aquacult. 2023, 15, 112–129. [Google Scholar] [CrossRef]
- Andreyev, A.; Kushnareva, Y.; Starkova, N.; Starkov, A. Metabolic ROS signaling: To immunity and beyond. Biochemistry 2020, 85, 1650–1667. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. [Google Scholar] [CrossRef]
- Banerjee, M.; Chakravarty, D.; Kalwani, P.; Ballal, A. Voyage of selenium from environment to lie: Beneficial or toxic? J. Biochem. Mol. Toxic. 2022, 36, e23195. [Google Scholar] [CrossRef]
- Waqas, W.; Yuan, Y.; Ali, S.; Zhang, M.; Shafiq, M.; Ali, W.; Chen, Y.; Xiang, Z.; Chen, R.; Ikhwanuddin, M. Toxic effects of heavy metals on crustaceans and associated health risks in humans: A review. Environ. Chem. Let. 2024, 22, 1391–1411. [Google Scholar] [CrossRef]
- Fu, Z.; Han, F.; Huang, K.; Zhang, J.; Qin, J.G.; Chen, L.; Li, E. Combined toxic effects of thiamethoxam on intestinal flora, transcriptome and physiology of Pacific white shrimp Litopenaeus vannamei. Sci. Total Environ. 2022, 830, 154799. [Google Scholar] [CrossRef]
- Derrick, A.; Yohana, M.A.; Yudong, Z.; Li, G.; Tan, B.; Zhang, S. Understanding the detrimental effects of heavy metal pollution in shrimp farming and treatment methods–a review. Ann. Anim. Sci. 2025, 25, 35–56. [Google Scholar] [CrossRef]
- Peng, T.; Wang, W.-N.; Gu, M.-M.; Xie, C.-Y.; Xiao, Y.-C.; Liu, Y.; Wang, L. Essential roles of Cdc42 and MAPK in cadmium-induced apoptosis in Litopenaeus vannamei. Aquat. Toxicol. 2015, 163, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, Y.; Huang, J.; Li, H.; Dong, H.; Zhang, J. Toxic effects of cadmium and lead exposure on intestinal histology, oxidative stress response, and microbial community of Pacific white shrimp Litopenaeus vannamei. Mar. Pollut. Bull. 2021, 167, 112220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhang, J.; He, Y.; Liao, C.; Wang, L.; Zhang, X. Parental exposure to waterborne selenite induces transgenerational development toxicity in zebrafish offspring. Chemosphere 2022, 303, 134838. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.A.; Russo, R.C.; Thurston, R.V. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977, 11, 714–719. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome. Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Granada, L.; Sousa, N.; Lopes, S.; Lemos, M.F. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges?—A review. Rev. Aquacult. 2016, 8, 283–300. [Google Scholar] [CrossRef]
- Wang, L.; Sagada, G.; Wang, R.; Li, P.; Xu, B.; Zhang, C.; Qiao, J.; Yan, Y. Different forms of selenium supplementation in fish feed: The bioavailability, nutritional functions, and potential toxicity. Aquaculture 2022, 549, 737819. [Google Scholar] [CrossRef]
- Lemly, A.D. Symptoms and implications of selenium toxicity in fish: The Belews Lake case example. Aquat. Toxicol. 2002, 57, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Spallholz, J.E.; Hoffman, D.J. Selenium toxicity: Cause and effects in aquatic birds. Aquat. Toxicol. 2002, 57, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dhara, K.; Chukwuka, A.V.; Saha, S.; Saha, N.C.; Faggio, C. Effects of short-term selenium exposure on respiratory activity and proximate body composition of early-life stages of Catla catla, Labeo rohita and Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2022, 90, 103805. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Xu, C.; Chen, C.; Qin, J.G.; Chen, L.; Li, E. Toxic effect of chronic waterborne copper exposure on growth, immunity, anti-oxidative capacity and gut microbiota of Pacific white shrimp Litopenaeus vannamei. Fish. Shellfish Immunol. 2020, 100, 445–455. [Google Scholar] [CrossRef]
