Physiological Responses of Laying Hens to Chronic Cold Stress and Ammonia Exposure: Implications for Environmental Management and Poultry Welfare
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Management and Experimental Design
2.2. Environmentally Controlled Chambers
2.3. Blood Sample Collection and Biochemical Analyses
2.4. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.5. Statistical Analysis
3. Results
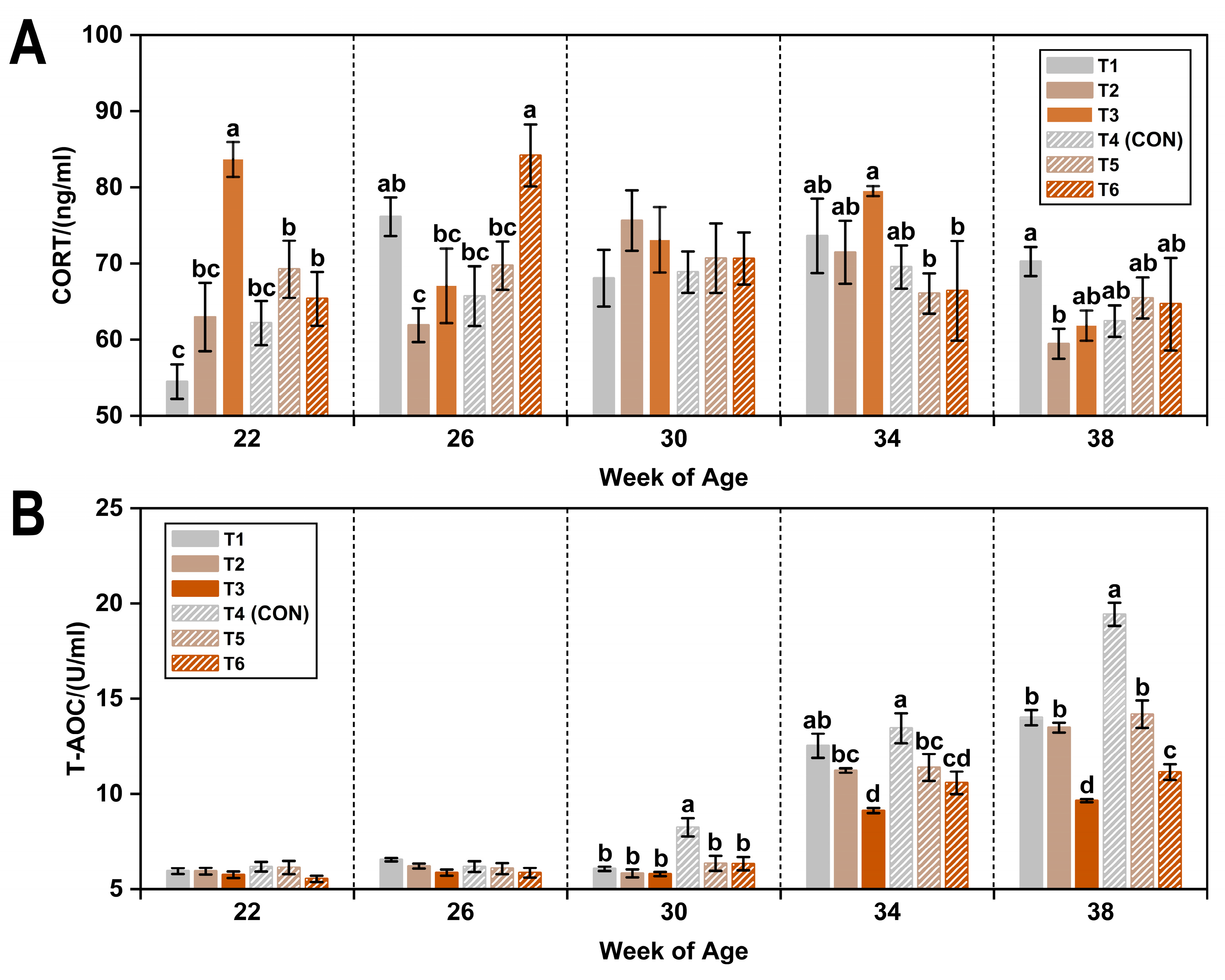
3.1. CORT and T-AOC
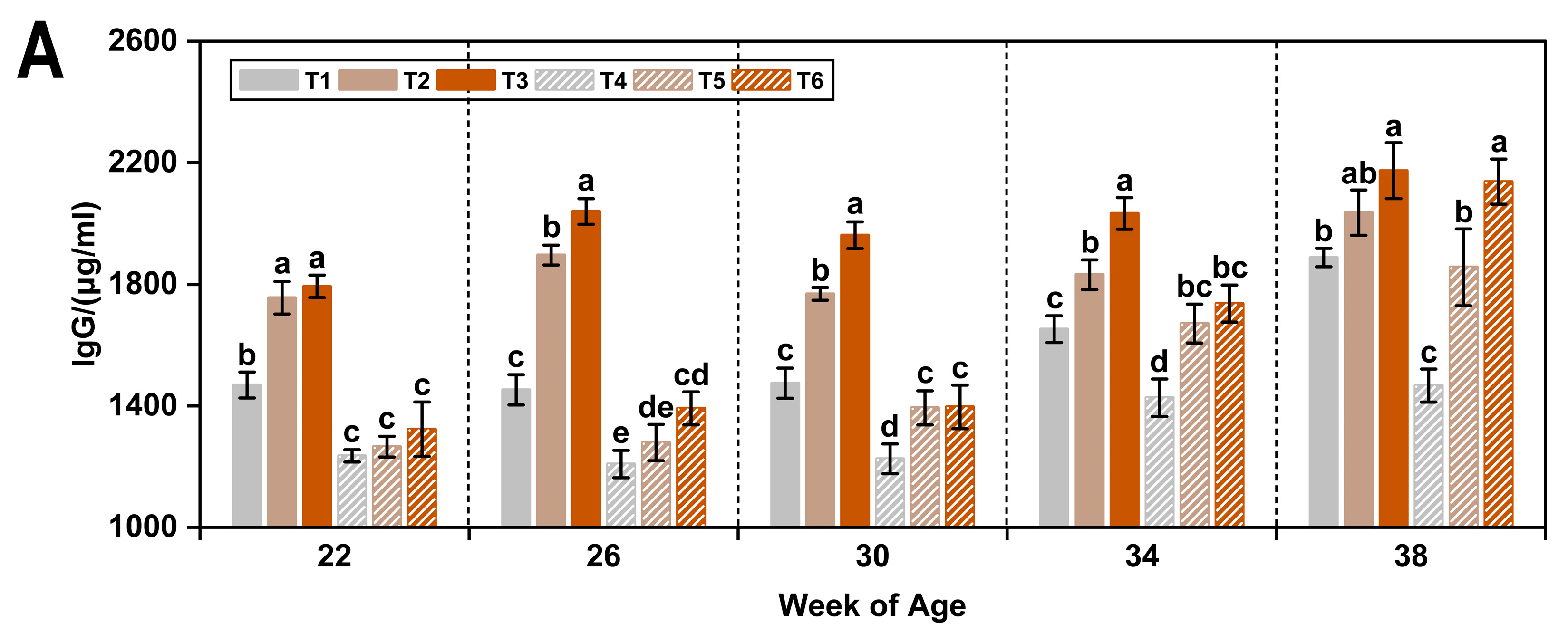
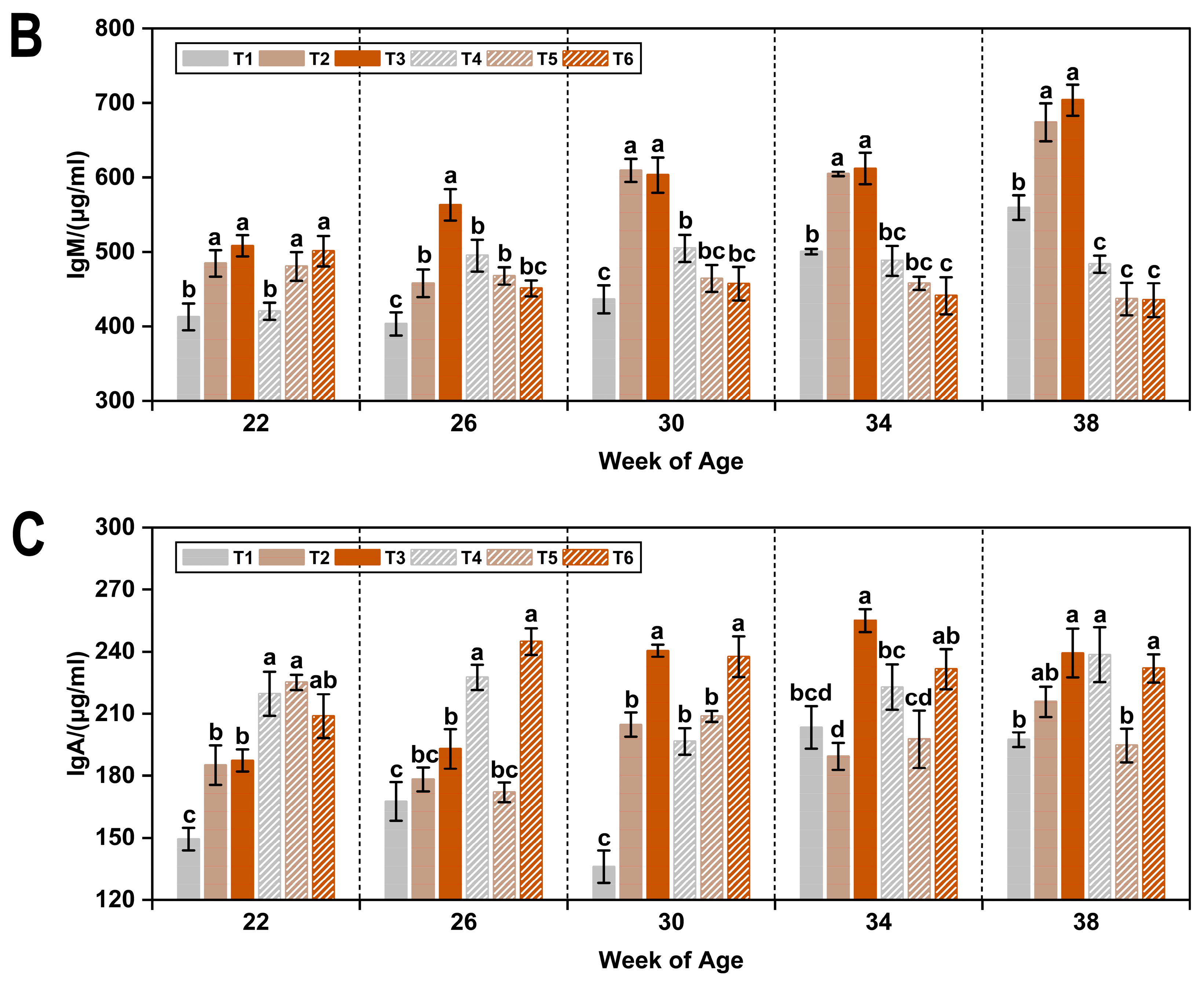
3.2. Immunoglobulins
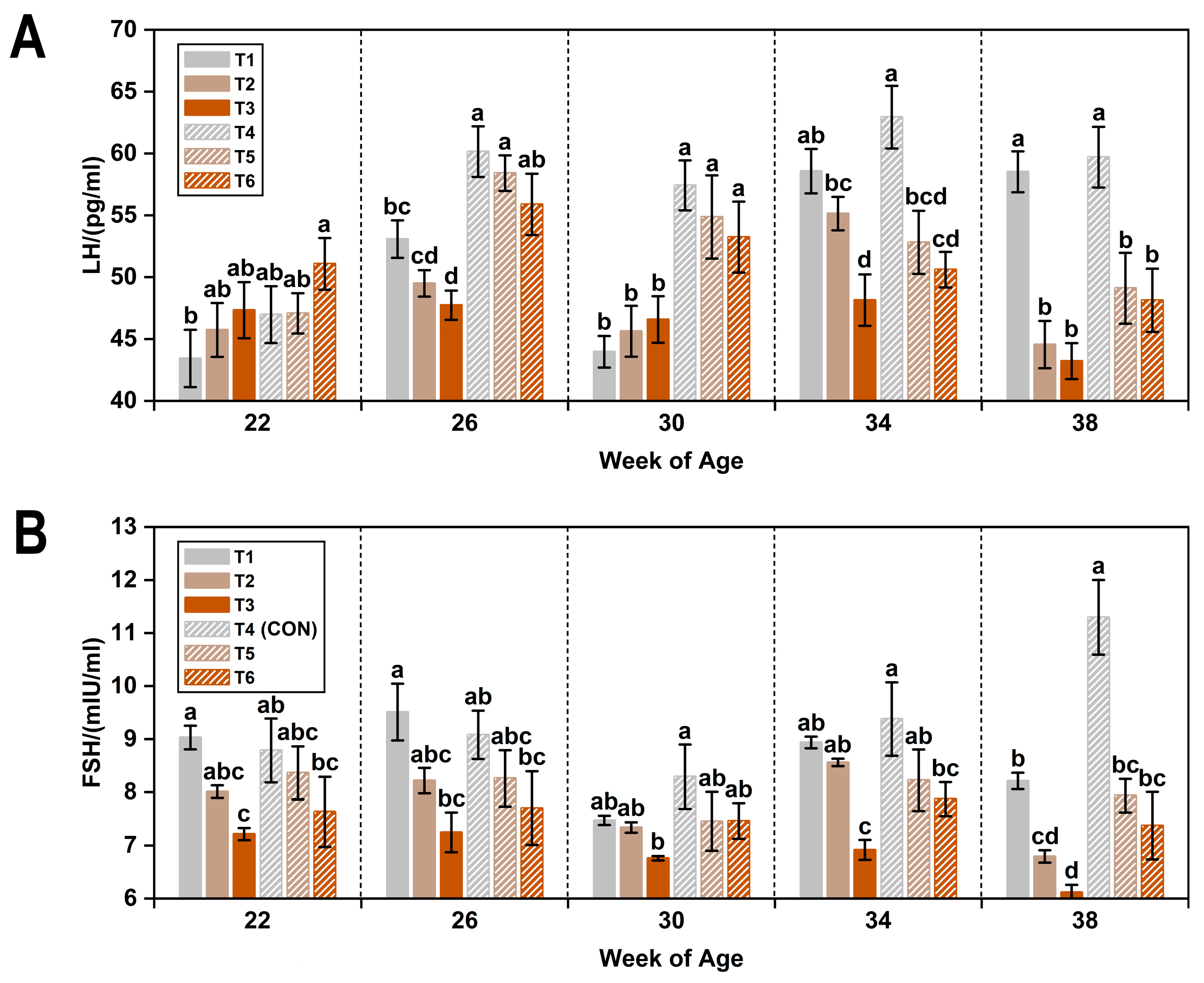
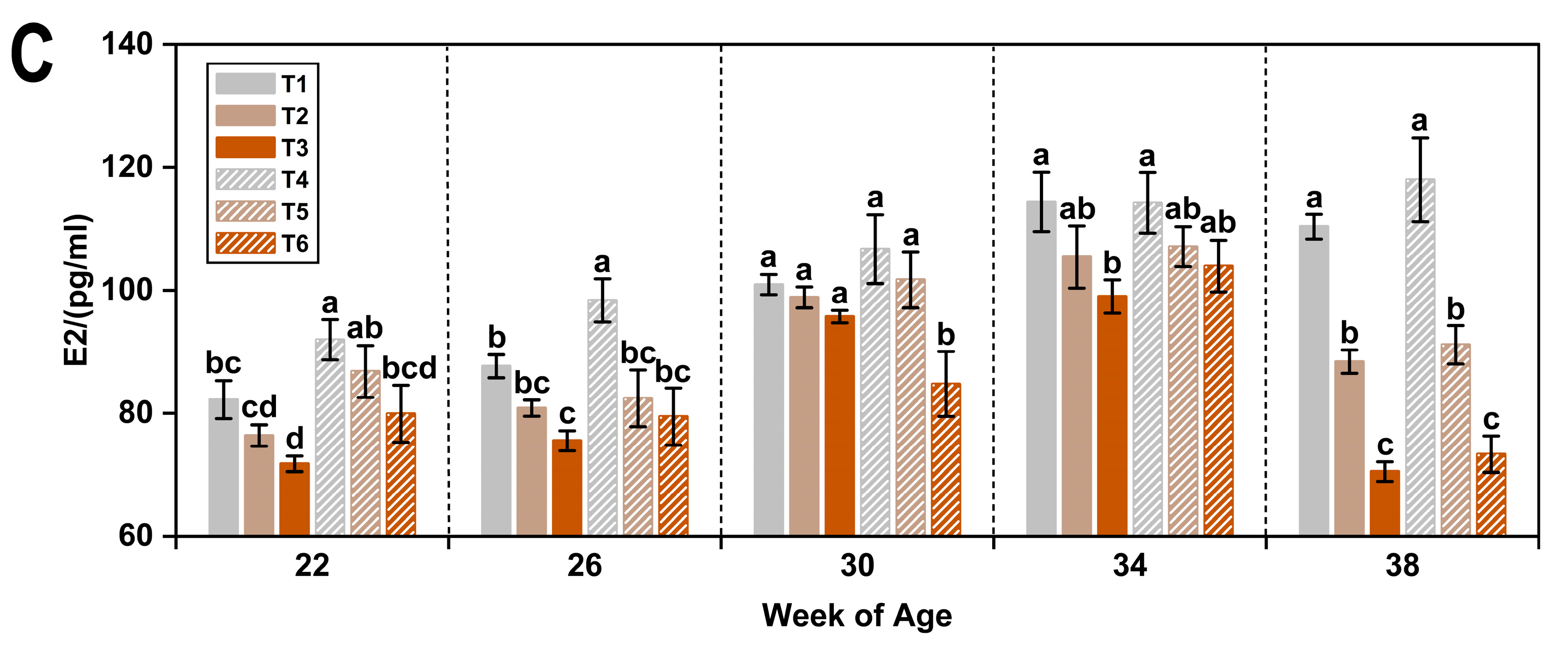
3.3. Reproductive Hormones
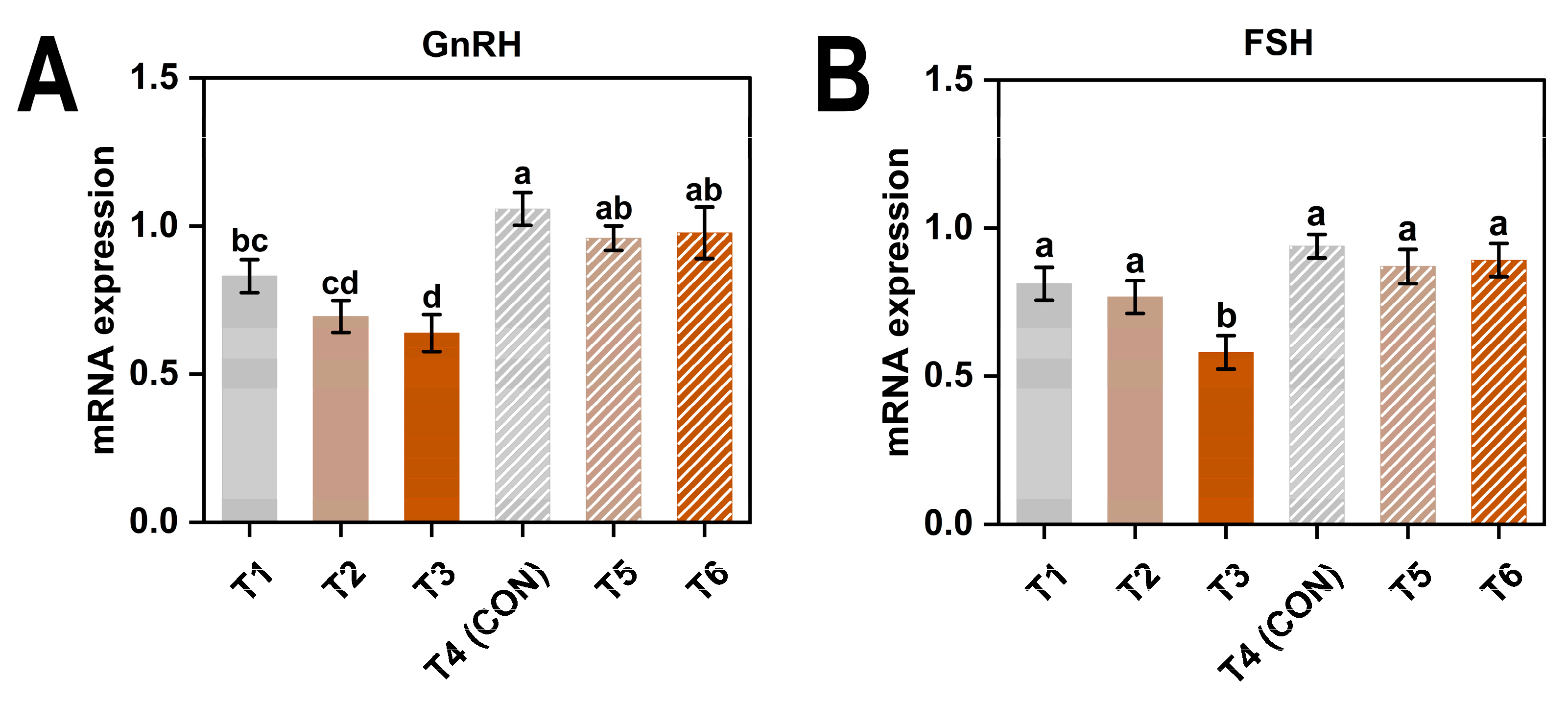
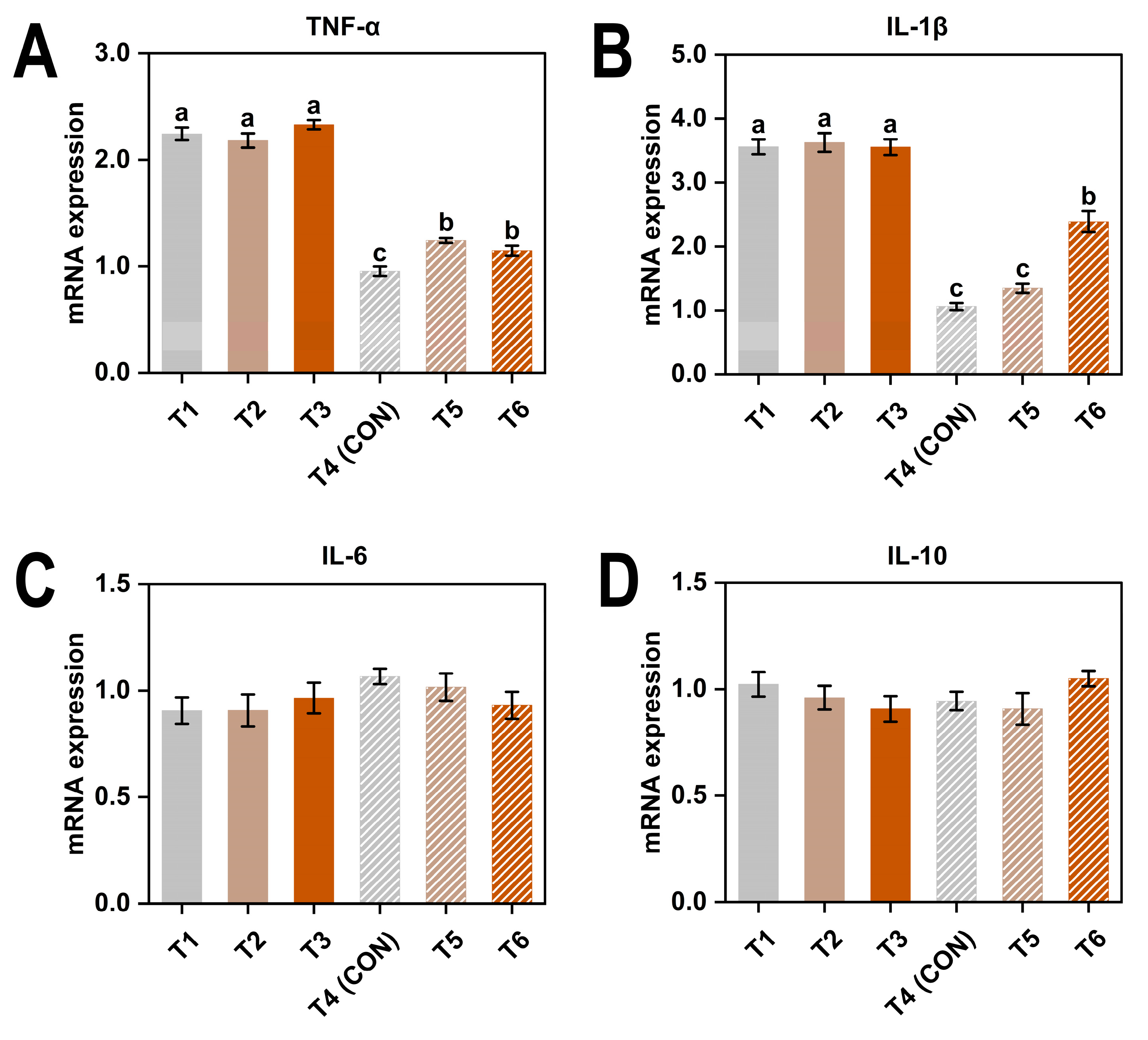
3.4. Gene Expression
4. Discussion
4.1. Physiological Stress Biomarkers
4.2. Immune Responses to Cold and Ammonia Stress
4.3. Reproductive Hormonal Regulation
4.4. Reproductive-Related Gene Expression Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CORT | Corticosterone |
| T-AOC | Total Antioxidant Capacity |
| Ig | Immunoglobulin |
| LH | Luteinizing Hormone |
| FSH | Follicle-stimulating Hormone |
| E2 | Estradiol |
| GnRH | Gonadotropin-releasing Hormone |
| TNF-α | Tumor Necrosis Factor-α |
| IL | Interleukin |
| TWA | Time-weighted Average |
| STEL | Short-term Exposure Limit |
| RH | Relative Humidity |
| CON | Control |
| ELISA | Enzyme-linked Immunosorbent Assay |
| WOA | Week of Age |
| AC | Ammonia Concentration |
| NS | Not Significant |
| HPA | Hypothalamic–Pituitary–Adrenal |
| HPG | Hypothalamic–Pituitary–Gonadal |
References
- Bist, R.B.; Bist, K.; Poudel, S.; Subedi, D.; Yang, X.; Paneru, B.; Mani, S.; Wang, D.Y.; Chai, L.L. Sustainable Poultry Farming Practices: A Critical Review of Current Strategies and Future Prospects. Poul. Sci. 2024, 103, 104295. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Campos, A.T.; Ferraz, P.F.P.; Bahuti, M.; Yanagi, T.J.r.; da Silva, J.P.; Ferreira, S.C. Dynamics of the Thermal Environment in Climate-Controlled Poultry Houses for Broiler Chickens. Agriengineering 2024, 6, 3891–3911. [Google Scholar] [CrossRef]
