Effects of Endotoxemia and Blood Pressure on Microcirculation and Noradrenaline Needs With or Without Dexmedetomidine in Beagle Dogs—A Blinded Cross-Over Study
Simple Summary
Abstract
1. Introduction
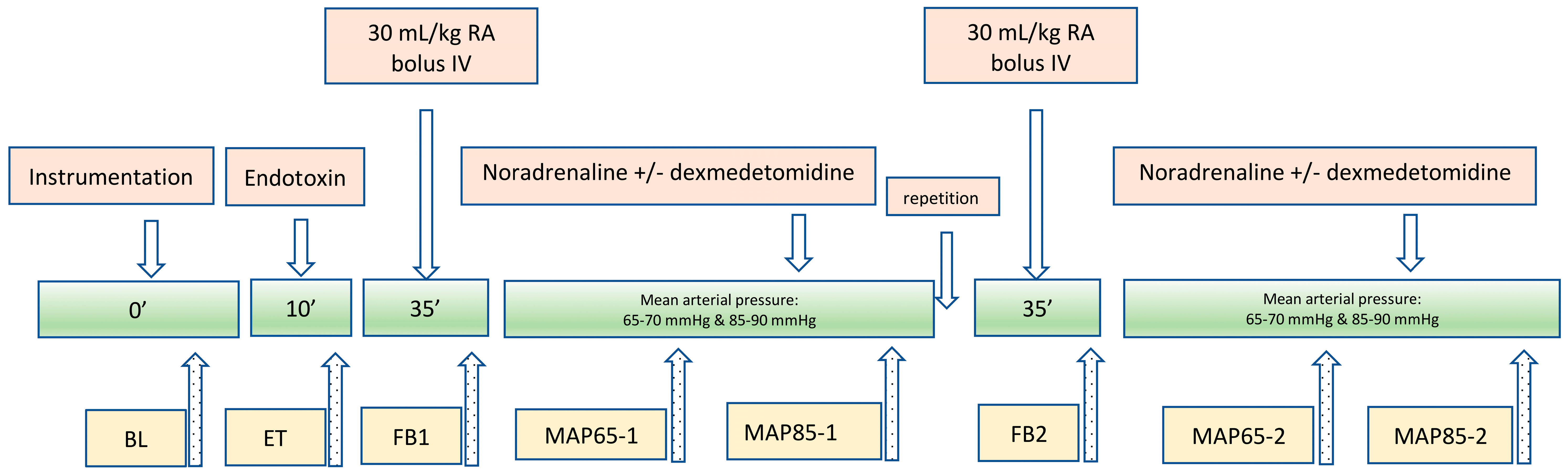
2. Materials and Methods
2.1. Anaesthesia, Instrumentation, and Measurements
2.2. Statistics
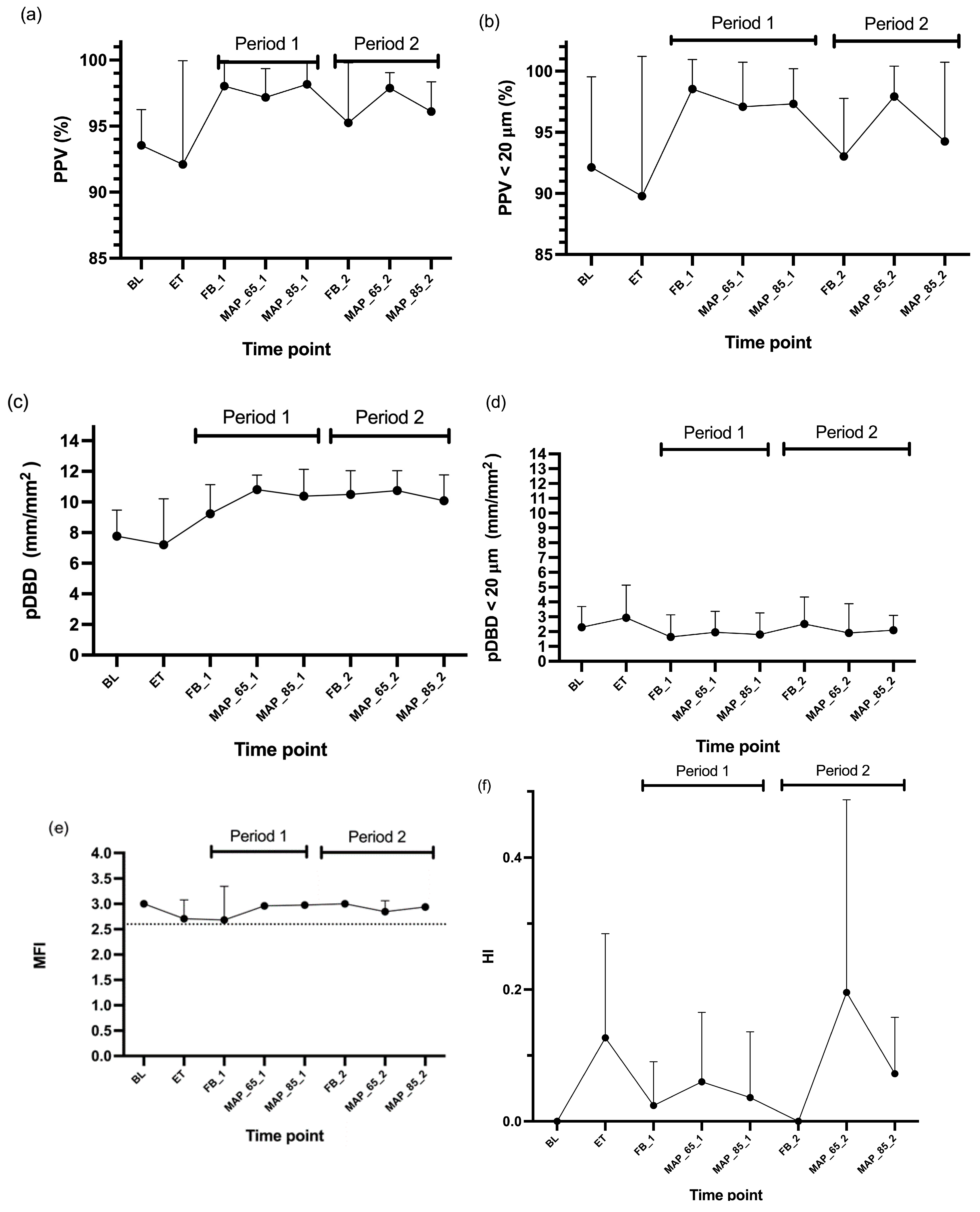
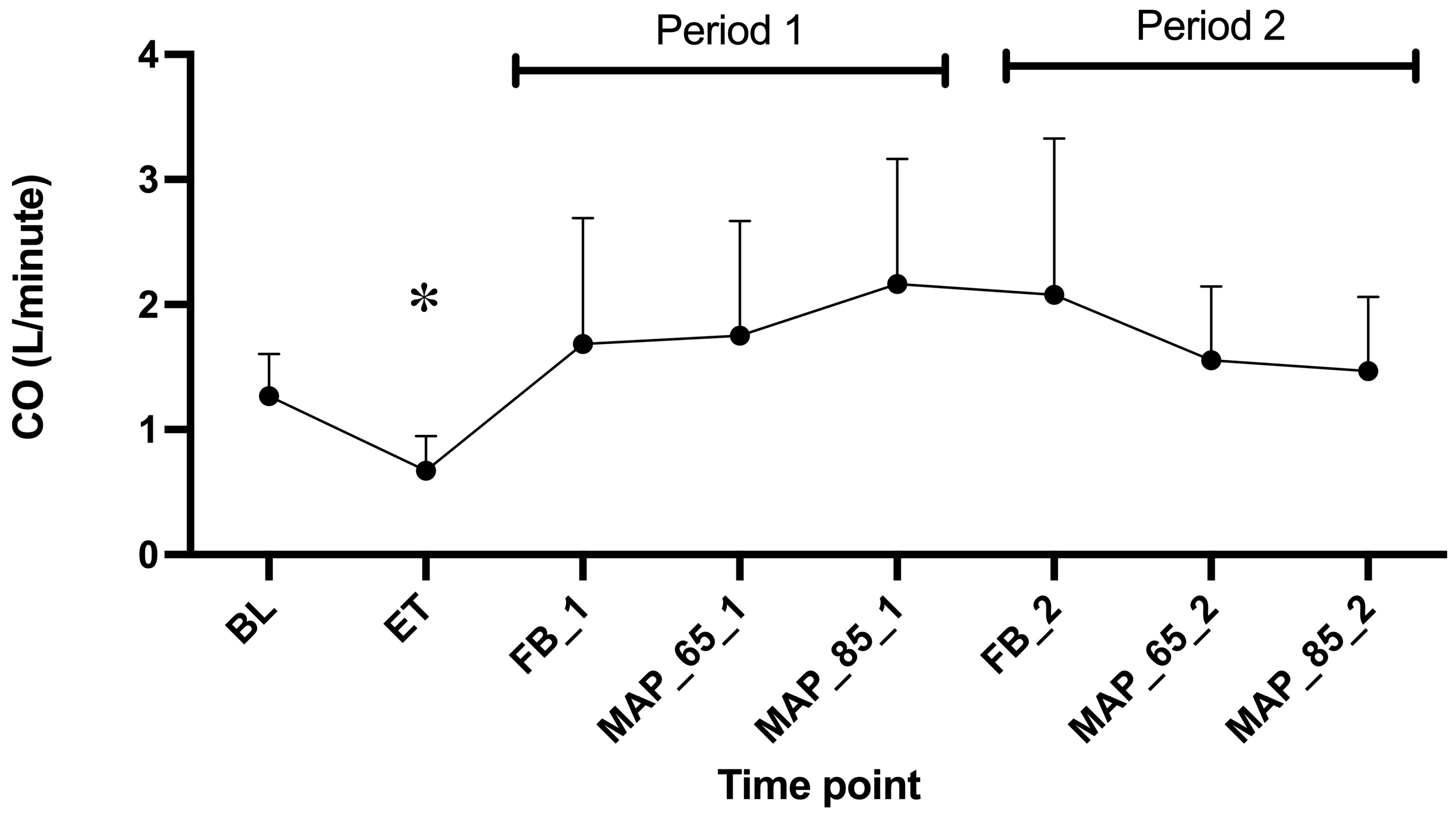
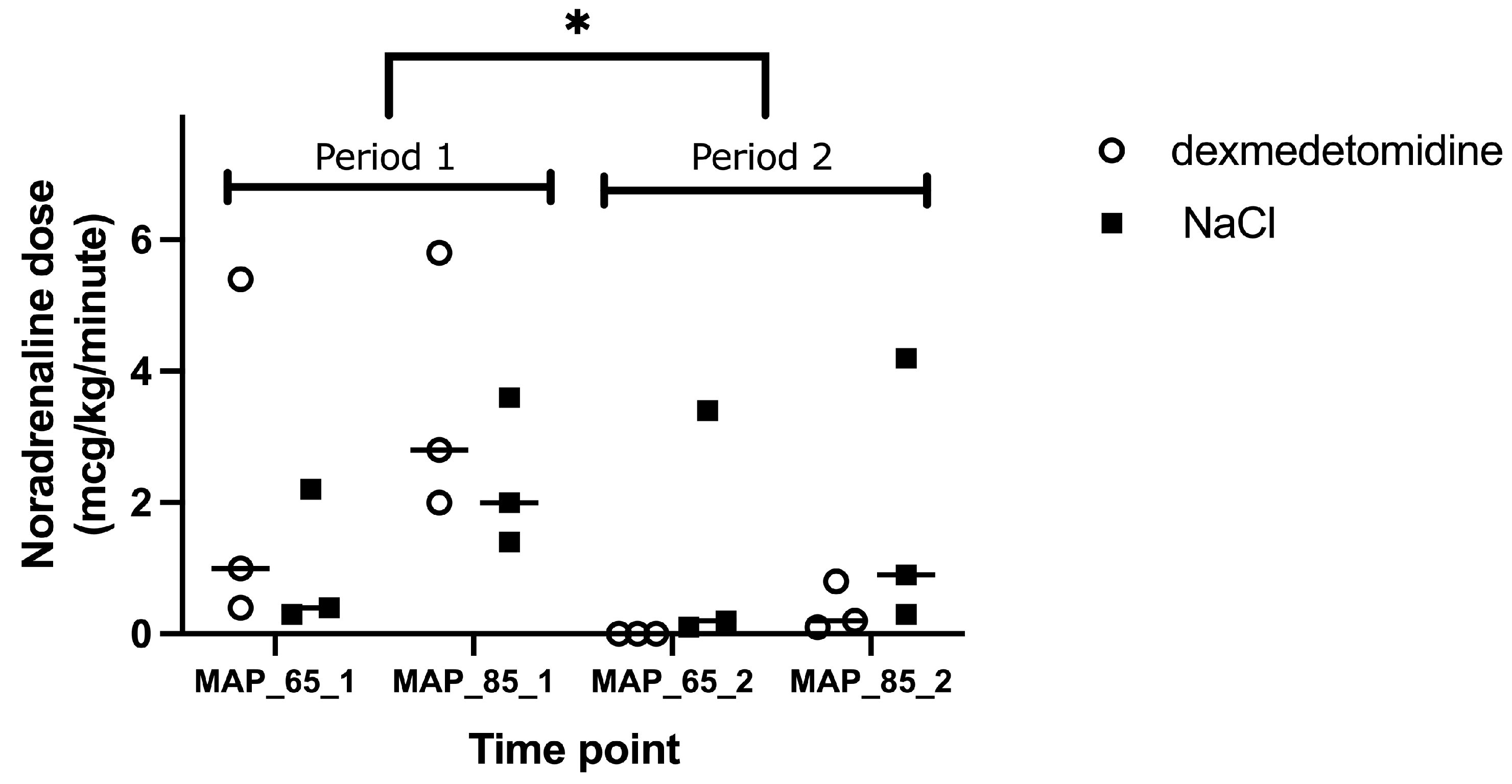
3. Results
4. Discussion
4.1. Microcirculatory Parameters
4.1.1. PPV
4.1.2. PPV < 20 μm
4.1.3. MFI and HI
4.2. Marker of Sepsis
4.2.1. CO
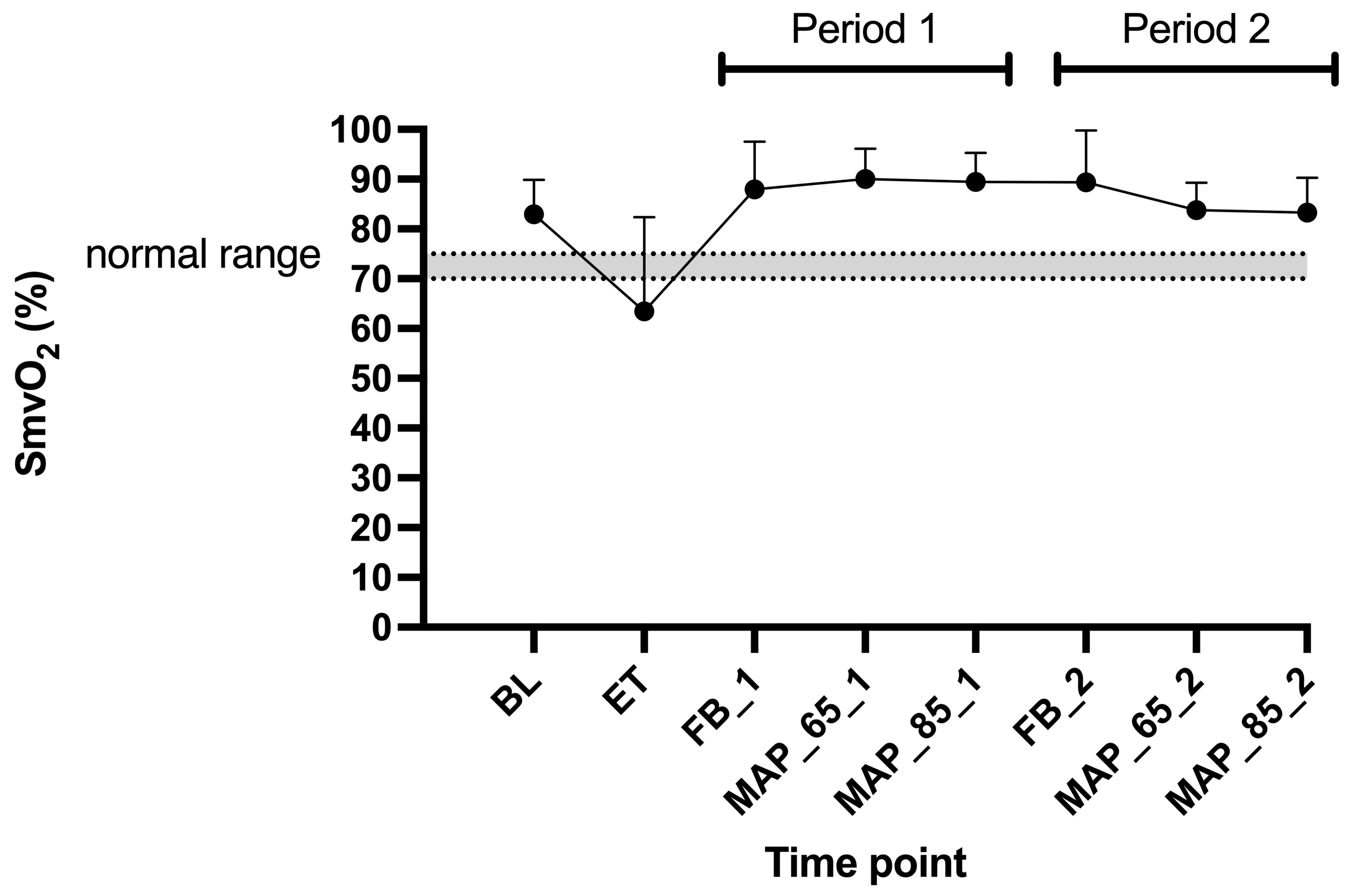
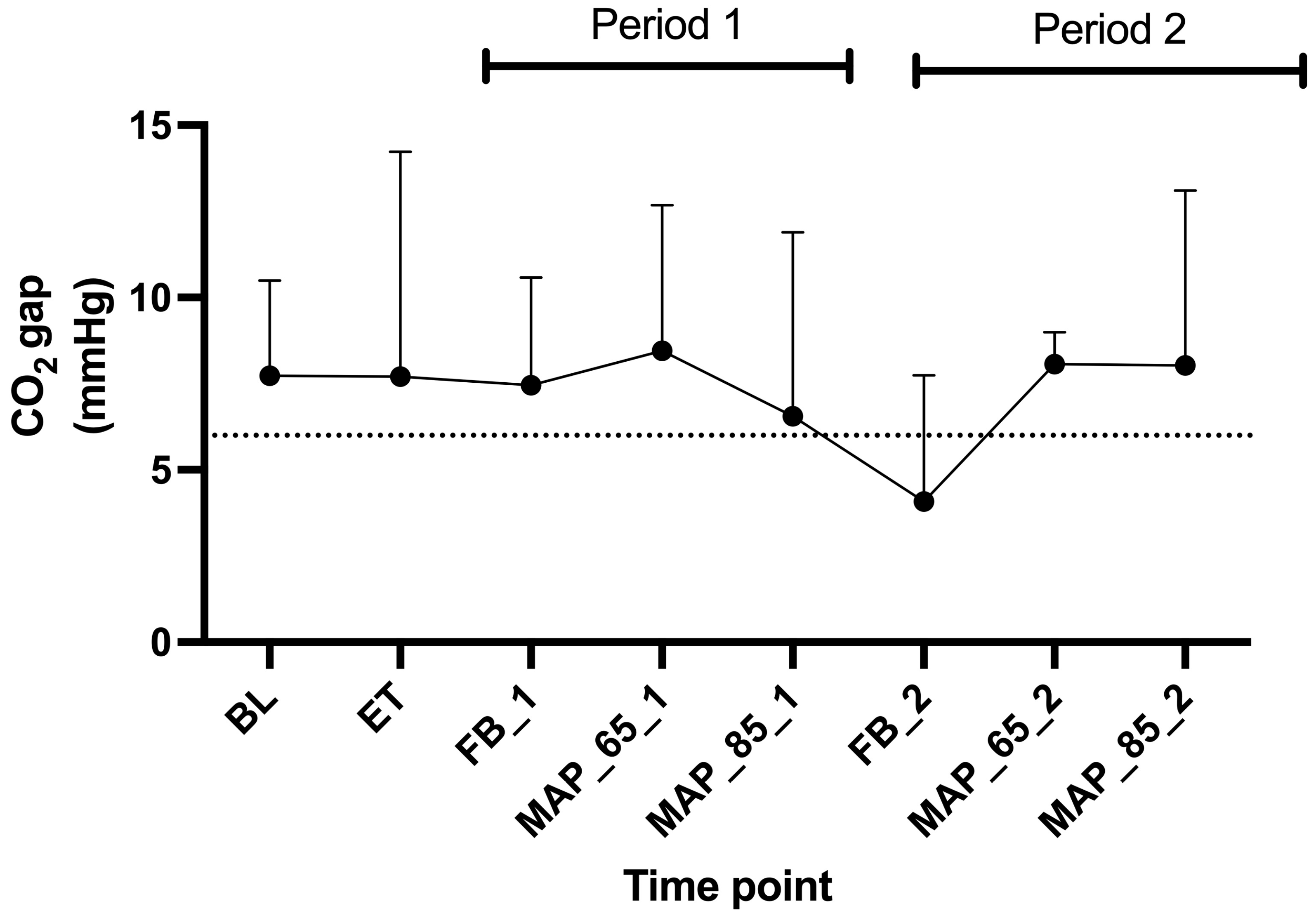
4.2.2. SmvO2 and CO2 Gap
4.2.3. Lactate
4.2.4. MAP 65 mmHg Versus 85 mmHg
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BL | baseline |
| CO | cardiac output |
| CO2 | carbon dioxide |
| CRI | constant rate infusion |
| ECG | electrocardiogram |
| ET | endotoxin |
| EtCO2 | end-tidal CO2 |
| FB | fluid bolus |
| HI | heterogeneity index |
| HR | heart rate |
| LPS | lipopolysaccharide |
| MAP | mean arterial pressure |
| MFI | microvascular flow index |
| PaCO2 | arterial partial pressure of CO2 |
| pDBD | Perfused de Backer density |
| PmvCO2 | mixed venous partial pressure of CO2 |
| PPV | Proportion of perfused vessels |
| T | temperature |
References
- Beal, A.L.; Cerra, F.B. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. JAMA 1994, 271, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M.; Early Goal-Directed Therapy Collaborative, G. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Tan, J.C.; Harvey, S.E.; Bell, D.; et al. Protocolised Management In Sepsis (ProMISe): A multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol. Assess. 