- Soegianto, A.; Winarni, D.; Handayani, U.S.; Hartati. Bioaccumulation, elimination, and toxic effect of cadmium on structure of gills and hepatopancreas of freshwater prawn Macrobrachium sintangese (De Man, 1898). Water, Air Soil. Poll. 2013, 224, 1–10. [Google Scholar] [CrossRef]
- Tavabe, K.R.; Abkenar, B.P.; Rafiee, G.; Frinsko, M. Effects of chronic lead and cadmium exposure on the oriental river prawn (Macrobrachium nipponense) in laboratory conditions. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 21–28. [Google Scholar] [CrossRef]
- Ding, Z.; Kong, Y.; Qi, C.; Liu, Y.; Zhang, Y.; Ye, J. The alleviative effects of taurine supplementation on growth, antioxidant enzyme activities, hepatopancreas morphology and mRNA expression of heat shock proteins in freshwater prawn Macrobrachium nipponense (De Haan) exposed to dietary lead stress. Aquacult Nutr. 2021, 27, 2195–2204. [Google Scholar] [CrossRef]
- Benko, I.; Nagy, G.; Tanczos, B.; Ungvari, E.; Sztrik, A.; Eszenyi, P.; Prokisch, J.; Banfalvi, G. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ. Toxicol. Chem. 2012, 31, 2812–2820. [Google Scholar] [CrossRef]
- Nagy, G.; Benko, I.; Kiraly, G.; Voros, O.; Tanczos, B.; Sztrik, A.; Takács, T.; Pocsi, I.; Prokisch, J.; Banfalvi, G. Cellular and nephrotoxicity of selenium species. J. Trace. Elem. Med. Bio. 2015, 30, 160–170. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.-L. Effect of acute exposure to nitrite on physiological parameters, oxidative stress, and apoptosis in Takifugu rubripes. Ecotoxicol. Environ. Saf. 2020, 188, 109878. [Google Scholar] [CrossRef]
- Kolbert, Z.; Lehotai, N.; Molnár, Á.; Feigl, G. “The roots” of selenium toxicity: A new concept. Plant Cell Rep. 2016, 11, e1241935. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Groman, D.B.; Wilson, S.P.; Ismail, P.; Neela, V.K. Biomarker responses in zebrafish (Danio rerio) larvae exposed to pristine low-density polyethylene fragments. Environ. Pollut. 2017, 223, 466–475. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Selimovic, D.; Haikel, Y.; Hassan, M. Crosstalk between apoptosis and autophagy: Molecular mechanisms and therapeutic strategies in cancer. J. Cell Death. 2013, 6, JCD.S11034. [Google Scholar] [CrossRef] [PubMed]
- Opferman, J.T.; Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef]
- Guo, H.; Li, K.; Wang, W.; Wang, C.; Shen, Y. Effects of copper on hemocyte apoptosis, ROS production, and gene expression in white shrimp Litopenaeus vannamei. Biol. Trace. Elem. Res. 2017, 179, 318–326. [Google Scholar] [CrossRef]
- Qian, B.; Xue, L.; Qi, X.; Bai, Y.; Wu, Y. Gene networks and toxicity/detoxification pathways in juvenile largemouth bass (Micropterus salmoides) liver induced by acute lead stress. Genomics 2020, 112, 20–31. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish. Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Hou, C.; Zhu, L.; Zheng, Y.; Shi, L.; Tan, B.; Zhang, S. Effects of dietary peptidoglycan on Litopenaeus vannamei: Growth performance, disease resistance, non-specific immunity and transcriptome analysis of immune response. Aquacult. Rep. 2023, 31, 101676. [Google Scholar] [CrossRef]