- Campo, J.L.; Prieto, M.T.; Dávila, S.G. Effects of Housing System and Cold Stress on Heterophil-to-lymphocyte Ratio, Fluctuating Asymmetry, and Tonic Immobility Duration of Chickens. Poult. Sci. 2008, 87, 621–626. [Google Scholar] [CrossRef]
- Torki, M.; Akbari, M.; Kaviani, K. Single and Combined Effects of Zinc and Cinnamon Essential Oil in Diet on Productive Performance, Egg Quality Traits, and Blood Parameters of Laying Hens Reared under Cold Stress Condition. Int. J. Biometeorol. 2015, 59, 1169–1177. [Google Scholar] [CrossRef]
- David, B.; Mejdell, C.; Michel, V.; Lund, V.; Moe, R.O. Air Quality in Alternative Housing Systems May Have an Impact on Laying Hen Welfare. Part II-Ammonia. Animals 2015, 5, 886–896. [Google Scholar] [CrossRef]
- Dietert, R.R.; Golemboski, K.A.; Austic, R.E. Environment-Immune Interactions. Poult. Sci. 1994, 73, 1062–1076. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmucker, S.S.; Bessei, W.; Grashorn, M.; Stefanski, V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals 2020, 10, 1138. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.K.; Kim, S.H.; Lee, K.W. The Impact of Temperature and Humidity on the Performance and Physiology of Laying Hens. Animals 2021, 11, 56. [Google Scholar] [CrossRef]
- Tsutsayeva, A.A.; Sevryukova, L.G. Effect of Cold Exposure on Survival and Stress Protein Expression of at Different Development Stages. Cryo Lett. 2001, 22, 145–150. [Google Scholar]
- Wichman, A.; De Groot, R.; Håstad, O.; Wall, H.; Rubene, D. Influence of Different Light Spectrums on Behaviour and Welfare in Laying Hens. Animals 2021, 11, 924. [Google Scholar] [CrossRef]
- Blahová, J.; Dobsiková, R.; Straková, E.; Suchy, P. Effect of Low Environmental Temperature on Performance and Blood System in Broiler Chickens. Acta. Vet. Brno. 2007, 76, S17–S23. [Google Scholar] [CrossRef]
- Sahin, K.; Kuçuk, O.; Sahin, N. Effects of Dietary Chromium Picolinate Supplementation on Performance and Plasma Concentrations of Insulin and Corticosterone in Laying Hens under Low Ambient Temperature. J. Anim. Physiol. Anim. Nutr. 2001, 85, 142–147. [Google Scholar] [CrossRef]