2015, 19, i–xxv, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Banks, S.M.; Costello, R.; Csako, G.; Eichacker, P.Q.; Danner, R.L.; Natanson, C.; Solomon, S.B. Differing effects of epinephrine, norepinephrine, and vasopressin on survival in a canine model of septic shock. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2545–H2554. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Wisniewski, S.R.; Pinsky, M.R. Effects of norepinephrine on the renal vasculature in normal and endotoxemic dogs. Am. J. Respir. Crit. Care Med. 1999, 159, 1186–1192. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kurita, A.; Kobayashi, K.; Yamamoto, K.; Inaba, H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J. Anesth. 2008, 22, 221–228. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Wu, C.Y.; Cheng, Y.J.; Liu, C.M.; Hsiao, J.K.; Chan, W.S.; Wu, Z.G.; Yu, L.C.; Sun, W.Z. Effects of Dexmedetomidine on Intestinal Microcirculation and Intestinal Epithelial Barrier in Endotoxemic Rats. Anesthesiology 2016, 125, 355–367. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.Z.; Chen, K.; Zhang, F.; Peng, M.; Wang, Y.L. Dexmedetomidine regulates inflammatory molecules contributing to ventilator-induced lung injury in dogs. J. Surg. Res. 2014, 187, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Balarini, M.M.; Bouskela, E. Dexmedetomidine attenuates the microcirculatory derangements evoked by experimental sepsis. Anesthesiology 2015, 122, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Geloen, A.; Chapelier, K.; Cividjian, A.; Dantony, E.; Rabilloud, M.; May, C.N.; Quintin, L. Clonidine and dexmedetomidine increase the pressor response to norepinephrine in experimental sepsis: A pilot study. Crit. Care Med. 2013, 41, e431–e438. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Wang, W.; Xie, G.; Cheng, B.; Wang, Y.; Hu, Y.; Fang, X. Dexmedetomidine Versus Propofol Sedation Improves Sublingual Microcirculation After Cardiac Surgery: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1509–1515. [Google Scholar] [CrossRef]