- Hoffman, D.J. Role of selenium toxicity and oxidative stress in aquatic birds. Aquat. Toxicol. 2002, 57, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Liu, B.; Gao, X.; Wang, X.; Liu, Y.; Bin, H. Effects of supplemental ultraviolet light on growth, oxidative stress responses, and apoptosis-related gene expression of the shrimp Litopenaeus vannamei. Aquaculture 2020, 520, 735013. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. Immune properties of invertebrate phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [CrossRef]
- Berntssen, M.; Sundal, T.; Olsvik, P.; Amlund, H.; Rasinger, J.; Sele, V.; Hamre, K.; Hillestad, M.; Buttle, L.; Ørnsrud, R. Sensitivity and toxic mode of action of dietary organic and inorganic selenium in Atlantic salmon (Salmo salar). Aquat. Toxicol. 2017, 192, 116–126. [Google Scholar] [CrossRef]
- Xia, L.; Chen, S.; Dahms, H.-U.; Ying, X.; Peng, X. Cadmium induced oxidative damage and apoptosis in the hepatopancreas of Meretrix meretrix. Ecotoxicology 2016, 25, 959–969. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Bautista-Covarrubias, J.C.; Osuna-Martínez, C.C.; Delgado-Alvarez, C.; Bojórquez, C.; Aguilar-Juárez, M.; Roos-Muñoz, S.; Osuna-López, I.; Páez-Osuna, F. Metals and oxidative stress in aquatic decapod crustaceans: A review with special reference to shrimp and crabs. Aquat. Toxicol. 2022, 242, 106024. [Google Scholar] [CrossRef]
- Jankowiak, D.; Pilarczyk, R.; Drozd, R.; Pilarczyk, B.; Tomza-Marciniak, A.; Wysocka, G.; Rzad, I.; Drozd, A.; Kuba, J. Activity of antioxidant enzymes in the liver of wild boars (Sus scrofa) from a selenium-deficient area depending on sex, age, and season of the year. Turk. J. Biol. 2015, 39, 129–138. [Google Scholar] [CrossRef]
- Wang, L.; Wu, N.; Zhang, Y.; Wang, G.; Pu, S.; Guan, T.; Zhu, C.; Wang, H.; Li, J. Effects of copper on non-specific immunity and antioxidant in the oriental river prawn (Macrobrachium nipponense). Ecotoxicol. Environ. Saf. 2022, 236, 113465. [Google Scholar] [CrossRef]
- Xie, D.; Li, Y.; Liu, Z.; Chen, Q. Inhibitory effect of cadmium exposure on digestive activity, antioxidant capacity and immune defense in the intestine of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 222, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Huang, Y.; Yan, G.; Pan, C.; Zhang, J. Antioxidative status, immunological responses, and heat shock protein expression in hepatopancreas of Chinese mitten crab, Eriocheir sinensis under the exposure of glyphosate. Fish. Shellfish Immunol. 2019, 86, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, J.; Wang, G.; Guan, T.; Zhu, C.; Li, J.; Wang, H. Effects of cadmium on antioxidant and non-specific immunity of Macrobrachium nipponense. Ecotoxicol. Environ. Saf. 2021, 224, 112651. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yi, Y. Effects of dietary heavy metals on the immune and antioxidant systems of Galleria mellonella larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 167, 131–139. [Google Scholar] [CrossRef]