- Li, D.P.; Tong, Q.; Shi, Z.X.; Zheng, W.C.; Wang, Y.; Li, B.M.; Yan, G.Q. Effects of Cold Stress and Ammonia Concentration on Productive Performance and Egg Quality Traits of Laying Hens. Animals 2020, 10, 2252. [Google Scholar] [CrossRef]
- Arad, Z.; Marder, J. Comparison of the Productive Performances of the Sinai Bedouin Fowl, the White Leghorn and Their Crossbreds: Study under Laboratory Conditions. Br. Poult. Sci. 1982, 23, 329–332. [Google Scholar] [CrossRef]
- Sahin, N.; Sahin, K.; Onderci, M. Vitamin E and Selenium Supplementation to Alleviate Cold-stress-associated Deterioration in Egg Quality and Egg Yolk Mineral Concentrations of Japanese Quails. Biol. Trace Elem. Res. 2003, 96, 179–189. [Google Scholar] [CrossRef]
- Spinu, M.; Degen, A.A. Effect of Cold Stress on Performance and Immune Responses of Bedouin and White Leghorn Hens. Br. Poult. Sci. 1993, 34, 177–185. [Google Scholar] [CrossRef]
- Akbari, M.; Torki, M.; Kaviani, K. Single and Combined Effects of Peppermint and Thyme Essential Oils on Productive Performance, Egg Quality Traits, and Blood Parameters of Laying Hens Reared under Cold Stress Condition (6.8 ± 3 A °C). Int. J. Biometeorol. 2016, 60, 447–454. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Devi, R.; Srikumar, R.; Manikandan, S.; Thangaraj, R. Protective Effect of Triphala on Cold Stress-induced Behavioral and Biochemical Abnormalities in Rats. Yakugaku Zasshi-J. Pharm. Soc. JPN. 2007, 127, 1863–1867. [Google Scholar] [CrossRef]
- Kucuk, O.; Sahin, N.; Sahin, K.; Gursu, M.F.; Gulcu, F.; Ozcelik, M.; Issi, M. Egg Production, Egg Quality, and Lipid Peroxidation Status in Laying Hens Maintained at a Low Ambient Temperature (6 °C) and Fed A Vitamin C and Vitamin E-supplemented Diet. Vet. Med.-Czech 2003, 48, 33–40. [Google Scholar] [CrossRef]