- Pichot, C.; Mathern, P.; Khettab, F.; Ghignone, M.; Geloen, A.; Quintin, L. Increased pressor response to noradrenaline during septic shock following clonidine? Anaesth. Intensive Care 2010, 38, 784–785. [Google Scholar]
- Lankadeva, Y.R.; Booth, L.C.; Kosaka, J.; Evans, R.G.; Quintin, L.; Bellomo, R.; May, C.N. Clonidine Restores Pressor Responsiveness to Phenylephrine and Angiotensin II in Ovine Sepsis. Crit. Care Med. 2015, 43, e221–e229. [Google Scholar] [CrossRef]
- Christen, M.A.; Schweizer-Gorgas, D.; Richter, H.; Joerger, F.B.; Dennler, M. Quantification of cerebrospinal fluid flow in dogs by cardiac-gated phase-contrast magnetic resonance imaging. J. Vet. Intern. Med. 2021, 35, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Muehlestein, M.B.; Steblaj, B.; Joerger, F.B.; Briganti, A.; Kutter, A.P.N. Evaluation of the ability of haemodynamic variables obtained with minimally invasive techniques to assess fluid responsiveness in endotoxaemic Beagles. Vet. Anaesth. Analg. 2021, 48, 645–653. [Google Scholar] [CrossRef]
- Kutter, A.P.N.; Joerger, F.B.; Riond, B.; Steblaj, B. Evaluation of the Effect of Induced Endotoxemia on ROTEM S((R)) and Platelet Parameters in Beagle Dogs Anaesthetized with Sevoflurane. Animals 2023, 13, 2997. [Google Scholar] [CrossRef]
- Steblaj, B.; Kutter, A.P.N.; Stirn, M.; Daminet, S.; Major, A.; Zini, E. Endotoxic kidney injury in Beagle dogs assessed by serum creatinine and symmetric dimethylarginine, and urinary neutrophil gelatinase-associated lipocalin and clusterin. Res. Vet. Sci. 2023, 162, 104966. [Google Scholar] [CrossRef]
