Effects of a Capsaicin-Based Phytogenic Solution on Intestinal Permeability, Serum Amino Acid Concentrations, and Digestibility in Heat-Stressed Growing Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Experiment 1
2.2.1. Animals, Diet, and Experimental Procedure
2.2.2. Serum Concentration (SC) of Amino Acids
2.2.3. Gene Expression
Extraction and Purification of Total RNA
Reverse Transcription
Quantitative PCR
2.3. Experiment 2
2.3.1. Animals, Diet, and Experimental Procedure
2.3.2. Chemical Analyses and Calculations
2.4. Statistical Analysis
3. Results
3.1. Serum Concentration of Free Essential Amino Acids
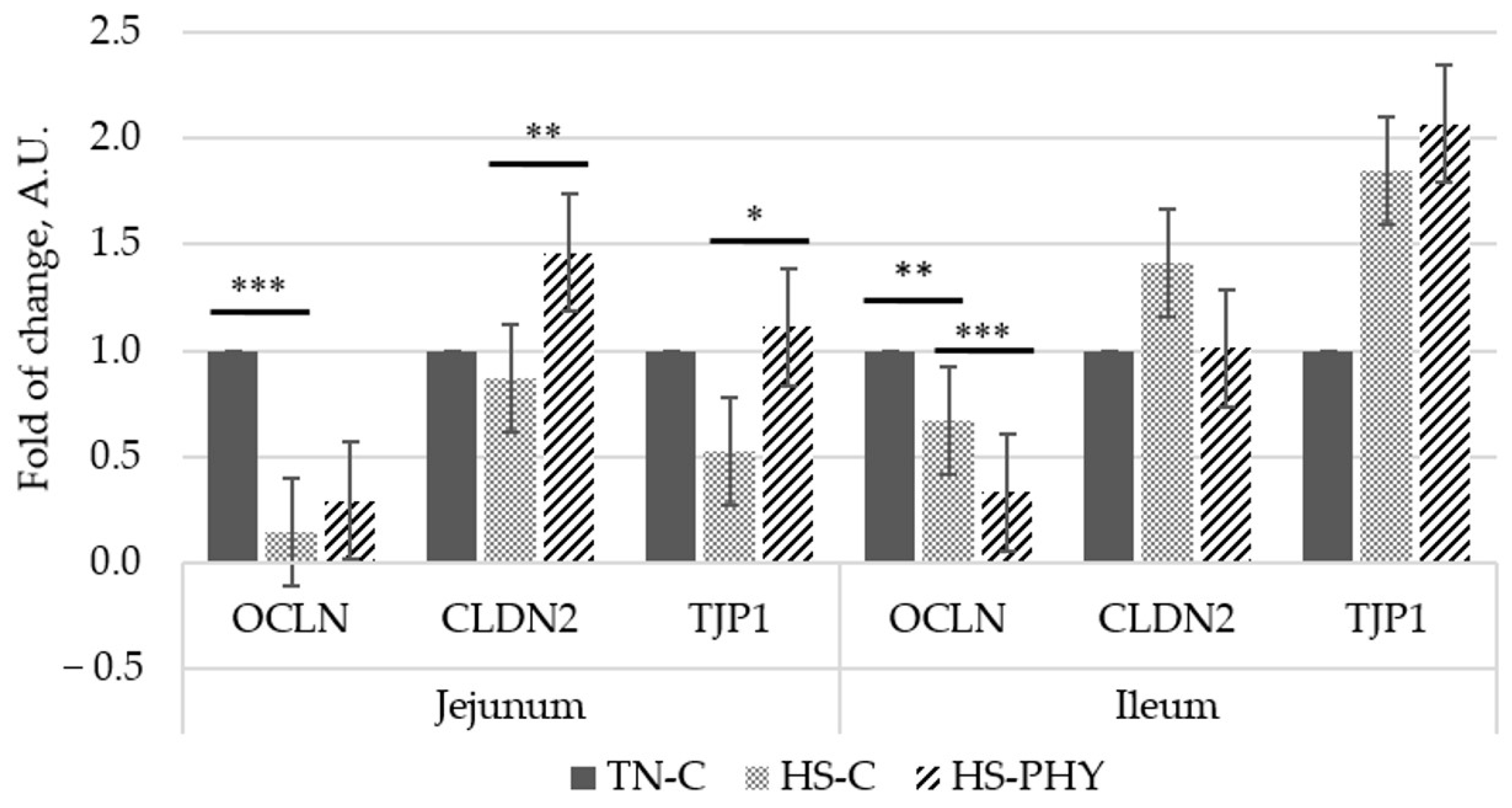
3.2. Tight Junction Proteins in Jejunum and Ileum
3.3. Apparent Ileal Digestibility (AID) of Essential and Non-Essential Amino Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-Term Exposure to Heat Stress Attenuates Appetite and Intestinal Integrity in Growing Pigs1. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, M.; Cota, M.; Arce, N.; Castillo, G.; Avelar, E.; Espinoza, S.; Morales, A. Effect of Heat Stress on Performance and Expression of Selected Amino Acid and Glucose Transporters, HSP90, Leptin and Ghrelin in Growing Pigs. J. Therm. Biol. 2016, 59, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Keating, A.; Ross, J.W.; Rhoads, R.P. Effects of Heat Stress on the Immune System, Metabolism and Nutrient Partitioning: Implications on Reproductive Success. Rev. Bras. Reprod. Anim. 2015, 39, 173–183. [Google Scholar]
- Collin, A.; Van Milgen, J.; Dubois, S.; Noblet, J. Effect of High Temperature and Feeding Level on Energy Utilization in Piglets. J. Anim. Sci. 2001, 79, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive Oxygen Species, Heat Stress and Oxidative-Induced Mitochondrial Damage. A Review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Kikusato, M.; Toyomizu, M. Crucial Role of Membrane Potential in Heat Stress-Induced Overproduction of Reactive Oxygen Species in Avian Skeletal Muscle Mitochondria. PLoS ONE 2013, 8, e0064412. [Google Scholar] [CrossRef]
- Morales, A.; Pérez, M.; Castro, P.; Ibarra, N.; Bernal, H.; Baumgard, L.H.; Cervantes, M. Heat Stress Affects the Apparent and Standardized Ileal Digestibilities of Amino Acids in Growing Pigs. J. Anim. Sci. 2016, 94, 3362–3369. [Google Scholar] [CrossRef]
- Cervantes, M.; Ibarra, N.; Vásquez, N.; Reyes, F.; Avelar, E.; Espinoza, S.; Morales, A. Serum Concentrations of Free Amino Acids in Growing Pigs Exposed to Diurnal Heat Stress Fluctuations. J. Therm. Biol. 2017, 69, 69–75. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat Stress and Reduced Plane of Nutrition Decreases Intestinal Integrity and Function in Pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef]