- Sahin, N.; Onderci, M.; Sahin, K. Effects of Dietary Chromium and Zinc on Egg Production, Egg Quality, and Some Blood Metabolites of Laying Hens Reared under Low Ambient Temperature. Biol. Trace Elem. Res. 2002, 85, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.; Sahin, K. Optimal Dietary Concentrations of Vitamin C and Chromium Picolinate for Alleviating the Effect of Low Ambient Temperature (6.2 degrees C) on Egg Production, Some Egg Characteristics, and Nutrient Digestibility in Laying Hens. Vet. Med.-Czech 2001, 46, 229–236. [Google Scholar] [CrossRef]
- Deaton, J.W.; Reece, F.N.; Lott, B.D. Effect of Atmospheric Ammonia on Laying Hen Performance. Poult. Sci. 1982, 61, 1815–1817. [Google Scholar] [CrossRef] [PubMed]
- Beker, A.; Vanhooser, S.L.; Swartlzander, J.H.; Teeter, R.G. Atmospheric Ammonia Concentration Effects on Broiler Growth and Performance. J. Appl. Poult. Res. 2004, 13, 5–9. [Google Scholar] [CrossRef]
- Charles, D.R.; Payne, C.G. The Influence of Graded Levels of Atmospheric Ammonia on Chickens. I. Effects on Respiration and on the Performance of Broilers and Replacement Growing Stock. Br. Poult. Sci. 1966, 7, 177–187. [Google Scholar] [CrossRef]
- Deaton, J.W.; Reece, F.N.; Lott, B.D. Effect of Atmospheric Ammonia on Pullets at Point of Lay. Poult. Sci. 1984, 63, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.M.; Branton, S.L.; Lott, B.D. Atmospheric Ammonia is Detrimental to the Performance of Modern Commercial Broilers. Poult. Sci. 2004, 83, 1650–1654. [Google Scholar] [CrossRef]
- Miles, D.M.; Miller, W.W.; Branton, S.L.; Maslin, W.R.; Lott, B.D. Ocular Responses to Ammonia in Broiler Chickens. Avian Dis. 2006, 50, 45–49. [Google Scholar] [CrossRef]
- Wei, F.X.; Hu, X.F.; Sa, R.N.; Liu, F.Z.; Li, S.Y.; Sun, Q.Y. Antioxidant Capacity and Meat Quality of Broilers Exposed to Different Ambient Humidity and Ammonia Concentrations. Genet. Mol. Res. 2014, 13, 3117–3127. [Google Scholar] [CrossRef]
- Cotterill, O.J.; Nordskog, A.W. Influence of Ammonia on Egg White Quality. Poult. Sci. 1954, 33, 432–434. [Google Scholar] [CrossRef]
- Kristensen, H.H.; Wathes, C.M. Ammonia and Poultry Welfare: A Review. World Poult. Sci. J. 2000, 56, 235–245. [Google Scholar] [CrossRef]