- Steblaj, B.; Campagna, I.; Hartnack, S.; Kutter, A.P. Effects of acepromazine and dexmedetomidine, followed by propofol induction and maintenance with isoflurane anaesthesia, on the microcirculation of Beagle dogs evaluated by sidestream dark field imaging: An experimental trial. Vet. Anaesth. Analg. 2022, 49, 364–371. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Hollenberg, S.; Boerma, C.; Goedhart, P.; Buchele, G.; Ospina-Tascon, G.; Dobbe, I.; Ince, C. How to evaluate the microcirculation: Report of a round table conference. Crit. Care 2007, 11, R101. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Boerma, E.C.; Cecconi, M.; De Backer, D.; Shapiro, N.I.; Duranteau, J.; Pinsky, M.R.; Artigas, A.; Teboul, J.L.; Reiss, I.K.M.; et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018, 44, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; DebRoy, B.; DebRoy, S.; Sarkar, D.; EISPACK Authors; Heisterkamp, S.; Van Willigen, B.; Ranke, J.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-168; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- De Backer, D.; Creteur, J.; Preiser, J.C.; Dubois, M.J.; Vincent, J.L. Microvascular Blood Flow Is Altered in Patients with Sepsis. Am. J. Respir. Crit. Care Med. 2002, 166, 98–104. [Google Scholar] [CrossRef]
- Cooper, E.S.; Silverstein, D.C. Fluid Therapy and the Microcirculation in Health and Critical Illness. Front. Vet. Sci. 2021, 8, 625708. [Google Scholar] [CrossRef]
- Ince, C. The microcirculation is the motor of sepsis. Crit. Care 2005, 9 (Suppl. 4), S13–S19. [Google Scholar] [CrossRef]
- Massey, M.J.; Hou, P.C.; Filbin, M.; Wang, H.; Ngo, L.; Huang, D.T.; Aird, W.C.; Novack, V.; Trzeciak, S.; Yealy, D.M.; et al. Microcirculatory perfusion disturbances in septic shock: Results from the ProCESS trial. Crit. Care 2018, 22, 308. [Google Scholar] [CrossRef]