- Xia, H.; Ding, C.; Zhang, Y.; Liu, L.; He, H.; Zhou, W.; Liu, H.; Chen, F.; Bu, J.; Yu, J. Adverse effects of cadmium exposure on the immune and antioxidant system functions of dongtingking crucian carp (Carassius auratus indigentiaus). Aquacult. Rep. 2024, 39, 102516. [Google Scholar] [CrossRef]
- Maekawa, S.; Wang, P.-C.; Chen, S.-C. Differential expression genes of the head kidney and spleen in Streptococcus iniae-infected East Asian fourfinger threadfin fish (Eleutheronema tetradactylum). Int. J. Mol. Sci. 2023, 24, 3832. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox. Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar] [CrossRef]
- Zhu, J.; Zou, Z.; Li, D.; Xiao, W.; Yu, J.; Chen, B.; Xue, L.; Yang, H. Transcriptome profiling revealed basis for growth heterosis in hybrid tilapia (Oreochromis niloticus♀× O. aureus♂). Fishes 2022, 7, 43. [Google Scholar] [CrossRef]
- Jiao, L.; Dai, T.; Jin, M.; Sun, P.; Zhou, Q. Transcriptome analysis of the hepatopancreas in the Litopenaeus vannamei responding to the lead stress. Biol. Trace Elem. Res. 2021, 199, 1100–1109. [Google Scholar] [CrossRef]
- Hu, W.; Zhu, Q.-L.; Zheng, J.-L.; Wen, Z.-Y. Cadmium induced oxidative stress, endoplasmic reticulum (ER) stress and apoptosis with compensative responses towards the up-regulation of ribosome, protein processing in the ER, and protein export pathways in the liver of zebrafish. Aquat. Toxicol. 2022, 242, 106023. [Google Scholar] [CrossRef]
- Luo, L.; Huang, J.-H.; Liu, D.-L.; Jiang, S.-G.; Zhou, F.-L.; Jiang, S.; Yang, Q.-B.; Li, Y.-D.; Li, T.; Tan, L.-Q. Transcriptome reveals the important role of metabolic imbalances, immune disorders and apoptosis in the treatment of Procambarus clarkii at super high temperature. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100781. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Mahanty, A.; Ganguly, S.; Mohanty, B.P. Transcriptomic responses to pollution in natural riverine environment in Rita rita. Environ. Res. 2020, 186, 109508. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, J.E.; Lagos, J.; Vázquez, M.C.; Valls, C.; De la Fuente, C.; Yuseff, M.I.; Alvarez, A.R.; Zanlungo, S. Lysosome motility and distribution: Relevance in health and disease. BBA-Mol. Basis Dis. 2019, 1865, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mukherjee, S.; Bhunia, N.S.; Bhunia, A.S.; Ray, M. Immunotoxicological Threats of Pollutants in Aquatic Invertebrates. In Emerging Pollutants in the Environment—Current and Further Implications; Intechopen: London, UK, 2015; pp. 149–167. [Google Scholar] [CrossRef]
- Gu, X.; Yang, H.; Sheng, X.; Ko, Y.-A.; Qiu, C.; Park, J.; Huang, S.; Kember, R.; Judy, R.L.; Park, J. Kidney disease genetic risk variants alter lysosomal beta-mannosidase (MANBA) expression and disease severity. Sci. Transl. Med. 2021, 13, eaaz1458. [Google Scholar] [CrossRef]
- Okeke, E.S.; Feng, W.; Song, C.; Mao, G.; Chen, Y.; Xu, H.; Qian, X.; Luo, M.; Wu, X.; Yang, L. Transcriptomic profiling reveals the neuroendocrine-disrupting effect and toxicity mechanism of TBBPA-DHEE exposure in zebrafish (Danio rerio) during sexual development. Sci. Total Environ. 2023, 858, 160089. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Wang, R.; Chen, S. The linkage of cell cycle and DNA replication with growth difference in female Chinese tongue sole (Cynoglossus semilaevis): Analysis from transcriptomic study and WGCNA. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2021, 39, 100833. [Google Scholar] [CrossRef]