- Nagaraja, K.V.; Emery, D.A.; Jordan, K.A.; Newman, J.A.; Pomeroy, B.S. Scanning Electron-Microscopic Studies of Adverse-Effects of Ammonia on Tracheal Tissues of Turkeys. Am. J. Vet. Res. 1983, 44, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.P.; Beard, C.W.; Hanson, R.P. The Adverse Effects of Ammonia on Chickens Including Resistance to Infection with Newcastle Disease Virus. Avian Dis. 1964, 8, 369–379. [Google Scholar] [CrossRef]
- Oyetunde, O.O.F.; Thomson, R.G.; Carlson, H.C. Aerosol Exposure of Ammonia, Dust and Escherichia-Coli in Broiler-Chickens. Can. Vet. J. 1978, 19, 187–193. [Google Scholar] [PubMed]
- Sato, S.; Shoya, S.; Kobayashi, H. Effect of Ammonia on Mycoplasma-Gallisepticum Infection in Chickens. Natl. I. Anim. Health Q. 1973, 13, 45–53. [Google Scholar]
- Wang, Y.M.; Meng, Q.P.; Guo, Y.M.; Wang, Y.Z.; Wang, Z.; Yao, Z.L.; Shan, T.Z. Effect of Atmospheric Ammonia on Growth Performance and Immunological Response of Broiler Chickens. J. Anim. Vet. Adv. 2010, 9, 2802–2806. [Google Scholar] [CrossRef]
- Koreleski, J.; Swiatkiewicz, S.; Arczewska-Wlosek, A. Rapeseed Cake, Glycerin and Distillers Dried Grains with Solubles Used Simultaneously as a Source of Nutrients for Hens in Their Second Laying Season. Ann. Anim. Sci. 2011, 11, 125–133. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive 2000/54/EC on the Protection of Workers from Risks Related to Exposure to Biological Agents at Work. Off. J. Eur. Communities 2000, L262, 21–45. [Google Scholar]
- Occupational Safety and Health Administration (OSHA). Ammonia Safety Limits and Exposure Guidelines. OSHA Standard No. 1910.111. 2012. Available online: https://www.osha.gov/laws-regs (accessed on 5 June 2025).
- National Institute for Occupational Safety and Health (NIOSH). Ammonia: Workplace Safety and Exposure Recommendations. NIOSH Publication No. 2016-111. 2016. Available online: https://www.cdc.gov/niosh (accessed on 5 June 2025).
- NY/T 388-1999; Environmental Quality Standards for Livestock and Poultry Farms. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 1999.