- Peruski, A.M.; Cooper, E.S. Assessment of microcirculatory changes by use of sidestream dark field microscopy during hemorrhagic shock in dogs. Am. J. Vet. Res. 2011, 72, 438–445. [Google Scholar] [CrossRef]
- Niemann, L.; Kutter, A.P.; Joerger, F.B.; Wieser, M.L.; Hartnack, S.; Steblaj, B. The impact of vatinoxan on microcirculation after intramuscular co-administration with medetomidine in Beagle dogs: A blinded crossover study. Vet. Anaesth. Analg. 2022, 49, 336–343. [Google Scholar] [CrossRef]
- Ozarslan, N.G.; Ayhan, B.; Kanbak, M.; Celebioglu, B.; Demircin, M.; Ince, C.; Aypar, U. Comparison of the effects of sevoflurane, isoflurane, and desflurane on microcirculation in coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2012, 26, 791–798. [Google Scholar] [CrossRef]
- Donati, A.; Damiani, E.; Botticelli, L.; Adrario, E.; Lombrano, M.R.; Domizi, R.; Marini, B.; Van Teeffelen, J.W.; Carletti, P.; Girardis, M.; et al. The aPC treatment improves microcirculation in severe sepsis/septic shock syndrome. BMC Anesthesiol. 2013, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Domizi, R.; Adrario, E.; Damiani, E.; Scorcella, C.; Carsetti, A.; Giaccaglia, P.; Casarotta, E.; Gabbanelli, V.; Pantanetti, S.; Lamura, E.; et al. IgM-enriched immunoglobulins (Pentaglobin) may improve the microcirculation in sepsis: A pilot randomized trial. Ann. Intensive Care 2019, 9, 135. [Google Scholar] [CrossRef]