- Gabler, N.K.; Koltes, D.; Schaumberger, S.; Murugesan, G.R.; Reisinger, N. Diurnal Heat Stress Reduces Pig Intestinal Integrity and Increases Endotoxin Translocation. Transl. Anim. Sci. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Habimana, V.; Nguluma, A.S.; Nziku, Z.C.; Ekine-Dzivenu, C.C.; Morota, G.; Mrode, R.; Chenyambuga, S.W. Heat Stress Effects on Milk Yield Traits and Metabolites and Mitigation Strategies for Dairy Cattle Breeds Reared in Tropical and Sub-Tropical Countries. Front. Vet. Sci. 2023, 7, 1121499. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Hosseindoust, A.; Ha, S.H.; Mun, J.Y.; Moturi, J.; Tajudeen, H.; Choi, Y.H.; Lee, S.H.; Kim, J.S. Importance of Dietary Supplementation of Soluble and Insoluble Fibers to Sows Subjected to High Ambient Temperatures during Late Gestation and Effects on Lactation Performance. Anim. Nutr. 2023, 14, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat Stress and Poultry Production: Impact and Amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Chávez, M.; Vásquez, N.; Htoo, J.K.; Buenabad, L.; Espinoza, S.; Cervantes, M. Increased Dietary Protein or Free Amino Acids Supply for Heat Stress Pigs: Effect on Performance and Carcass Traits. J. Anim. Sci. 2018, 96, 1419–1429. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M.; et al. Nutritional Manipulation to Combat Heat Stress in Poultry—A Comprehensive Review. J. Therm. Biol. 2021, 98, 102915. [Google Scholar] [CrossRef]
- Pratel, J.A.M. Impact of Heat Stress on Carcass Traits, Meat Quality, and Nutritional Value in Monogastric Animals: Underlying Mechanisms and Nutritional Mitigation Strategies. Foods 2025, 14, 1612. [Google Scholar] [CrossRef]
- Sakkas, P. The Effects of Feed Additives on Farm Animals Under Heat Stress Conditions. In Sustainable Use of Feed Additives in Livestock; Arsenos, G., Giannenas, I., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 285–326. ISBN 9783031428555. [Google Scholar]
- Cervantes, M.; Sakkas, P.; Soto, M.; Gómez, A.J.; Camacho, R.L.; Arce, N.; Quilichini, N.; Morales, A. A Capsaicin-Based Phytogenic Solution Improves Performance and Thermal Tolerance of Heat-Stressed Growing Pigs. Animals 2024, 14, 973. [Google Scholar] [CrossRef]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef]
- Prakash, U.N.S.; Srinivasan, K. Fat Digestion and Absorption in Spice-Pretreated Rats. J. Sci. Food Agric. 2012, 92, 503–510. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Influence of Dietary Spices or Their Active Principles on Digestive Enzymes of Small Intestinal Mucosa in Rats. Int. J. Food Sci. Nutr. 1996, 47, 55–59. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, B.; Friesen, M.; Liu, S.; Zhu, C.; Yang, C. Capsaicin Attenuates Lipopolysaccharide-Induced Inflammation and Barrier Dysfunction in Intestinal Porcine Epithelial Cell Line-J2. Front. Physiol. 2021, 12, 715469. [Google Scholar] [CrossRef]