- Lu, J.; Wang, Q.; Ma, M.; Li, Y.; Guo, W.; Zhang, X.; Yang, X.; Qu, L. Influence of Body Weight at the End of the Brooding Period on the Productive Performance in Hyline Brown Laying Hens from 6 to 72 Weeks of Age. Animals 2025, 15, 1292. [Google Scholar] [CrossRef]
- Li, D.P.; Tong, Q.; Shi, Z.X.; Li, H.; Wang, Y.; Li, B.M.; Yan, G.Q.; Chen, H.; Zheng, W.C. Effects of Chronic Heat Stress and Ammonia Concentration on Blood Parameters of Laying Hens. Poult. Sci. 2020, 99, 3784–3792. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yan, F.F.; Hu, J.Y.; Chen, H.; Tucker, C.M.; Green, A.R.; Cheng, H.W. The Effect of Chronic Ammonia Exposure on Acute-phase Proteins, Immunoglobulin, and Cytokines in Laying Hens. Poult. Sci. 2017, 96, 1524–1530. [Google Scholar] [CrossRef]
- Akinyemi, F.; Adewole, D. Environmental Stress in Chickens and the Potential Effectiveness of Dietary Vitamin Supplementation. Front. Anim. Sci. 2021, 2, 775311. [Google Scholar] [CrossRef]
- Nazar, F.N.; Estevez, I. The Immune-neuroendocrine System, A Key Aspect of Poultry Welfare and Resilience. Poult. Sci. 2022, 101, 101919. [Google Scholar] [CrossRef] [PubMed]
- Post, J.; Rebel, J.M.J.; ter Huurne, A.A.H.M. Physiological Effects of Elevated Plasma Corticosterone Concentrations in Broiler Chickens. An Alternative Means by which to Assess the Physiological Effects of Stress. Poult. Sci. 2003, 82, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Shini, A.; Huff, G.R. Effects of Chronic and Repeated Corticosterone Administration in Rearing Chickens on Physiology, the Onset of Lay and Egg Production of Hens. Physiol. Behav. 2009, 98, 73–77. [Google Scholar] [CrossRef]
- Calefi, A.S.; Honda, B.T.B.; Costola-de-Souza, C.; de Siqueira, A.; Namazu, L.B.; Quinteiro, W.M.; Fonseca, J.G.D.; Aloia, T.P.A.; Piantino-Ferreira, A.J.; Palermo-Neto, J. Effects of Long-term Heat stress in an Experimental Model of Avian Necrotic Enteritis. Poult. Sci. 2014, 93, 1344–1353. [Google Scholar] [CrossRef]
- He, X.F.; Lu, Z.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Chronic Heat Stress Alters Hypothalamus Integrity, the Serum Indexes and Attenuates Expressions of Hypothalamic Appetite Genes in Broilers. J. Therm. Biol. 2019, 81, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Chao, X.H.; Zhang, W.W.; Zhang, X.Q.; Wu, J.W.; Ye, C.T.; Ma, X.R.; Fan, Z.X.; Liu, M.Q.; Fang, C.; et al. Heat Stress Influences Immunity Through and Mediated Antigen Presentation in Chickens. Animals 2025, 15, 1141. [Google Scholar] [CrossRef]
- Kim, H.R.; Ryu, C.; Lee, S.D.; Cho, J.H.; Kang, H. Effects of Heat Stress on the Laying Performance, Egg Quality, and Physiological Response of Laying Hens. Animals 2024, 14, 1076. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The Sympathetic Nerve–An Integrative Interface between Two Supersystems: The Brain and the Immune System. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [CrossRef]
- Quinteiro, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat Stress Impairs Performance Parameters, Induces Intestinal Injury, and Decreases Macrophage Activity in Broiler Chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; Decuypere, E.; Buyse, J. Acute Heat Stress Induces Oxidative Stress in Broiler Chickens. Comp. Biochem. Phys. A. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Devi, G.S.; Prasad, M.H.; Saraswathi, I.; Raghu, D.; Rao, D.N.; Reddy, P.P. Free Radicals Antioxidant Enzymes and Lipid Peroxidation in Different Types of Leukemias. Clin. Chim. Acta 2000, 293, 53–62. [Google Scholar] [CrossRef]
- Thomas, M.J. The Role of Free Radicals and Antioxidants. Nutrition 2000, 16, 716–718. [Google Scholar] [CrossRef]
- Oke, O.E.; Akosile, O.A.; Oni, A.I.; Opowoye, I.O.; Ishola, C.A.; Adebiyi, J.O.; Odeyemi, A.J.; Adjei-Mensah, B.; Uyanga, V.A.; Abioja, M.O. Oxidative Stress in Poultry Production. Poult. Sci. 2024, 103, 104003. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Ma, X.Y.; Lin, Y.C.; Zhang, H.X.; Chen, W.; Wang, S.; Ruan, D.; Jiang, Z.Y. Heat Stress Impairs the Nutritional Metabolism and Reduces the Productivity of Egg-laying Ducks. Anim. Reprod. Sci. 2014, 145, 182–190. [Google Scholar] [CrossRef]
- Gomes, A.V.S.; Quinteiro, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Baskeville, E.; Akamine, A.T.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Palermo-Neto, J. Overcrowding Stress Decreases Macrophage Activity and Increases Enteritidis Invasion in Broiler Chickens. Avian Pathol. 2014, 43, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lebacqve, A.M.; Vaerman, J.P.; Heremans, J.F. Quantification and Distribution of Chicken Immunoglobulins Iga, Igm and Igg in Serum and Secretions. Immunology 1974, 27, 683–692. [Google Scholar]
- Sanders, B.G.; Case, W.L. Chicken Secretory Immunoglobulin—Chemical and Immunological Characterization of Chicken Iga. Comp. Biochem. Phys. B. 1977, 56, 273–278. [Google Scholar] [CrossRef]
- Honda, B.T.B.; Calefi, A.S.; Costola-de-Souza, C.; Quinteiro, W.M.; Fonseca, J.G.D.; de Paula, V.F.; Palermo-Neto, J. Vaccinated for Newcastle Disease Virus. Poult. Sci. 2015, 94, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, M.; Khan, M.Z.I.; Siddiqi, M.N.H.; Masum, M.A. Mobilization of Immunoglobulin (Ig)-containing Plasma Cells in Harderian Gland, Cecal Tonsil and Trachea of Broilers Vaccinated with Newcastle Disease Vaccine. Tissue Cell 2013, 45, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hangalapura, B.N.; Nieuwland, M.G.B.; Buyse, J.; Kemp, B.; Parmentier, H.K. Effect of Duration of Cold Stress on Plasma Adrenal and Thyroid Hormone Levels and Immune Responses in Chicken Lines Divergently Selected for Antibody Responses. Poult. Sci. 2004, 83, 1644–1649. [Google Scholar] [CrossRef]