- Pottecher, J.; Deruddre, S.; Teboul, J.L.; Georger, J.F.; Laplace, C.; Benhamou, D.; Vicaut, E.; Duranteau, J. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010, 36, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, J.K.; Goncalves, L.A.; Pereira, M.A.; Talib, M.S.; de Olveira, C.M.; Ambrosio, A.M.; Fantoni, D.T. Microcirculation assessment of dexmedetomidine constant rate infusion during anesthesia of dogs with sepsis from pyometra: A randomized clinical study. Vet. Anaesth. Analg. 2022, 49, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascon, G.; Neves, A.P.; Occhipinti, G.; Donadello, K.; Buchele, G.; Simion, D.; Chierego, M.L.; Silva, T.O.; Fonseca, A.; Vincent, J.L.; et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010, 36, 949–955. [Google Scholar] [CrossRef]
- Vincent, J.L.; Bakker, J. Blood lactate levels in sepsis: In 8 questions. Curr. Opin. Crit. Care 2021, 27, 298–302. [Google Scholar] [CrossRef]
- Ryoo, S.M.; Lee, J.; Lee, Y.S.; Lee, J.H.; Lim, K.S.; Huh, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, W.Y. Lactate Level Versus Lactate Clearance for Predicting Mortality in Patients With Septic Shock Defined by Sepsis-3. Crit. Care Med. 2018, 46, e489–e495. [Google Scholar] [CrossRef]
- Valeanu, L.; Bubenek-Turconi, S.I.; Ginghina, C.; Balan, C. Hemodynamic Monitoring in Sepsis-A Conceptual Framework of Macro- and Microcirculatory Alterations. Diagnostics 2021, 11, 1559. [Google Scholar] [CrossRef]
- De Backer, D. Detailing the cardiovascular profile in shock patients. Crit. Care 2017, 21, 311. [Google Scholar] [CrossRef]
- Asfar, P.; Meziani, F.; Hamel, J.F.; Grelon, F.; Megarbane, B.; Anguel, N.; Mira, J.P.; Dequin, P.F.; Gergaud, S.; Weiss, N.; et al. High versus low blood-pressure target in patients with septic shock. N. Engl. J. Med. 2014, 370, 1583–1593. [Google Scholar] [CrossRef]
- Tan, J.A.; Ho, K.M. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: A meta-analysis. Intensive Care Med. 2010, 36, 926–939. [Google Scholar] [CrossRef] [PubMed]