- Long, S.; Liu, S.; Wang, J.; Mahfuz, S.; Piao, X. Natural Capsicum Extract Replacing Chlortetracycline Enhances Performance via Improving Digestive Enzyme Activities, Antioxidant Capacity, Anti-Inflammatory Function, and Gut Health in Weaned Pigs. Anim. Nutr. 2021, 7, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Nom-062-Zoo Norma Oficial Mexicana. Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio. Nom-062-Zoo 1999, 477, 1–58. [Google Scholar]
- Rothfusz, L.P. NWS Southern Region Headquarters. In The Heat Index Equation (or, More Than You Ever Wanted to Know About Heat Index); National Oceanic and Atmospheric Administration: Fort Worth, TX, USA, 1990; pp. 23–90. [Google Scholar]
- National Research Council. Nutrient Requirements of Swine, 12th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Sunde, J.; Kiessling, A.; Higgs, D.; Opstvedt, J.; Venturini, G.; Rungruangsaktorrissen, K. Evaluation of Feed Protein Quality by Measuring Plasma Free Amino Acids in Atlantic Salmon (Salmo salar L.) after Dorsal Aorta Cannulation. Aquac. Nutr. 2003, 9, 351–360. [Google Scholar] [CrossRef][Green Version]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989; Volume 1, pp. 931–957. [Google Scholar]
- Sauer, W.C.; Ozimek, L. Digestibility of Amino Acids in Swine: Results and Their Practical Applications. A Review. Livest. Prod. Sci. 1986, 15, 367–388. [Google Scholar] [CrossRef]
- Fenton, T.W.; Fenton, M. An Improved Procedure for the Determination of Chromic Oxide in Feed and Feces. Can. J. Anim. Sci. 1979, 59, 631–634. [Google Scholar] [CrossRef]
- Vásquez, N.; Cervantes, M.; Bernal-Barragán, H.; Rodríguez-Tovar, L.E.; Morales, A. Short- and Long-Term Exposure to Heat Stress Differently Affect Performance, Blood Parameters, and Integrity of Intestinal Epithelia of Growing Pigs. Animals 2022, 12, 2529. [Google Scholar] [CrossRef]
- Ogoh, S.; Sato, K.; Okazaki, K.; Miyamoto, T.; Hirasawa, A.; Morimoto, K.; Shibasaki, M. Blood Flow Distribution during Heat Stress: Cerebral and Systemic Blood Flow. J. Cereb. Blood Flow Metab. 2013, 33, 1915–1920. [Google Scholar] [CrossRef]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of Heat Stress on the Porcine Small Intestine: A Morphological and Gene Expression Study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 119–128. [Google Scholar] [CrossRef]
- Fan, M.Z.; Matthews, J.C.; Etienne, N.M.P.; Stoll, B.; Lackeyram, D.; Burrin, D.G. Expression of Apical Membrane L-Glutamate Transporters in Neonatal Porcine Epithelial Cells along the Small Intestinal Crypt-Villus Axis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G385–G398. [Google Scholar] [CrossRef]