- Wang, C.; Bing, A.Y.; Liu, H.; Wang, X.J.; Zhao, J.P.; Lin, H.; Jiao, H.C. High Ambient Humidity Aggravates Ammonia-induced Respiratory Mucosal Inflammation by Eliciting Th1/Th2 Imbalance and NF-kB Pathway Activation in Laying Hens. Poult. Sci. 2022, 101, 102028. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory IgA: Designed for Anti-microbial Defense. Front. Immunol. 2013, 4, 222. [Google Scholar] [CrossRef]
- Duan, Y.F.; Xiao, M.; Zhu, R.J.; Nan, Y.X.; Yang, Y.K.; Huang, X.H.; Zhang, D.C. Effects of Ammonia Stress on the Antioxidant, Ferroptosis, and Immune Response in the Liver of Golden Pompano. Antioxidants 2025, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Moens, L.; Tangye, S.G. Cytokine-mediated Regulation of Plasma Cell Generation: IL-21 Takes Center Stage. Front. Immunol. 2014, 5, 65. [Google Scholar] [CrossRef]
- Greene, E.S.; Tabler, T.; Bottje, W.G.; Orlowski, S.; Dridi, S. Effect of Heat Stress on the Expression of Circulating Cyto(chemo)kine and Inflammatory Markers in Broiler Chickens Selected for High- or Low-water Efficiency. Front. Biosci-Landmrk. 2024, 29, 359. [Google Scholar] [CrossRef]
- Tang, L.P.; Li, W.H.; Liu, Y.L.; Lun, J.C.; He, Y.M. Heat Stress Aggravates Intestinal Inflammation through TLR4-NF-κB Signaling Pathway in Ma Chickens Infected with Escherichia coli O157:H7. Poult. Sci. 2021, 100, 101030. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Q.X.; Li, X.M.; Ma, D.D.; Xing, S.; Feng, J.H.; Zhang, M.H. Effects of Ammonia Exposure on Growth Performance and Cytokines in the Serum, Trachea, and Ileum of Broilers. Poult. Sci. 2020, 99, 2485–2493. [Google Scholar] [CrossRef]
- Hanlon, C.; Takeshima, K.; Bédécarrats, G.Y. Changes in the Control of the Hypothalamic-Pituitary Gonadal Axis Across Three Differentially Selected Strains of Laying Hens (Gallus gallus domesticus). Front. Physiol. 2021, 12, 651491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.B.; Pan, H.B.; Liu, Y.; He, Y.; Shi, H.M.; Ge, C.R. Interacting Networks of the Hypothalamic-Pituitary-Ovarian Axis Regulate Layer Hens Performance. Genes. 2023, 14, 141. [Google Scholar] [CrossRef]
- Novero, R.P.; Beck, M.M.; Gleaves, E.W.; Johnson, A.L.; Deshazer, J.A. Plasma Progesterone, Luteinizing-Hormone Concentrations, and Granulosa-Cell Responsiveness in Heat-Stressed Hens. Poult. Sci. 1991, 70, 2335–2339. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.Y.; Xu, W.J.; Zhu, C.H.; Zhang, S.J.; Shi, Z.H.; Song, W.T.; Liu, H.X.; Li, H.F. Effects of Ammonia on Intestinal Microflora and Productive Performance of Laying Ducks. Poult. Sci. 2019, 98, 1947–1959. [Google Scholar] [CrossRef]
- Rozenboim, I.; Tako, E.; Gal-Garber, O.; Proudman, J.A.; Uni, Z. The Effect of Heat Stress on Ovarian Function of Laying Hens. Poult. Sci. 2007, 86, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Foster, L.K.; Silsby, J.L.; Elhalawani, M.E.; Foster, D.N. Sequence-Analysis of the Turkey Lh Beta-Subunit and Its Regulation by Gonadotropin-Releasing-Hormone and Prolactin in Cultured Pituitary-Cells. J. Mol. Endocrinol. 1995, 14, 117–129. [Google Scholar] [CrossRef]
- Youngren, O.M.; Elhalawani, M.E.; Silsby, J.L.; Phillips, R.E. Intracranial Prolactin Perfusion Induces Incubation Behavior in Turkey Hens. Biol. Reprod. 1991, 44, 425–431. [Google Scholar] [CrossRef]
- Wolfenson, D.; Lew, B.J.; Thatcher, W.W.; Graber, Y.; Meidan, R. Seasonal and Acute Heat Stress Effects on Steroid Production by Dominant Follicles in Cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef]
- Blas, J. Stress in birds. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: London, UK, 2015; pp. 769–810. [Google Scholar]
- Wang, X.J.; Liu, L.; Zhao, J.P.; Jiao, H.C.; Lin, H. Stress Impairs the Reproduction of Laying Hens: An Involvement of Energy. World Poult. Sci. J. 2017, 73, 845–855. [Google Scholar] [CrossRef]
- Henriksen, R.; Groothuis, T.G.; Rettenbacher, S. Elevated Plasma Corticosterone Decreases Yolk Testosterone and Progesterone in Chickens: Linking Maternal Stress and Hormone-Mediated Maternal Effects. PLoS ONE 2011, 6, 8. [Google Scholar] [CrossRef]
- Hillebrecht, T.; Korbel, R.; Rinder, M.; Gahr, M. Circadian Corticosterone Profile in Laying Hens (Gallus gallus domesticus). Animals 2024, 14, 873. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wu, F.Y.; Yang, X.Y.; Liu, S.D.; Han, S.J.; Chen, B.J. Effects of Ammonia on Hypothalamic-pituitary-ovarian Axis in Female Rabbits. Ecotox. Environ. Safe 2021, 227, 112922. [Google Scholar] [CrossRef]
- Tu, W.L.; Cheng, C.Y.; Wang, S.H.; Tang, P.C.; Chen, C.F.; Chen, H.H.; Lee, Y.P.; Chen, S.E.; Huang, S.Y. Profiling of Differential Gene Expression in the Hypothalamus of Broiler-type Taiwan Country Chickens in Response to Acute Heat Stress. Theriogenology 2016, 85, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, Stress, and Fertility. Minerva Endocrinol. 2010, 35, 109–125. [Google Scholar] [PubMed]
- Oakley, A.E.; Breen, K.M.; Clarke, I.J.; Karsch, F.J.; Wagenmaker, E.R.; Tilbrook, A.J. Cortisol Reduces Gonadotropin-Releasing Hormone Pulse Frequency in Follicular Phase Ewes: Influence of Ovarian Steroids. Endocrinology 2009, 150, 341–349. [Google Scholar] [CrossRef]
- Breen, K.M.; Karsch, F.J. Does Season Alter Responsiveness of the Reproductive Neuroendocrine Axis to the Suppressive Actions of Cortisol in Ovariectomized Ewes? Biol. Reprod. 2006, 74, 41–45. [Google Scholar] [CrossRef][Green Version]