- de Sant’Ana Alves, L.; Arcoverde, K.N.; de Oliveira, C.V.A.; Cavalcante, J.M.; Araujo-Silva, G.; de Paula, V.V. Pharmacokinetics and pharmacodynamics of intravenous dexmedetomidine (2 μg∙kg−1) in dogs. Res. Vet. Sci. 2024, 171, 105229. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.; Karol, M.M. Pharmacokinetics and interaction pharmacodynamics of dexmedetomidine in humans. Best Pract. Res. Clin. Anaesthesiol. 2000, 14, 261–269. [Google Scholar] [CrossRef]
- Kirkendol, R.L.; Pearson, J.E.; Bower, J.D.; Holbert, R.D. Myocardial depressant effects of sodium acetate. Cardiovasc. Res. 1978, 12, 127–136. [Google Scholar] [CrossRef]
- Olinger, G.N.; Werner, P.H.; Bonchek, L.I.; Boerboom, L.E. Vasodilator effects of the sodium acetate in pooled protein fraction. Ann. Surg. 1979, 190, 305–311. [Google Scholar] [CrossRef]

| Microcirculatory Variables (Buccal) and Cardiac Output | Effect of Treatment Dexmedetomidine Versus NaCl | Effect of Endotoxin Versus Baseline | Effect of Period 2 Versus Period 1 | |||
|---|---|---|---|---|---|---|
| Estimated Effect | p-Value | Estimated Effect | p-Value | Estimated Effect | p-Value | |
| Proportion of perfused vessels (%) | 0.00 | 0.99 | −0.02 | 0.77 | −1.38 | 0.13 |
| Proportion of perfused vessels < 20 µm (%) | −1.49 | 0.38 | 0.00 | 0.98 | −2.58 | 0.07 |
| Perfused DeBacker density (mm/mm2) | −0.16 | 0.75 | 0.01 | 0.95 | 0.31 | 0.49 |
| Perfused DeBacker density < 20 µm (mm/mm2) | 0.44 | 0.47 | 0.09 | 0.58 | 0.37 | 0.45 |
| Microvascular flow index (no unit) | 0.01 | 0.92 | −0.14 | 0.43 | 0.06 | 0.51 |
| Heterogeneity index (no unit) | 0.05 | 0.39 | 0.19 | 0.30 | 0.05 | 0.28 |
| Cardiac output (L/minute) | −0.31 | 0.08 | 2.63 | 0.03 * | −0.17 | 0.22 |