- Ji, C.; Magnuson, J.T.; Zhang, W.; Zhao, M. New insight into the enantioselective cytotoxicity of cypermethrin: Imbalance between cell cycle and apoptosis. J. Hazard. Mater. 2021, 403, 123893. [Google Scholar] [CrossRef]
- Łukasik, P.; Załuski, M.; Gutowska, I. Cyclin-dependent kinases (CDK) and their role in diseases development—Review. Int. J. Mol. Sci. 2021, 22, 2935. [Google Scholar] [CrossRef]
- Pokhrel, N.; Genin, O.; Sela-Donenfeld, D.; Cinnamon, Y. HREM, RNAseq and cell cycle analyses reveal the role of the G2/M-regulatory protein, WEE1, on the survivability of chicken embryos during diapause. Biomedicines 2022, 10, 779. [Google Scholar] [CrossRef]
- Khan, A.R.; Awan, F.R. Metals in the pathogenesis of type 2 diabetes. J. Diabetes Metab. Disord. 2014, 13, 16. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Qi, T.; Ge, B.; Wang, Z.; Jiang, S.; Liu, Q.; Zhang, H.; Ding, G.; Tang, B. Transcriptome analysis of hepatopancreas from the Cr (VI)-stimulated mantis shrimp (Oratosquilla oratoria) by Illumina paired-end sequencing: Assembly, annotation, and expression analysis. J. Agric. Food Chem. 2018, 66, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, M.; Xu, W.; Chen, S.; Qin, Q.; Huang, X.; Huang, Y. Involvement of ATR-CHK1 pathway in fish megalocytivirus infection induced DNA-damage response in vitro. Aquaculture 2023, 575, 739792. [Google Scholar] [CrossRef]
- Hou, T.; Zhou, L.; Wang, L.; Kazobinka, G.; Zhang, X.; Chen, Z. CLCA4 inhibits bladder cancer cell proliferation, migration, and invasion by suppressing the PI3K/AKT pathway. Oncotarget 2017, 8, 93001. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Huang, Y.; Wu, S.; Yang, X.; Dong, Y.; Xu, D.; Huang, Z. Effects of imidacloprid on the oxidative stress, detoxification and gut microbiota of Chinese mitten crab, Eriocheir sinensis. Sci. Total Environ. 2020, 729, 138276. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, Y.; Chen, W.; Jin, Z.; Yao, Z.; Zhang, D. Strain-specific changes in the gut microbiota profiles of the white shrimp Litopenaeus vannamei in response to cold stress. Aquaculture 2019, 503, 357–366. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, L.; Xu, Z.; Wang, L.; Wang, S.; Seif, M.; Xu, X. Toxic effect of chromium on nonspecific immune, bioaccumulation, and tissue structure of Urechis unicinctus. Environ. Sci. Pollut. Res. Int. 2024, 31, 23077–23090. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; De Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in human health and gut microflora: Bioavailability of selenocompounds and relationship with diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Ma, H.-L.; Liu, G.-X.; Fan, S.-G.; Deng, Y.-Q.; Jiang, J.-J.; Feng, J.; Guo, Z.-X. Toxic effects of cadmium exposure on intestinal histology, oxidative stress, microbial community, and transcriptome change in the mud crab (Scylla paramamosain). Chemosphere 2023, 326, 138464. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Guo, W.; Fu, Y.; Ruan, G.; Fang, L.; Wang, Q. Effects of lead contamination on histology, antioxidant and intestinal microbiota responses in freshwater crayfish, Procambarus clarkii. Aquat. Toxicol. 2023, 265, 106768. [Google Scholar] [CrossRef]