| Item 1 | T1 | T2 | T3 | T4 (CON) | T5 | T6 |
|---|---|---|---|---|---|---|
| T (°C) | 8 | 8 | 8 | 20 | 20 | 20 |
| AC (ppm) | ≤5 | 20 | 45 | ≤5 | 20 | 45 |
| Target Gene | Primer | Primer Sequences (5′-3′) | Product Length (bp) |
|---|---|---|---|
| β-actin | Forward | CACGATCATGTTTGAGACCTT | 100 |
| Reverse | CATCACAATACCAGTGGTACG | ||
| FSH | Forward | TTATGTAGCCCACTGAGGAAAGCCA | 257 |
| Reverse | TAGCTGAGCCGTTCTACACTTTGGA | ||
| GnRH | Forward | AATCTGCTTGGCTCAACACTG | 220 |
| Reverse | AATCTCCTTTCTTCTGGCTTCT | ||
| TNF-α | Forward | ATGAACCCTCCGCAGTACTC | 200 |
| Reverse | AAGAGGCCACCACACGACA | ||
| IL-1β | Forward | GGGCTACAAGCTCTACATGT | 118 |
| Reverse | GTAGAAGATGAAGCGGGTCA | ||
| IL-6 | Forward | AGAAATCCCTCCTCGCCAAT | 121 |
| Reverse | AAATAGCGAACGGCCCTCA | ||
| IL-10 | Forward | CGCTGTCACCGCTTCTTCA | 88 |
| Reverse | TCCCGTTCTCATCCATCTTCTC |
| Item 1 | CORT (ng/mL) | T-AOC (U/mL) |
|---|---|---|
| WOA | ||
| 22 | 66.33 ± 1.68 b | 5.92 ± 0.09 c |
| 26 | 70.80 ± 1.66 a | 6.13 ± 0.09 c |
| 30 | 71.19 ± 1.54 a | 6.44 ± 0.16 c |
| 34 | 71.11 ± 1.69 a | 11.39 ± 0.28 b |
| 38 | 64.03 ± 1.31 b | 13.65 ± 0.41 a |
| T (°C) | ||
| 8 | 69.30 ± 1.01 | 8.27 ± 0.23 b |
| 20 | 68.09 ± 1.03 | 9.11 ± 0.32 a |
| AC (ppm) | ||
| ≤5 | 67.14 ± 1.09 b | 9.86 ± 0.43 a |
| 20 | 67.27 ± 1.14 b | 8.69 ± 0.32 b |
| 45 | 71.66 ± 1.45 a | 7.57 ± 0.22 c |
| p-value 2 | ||
| WOA | <0.05 | <0.05 |
| T | NS | <0.05 |
| AC | <0.05 | <0.05 |
| WOA × T | NS | <0.05 |
| WOA × AC | <0.05 | <0.05 |
| T × AC | NS | <0.05 |
| Item 1 | IgG (μg/mL) | IgM (μg/mL) | IgA (μg/mL) |
|---|---|---|---|
| WOA | |||
| 22 | 1473.51 ± 33.46 c | 468.02 ± 8.09 c | 195.89 ± 4.40 b |
| 26 | 1544.36 ± 41.55 c | 473.12 ± 8.87 c | 197.19 ± 4.47 b |
| 30 | 1536.65 ± 35.79 c | 512.62 ± 11.31 b | 203.99 ± 4.83 b |
| 34 | 1725.28 ± 30.95 b | 517.44 ± 10.21 b | 216.61 ± 4.71 a |
| 38 | 1925.78 ± 42.42 a | 548.86 ± 15.07 a | 219.58 ± 4.18 a |
| T (°C) | |||
| 8 | 1814.70 ± 20.48 a | 542.29 ± 8.08 a | 196.12 ± 3.01 b |
| 20 | 1467.53 ± 25.32 b | 465.74 ± 4.91 b | 217.18 ± 2.64 a |
| AC (ppm) | |||
| ≤5 | 1449.88 ± 22.87 c | 470.48 ± 6.61 b | 195.88 ± 4.03 b |
| 20 | 1675.12 ± 30.34 b | 513.86 ± 8.90 a | 197.12 ± 2.70 b |
| 45 | 1798.35 ± 34.41 a | 527.69 ± 10.05 a | 226.96 ± 3.21 a |
| p-value 2 | |||
| WOA | <0.05 | <0.05 | <0.05 |
| T | <0.05 | <0.05 | <0.05 |
| AC | <0.05 | <0.05 | <0.05 |
| WOA × T | <0.05 | <0.05 | <0.05 |
| WOA × AC | NS | NS | <0.05 |
| T × AC | NS | <0.05 | <0.05 |
| Item 1 | LH (pg/mL) | FSH (mIU/mL) | E2 (pg/mL) |
|---|---|---|---|
| WOA | |||
| 22 | 46.94 ± 0.89 c | 8.17 ± 0.19 a | 81.50 ± 1.51 d |
| 26 | 54.12 ± 0.85 a | 8.33 ± 0.21 a | 84.04 ± 1.55 d |
| 30 | 50.28 ± 1.10 b | 7.46 ± 0.15 b | 98.11 ± 1.70 b |
| 34 | 54.70 ± 0.99 a | 8.31 ± 0.18 a | 107.33 ± 1.80 a |
| 38 | 50.54 ± 1.15 b | 7.95 ± 0.26 ab | 91.94 ± 2.50 c |
| T (°C) | |||
| 8 | 48.74 ± 0.58 b | 7.76 ± 0.09 b | 90.52 ± 1.20 b |
| 20 | 53.89 ± 0.69 a | 8.34 ± 0.16 a | 94.64 ± 1.49 a |
| AC (ppm) | |||
| ≤5 | 54.47 ± 0.88 a | 9.00 ± 0.17 a | 102.47 ± 1.64 a |
| 20 | 50.28 ± 0.77 b | 7.92 ± 0.12 b | 91.89 ± 1.40 b |
| 45 | 49.19 ± 0.71 b | 7.23 ± 0.13 c | 83.39 ± 1.46 c |
| p-value 2 | |||
| WOA | <0.05 | <0.05 | <0.05 |
| T | <0.05 | <0.05 | <0.05 |
| AC | <0.05 | <0.05 | <0.05 |
| WOA × T | <0.05 | <0.05 | NS |
| WOA × AC | <0.05 | <0.05 | <0.05 |
| T × AC | NS | NS | NS |
| Item 1 | GnRH | FSH |
|---|---|---|
| T (°C) | ||
| 8 | 0.72 ± 0.16 b | 0.73 ± 0.16 b |
| 20 | 0.99 ± 0.15 a | 0.90 ± 0.12 a |
| AC (ppm) | ||
| ≤5 | 0.94 ± 0.18 a | 0.88 ± 0.13 a |
| 20 | 0.83 ± 0.18 ab | 0.82 ± 0.14 ab |
| 45 | 0.81 ± 0.25 b | 0.74 ± 0.21 b |
| p-value 2 | ||
| T | <0.05 | <0.05 |
| AC | <0.05 | <0.05 |
| T × AC | NS | NS |
| Item 1 | TNF-α | IL-1β | IL-6 | IL-10 |
|---|---|---|---|---|
| T (°C) | ||||
| 8 | 2.25 ± 0.15 a | 3.58 ± 0.30 a | 0.93 ± 0.16 | 0.96 ± 0.14 |
| 20 | 1.11 ± 0.15 b | 1.60 ± 0.64 b | 1.00 ± 0.14 | 0.97 ± 0.14 |
| AC (ppm) | ||||
| ≤5 | 1.60 ± 0.69 b | 2.31 ± 1.32 b | 0.99 ± 0.15 | 0.98 ± 0.13 |
| 20 | 1.71 ± 0.50 a | 2.50 ± 1.22 b | 0.96 ± 0.17 | 0.93 ± 0.16 |
| 45 | 1.74 ± 0.63 a | 2.97 ± 0.70 a | 0.95 ± 0.16 | 0.98 ± 0.14 |
| p-value 2 | ||||
| T | <0.05 | <0.05 | NS | NS |
| AC | <0.05 | <0.05 | NS | NS |
| T × AC | <0.05 | <0.05 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, F.; Liu, W.; Han, H.; Wang, J.; Hao, D.; Sun, Y. Physiological Responses of Laying Hens to Chronic Cold Stress and Ammonia Exposure: Implications for Environmental Management and Poultry Welfare. Animals 2025, 15, 1769. https://doi.org/10.3390/ani15121769
Li D, Li F, Liu W, Han H, Wang J, Hao D, Sun Y. Physiological Responses of Laying Hens to Chronic Cold Stress and Ammonia Exposure: Implications for Environmental Management and Poultry Welfare. Animals. 2025; 15(12):1769. https://doi.org/10.3390/ani15121769
Chicago/Turabian StyleLi, Dapeng, Fuwei Li, Wei Liu, Haixia Han, Jie Wang, Dan Hao, and Yan Sun. 2025. "Physiological Responses of Laying Hens to Chronic Cold Stress and Ammonia Exposure: Implications for Environmental Management and Poultry Welfare" Animals 15, no. 12: 1769. https://doi.org/10.3390/ani15121769
APA StyleLi, D., Li, F., Liu, W., Han, H., Wang, J., Hao, D., & Sun, Y. (2025). Physiological Responses of Laying Hens to Chronic Cold Stress and Ammonia Exposure: Implications for Environmental Management and Poultry Welfare. Animals, 15(12), 1769. https://doi.org/10.3390/ani15121769