| Microcirculatory Variables (Buccal) and Cardiac Output | Effect of MAP 85 mmHg Versus MAP 65 mmHg | |
|---|---|---|
| Estimated Effect | p-Value | |
| Proportion of perfused vessels (%) | −0.38 | 0.64 |
| Proportion of perfused vessels < 20 µm (%) | −1.72 | 0.32 |
| Perfused DeBacker density (mm/mm2) | −0.54 | 0.24 |
| Perfused DeBacker density < 20 µm (mm/mm2) | 0.02 | 0.97 |
| Microvascular flow index (no unit) | 0.01 | 0.79 |
| Heterogeneity index (no unit) | −0.06 | 0.28 |
| Cardiac output (L/minute) | −0.08 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steblaj, B.; Joerger, F.B.; Hartnack, S.; Briganti, A.; Kutter, A.P.N. Effects of Endotoxemia and Blood Pressure on Microcirculation and Noradrenaline Needs With or Without Dexmedetomidine in Beagle Dogs—A Blinded Cross-Over Study. Animals 2025, 15, 1779. https://doi.org/10.3390/ani15121779
Steblaj B, Joerger FB, Hartnack S, Briganti A, Kutter APN. Effects of Endotoxemia and Blood Pressure on Microcirculation and Noradrenaline Needs With or Without Dexmedetomidine in Beagle Dogs—A Blinded Cross-Over Study. Animals. 2025; 15(12):1779. https://doi.org/10.3390/ani15121779
Chicago/Turabian StyleSteblaj, Barbara, Fabiola Binia Joerger, Sonja Hartnack, Angela Briganti, and Annette P. N. Kutter. 2025. "Effects of Endotoxemia and Blood Pressure on Microcirculation and Noradrenaline Needs With or Without Dexmedetomidine in Beagle Dogs—A Blinded Cross-Over Study" Animals 15, no. 12: 1779. https://doi.org/10.3390/ani15121779
APA StyleSteblaj, B., Joerger, F. B., Hartnack, S., Briganti, A., & Kutter, A. P. N. (2025). Effects of Endotoxemia and Blood Pressure on Microcirculation and Noradrenaline Needs With or Without Dexmedetomidine in Beagle Dogs—A Blinded Cross-Over Study. Animals, 15(12), 1779. https://doi.org/10.3390/ani15121779