- Sha, H.; Lu, J.; Chen, J.; Xiong, J. A meta-analysis study of the robustness and universality of gut microbiota–shrimp diseases relationship. Environ. Microbiol. 2022, 24, 3924–3938. [Google Scholar] [CrossRef]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life strategies, ecology, and risks in a changing environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- Wang, N.; Guo, Z.; Zhang, Y.; Zhang, P.; Liu, J.; Cheng, Y.; Zhang, L.; Li, Y. Effect on intestinal microbiota, bioaccumulation, and oxidative stress of Carassius auratus gibelio under waterborne cadmium exposure. Fish. Physiol. Biochem. 2020, 46, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Kakade, A.; Salama, E.-S.; Pengya, F.; Liu, P.; Li, X. Long-term exposure of high concentration heavy metals induced toxicity, fatality, and gut microbial dysbiosis in common carp, Cyprinus carpio. Environ. Pollut. 2020, 266, 115293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, X.; Huang, W.; Li, Y.; Lin, X.; Li, Y.; Yang, Z. Photoaging of biodegradable nanoplastics regulates their toxicity to aquatic insects (Chironomus kiinensis) by impairing gut and disrupting intestinal microbiota. Environ. Int. 2024, 185, 108483. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Cheng, C.-X.; Yang, L.; Ye, Q.-Q.; Li, W.-H.; Jiang, J.-Y. Characterization of gut microbiome in the mud snail Cipangopaludina cathayensis in response to high-temperature stress. Animals 2022, 12, 2361. [Google Scholar] [CrossRef]

| Primer | Sequences (5′-3′) | Sequences (5′-3′) |
|---|---|---|
| ncbi_113823493 | F: TCGAGAGTGGTTGTGCAGAC | R: GTCGTTGTTCGGGTGTAGGT |
| ncbi_113819543 | F: ACCTTCGAGAGTGGTTGTGC | R: TGAAGTGTTCGACGGAGACG |
| ncbi_113830564 | F: TGTGAAGACGGTCTGAAGCC | R: ACACATCCAGTGTCTGCGAG |
| ncbi_113821441 | F: TCTTTAGCTGCTCTGCCACC | R: TGCAGCCACCCATACTGAAG |
| ncbi_113805388 | F: ATGTCCAGCTCGGGCTAATG | R: AGCCACTAACAGGGTCAAGC |
| ncbi_113825012 | F: TCTAACGAGAACTACAGCGAGTGG | R: CGGTCTTGATGGTGACGACATTG |
| ncbi_113814977 | F: AGGCTGGAGCTGATCCTAACATC | R: GCCGTGTCGCCATCATTATCG |
| ncbi_113821612 | F: CCGACGCCGACCCTTGG | R: CGTAGCCTCCTCTTCCGTAGTAG |
| ncbi_113809493 | F: GAAGGAAAGCGACACTCACACTG | R: CGTAGCGGAGGCGAAGGAC |
| ncbi_113802274 | F: GTGATGTGCTGTGGATGTGACTC | R: CTGACTGGTGATCTGCTTCTTGAC |
| LvEF-1α | F: TGCACCACGAAGCCCTTAC | R: CAGGGTGGTTGAGGACGATC |
| Exposure Time (h) | Se Concentration (mg/L) | LC50 (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| 0.1 | 0.5 | 2.5 | 12.5 | 62.5 | 125 | ||
| 24 | 0 | 0 | 5 | 80 | 100 | 100 | 7.12 (5.16–9.81) |
| 48 | 0 | 5 | 20 | 100 | 100 | 100 | 3.74 (2.71–5.15) |
| 72 | 5 | 10 | 40 | 100 | 100 | 100 | 2.69 (1.74–4.16) |
| 96 | 5 | 10 | 40 | 100 | 100 | 100 | 2.69 (1.74–4.16) |
| Samples | Raw Data (bp) | Clean Data (bp) | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|
| CON1-1 | 6500717400 | 6417446174 | 97.20% | 92.74% | 47.67% |
| CON1-2 | 6030712500 | 5956039994 | 96.87% | 92.02% | 46.36% |
| CON1-3 | 7480366500 | 7376684029 | 97.18% | 92.66% | 45.94% |
| S2-1 | 6423535500 | 6351128194 | 97.07% | 92.36% | 45.90% |
| S2-2 | 6968606700 | 6884621547 | 97.18% | 92.58% | 46.78% |
| S2-3 | 7259163600 | 7172233369 | 97.09% | 92.42% | 46.11% |
| Gene ID | Log2(fc) | Symbol | Description |
|---|---|---|---|
| Lysosome | |||
| ncbi_113801959 | −4.42 | LIPS | triacylglycerol lipase |
| ncbi_113805268 | −1.11 | PLA2G15 | PREDICTED: group XV phospholipase A2-like |
| ncbi_113806002 | −1.31 | GBA | PREDICTED: glucosylceramidase-like isoform X2 |
| ncbi_113808797 | −1.88 | LCP2 | cathepsin l |
| ncbi_113809383 | −1.31 | MANBA | PREDICTED: beta-mannosidase-like |
| Ribosome biogenesis in eukaryo | |||
| ncbi_113800232 | 1.33 | Nop60B | PREDICTED: H/ACA ribonucleoprotein complex subunit 4 |
| ncbi_113802117 | 1.26 | NOP58 | Nucleolar protein NOP5 |
| ncbi_113803266 | 1.41 | NOP56 | Nucleolar kke/d repeat protein |
| ncbi_113804709 | 1.98 | Utp14a | PREDICTED: U3 small nucleolar RNA-associated protein 14 homolog A-like |
| ncbi_113805050 | 1.48 | PWP2 | PREDICTED: periodic tryptophan protein 2 homolog |
| Pancreatic secretion | |||
| ncbi_113802097 | −1.49 | Cpa2 | carboxypeptidase B, partial |
| ncbi_113805576 | 3.47 | CLCA4 | Calcium-activated chloride channel regulator 4, 30 kDa form |
| ncbi_113807409 | −1.02 | Oslo | calcium-activated potassium channel transcript variant 1 |
| ncbi_113809844 | −5.80 | Rho1 | RhoA |
| ncbi_113813375 | 3.40 | CLCA2 | Calcium-activated chloride channel regulator 4, 30 kDa form |
| Cell cycle | |||
| ncbi_113822117 | 1.77 | Wee1 | PREDICTED: wee1-like protein kinase |
| ncbi_113822118 | 1.30 | Atr | PREDICTED: serine/threonine-protein kinase ATR-like isoform X2 |
| ncbi_113808550 | 1.28 | CDK7 | cyclin-dependent kinase 7 |
| ncbi_113821379 | 1.99 | ccnb2 | cyclin B |
| ncbi_113828355 | 1.68 | ORC4 | PREDICTED: origin recognition complex subunit 4 |
| Pyrimidine metabolism | |||
| ncbi_113800828 | 1.21 | POLR3A | PREDICTED: DNA-directed RNA polymerase III subunit RPC1 isoform X2 |
| ncbi_113800946 | 1.62 | pola1 | PREDICTED: DNA polymerase alpha catalytic subunit-like |
| ncbi_113801001 | 1.35 | Pole | PREDICTED: DNA polymerase epsilon catalytic subunit A-like |
| ncbi_113802524 | 1.38 | Polr1b | DNA-directed RNA polymerase I subunit RPA2 |
| ncbi_113802842 | 1.60 | polr1e | PREDICTED: DNA-directed RNA polymerase I subunit RPA49-like |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Chen, J.; Derrick, A.; Li, G.; Wang, H.; Xue, Z.; Shi, L.; Zhang, S. Toxic Effects of Acute Water Selenium Exposure on Litopenaeus vannamei: Survival, Physiological Responses, Transcriptome, and Intestinal Microbiota. Animals 2025, 15, 1792. https://doi.org/10.3390/ani15121792
Luo X, Chen J, Derrick A, Li G, Wang H, Xue Z, Shi L, Zhang S. Toxic Effects of Acute Water Selenium Exposure on Litopenaeus vannamei: Survival, Physiological Responses, Transcriptome, and Intestinal Microbiota. Animals. 2025; 15(12):1792. https://doi.org/10.3390/ani15121792
Chicago/Turabian StyleLuo, Xinghui, Jian Chen, Asare Derrick, Gongyu Li, Hongming Wang, Zhihao Xue, Lili Shi, and Shuang Zhang. 2025. "Toxic Effects of Acute Water Selenium Exposure on Litopenaeus vannamei: Survival, Physiological Responses, Transcriptome, and Intestinal Microbiota" Animals 15, no. 12: 1792. https://doi.org/10.3390/ani15121792
APA StyleLuo, X., Chen, J., Derrick, A., Li, G., Wang, H., Xue, Z., Shi, L., & Zhang, S. (2025). Toxic Effects of Acute Water Selenium Exposure on Litopenaeus vannamei: Survival, Physiological Responses, Transcriptome, and Intestinal Microbiota. Animals, 15(12), 1792. https://doi.org/10.3390/ani15121792