- Bröer, S. Amino Acid Transport across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Yen, J.T.; Kerr, B.J.; Easter, R.A.; Parkhurst, A.M. Difference in Rates of Net Portal Absorption between Crystalline and Protein-Bound Lysine and Threonine in Growing Pigs Fed Once Daily. J. Anim. Sci. 2004, 82, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Moënnoz, D.; Montigon, F.; Fay, L.B.; Breuillé, D.; Finot, P.A.; Ballèvre, O.; Boza, J. Development of a Rapid and Convenient Method to Purify Mucins and Determine Their in Vivo Synthesis Rate in Rats. Anal. Biochem. 2002, 307, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Bar-Noy, S.; Williams, W.M.; Requena, J.; Berlett, B.S.; Stadtman, E.R. Methionine Sulfoxide Reductase (MsrA) Is a Regulator of Antioxidant Defense and Lifespan in Mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 12920–12925. [Google Scholar] [CrossRef] [PubMed]
- Schindeldecker, M.; Moosmann, B. Protein-Borne Methionine Residues as Structural Antioxidants in Mitochondria. Amino Acids 2015, 47, 1421–1432. [Google Scholar] [CrossRef]
- Ozorio, L.; Mellinger-Silva, C.; Cabral, L.M.C.; Jardin, J.; Boudry, G.; Dupont, D. The Influence of Peptidases in Intestinal Brush Border Membranes on the Absorption of Oligopeptides from Whey Protein Hydrolysate: An Ex Vivo Study Using an Ussing Chamber. Foods 2020, 9, 1415. [Google Scholar] [CrossRef]
- Gelberg, H.B. Comparative Anatomy, Physiology, and Mechanisms of Disease Production of the Esophagus, Stomach, and Small Intestine. Toxicol. Pathol. 2014, 42, 54–66. [Google Scholar] [CrossRef]
| Ingredient | Basal | PHY-Supplemented |
|---|---|---|
| Wheat | 84.46 | 84.26 |
| Soybean meal | 12 | 12 |
| L-Lysine.HCl | 0.54 | 0.54 |
| L-Threonine | 0.14 | 0.14 |
| DL-Methionine | 0.06 | 0.06 |
| Phytogenic solution 1 | 0.20 | |
| Ca carbonate | 1.40 | 1.40 |
| Di-Ca phosphate | 0.65 | 0.65 |
| Iodized salt | 0.35 | 0.35 |
| Vitamin and mineral premix 2 | 0.40 | 0.40 |
| Calculated composition, % | ||
| Net energy, MJ/kg | 10.10 | 10.10 |
| Standardized ileal digestible amino acid (SID), % | ||
| SID Arginine | 0.85 | 0.85 |
| SID Histidine | 0.38 | 0.38 |
| SID Isoleucine | 0.57 | 0.57 |
| SID Leucine | 1.06 | 1.06 |
| SID Lysine | 0.98 | 0.98 |
| SID Methionine | 0.28 | 0.28 |
| SID Methionine + Cysteine | 0.57 | 0.57 |
| SID Phenylalanine | 0.71 | 0.71 |
| SID Threonine | 0.62 | 0.62 |
| SID Tryptophan | 0.19 | 0.19 |
| SID Valine | 0.66 | 0.66 |
| mRNA | Primer Sequence | Amplicon (bp) |
|---|---|---|
| Sus scrofa claudin 2, mRNA (CLDN2, GenBank: NM_001161638.1) | ||
| Fw 5′AGCTGGCGAACGAGTTCTTA3′ | 343 | |
| Rv 5′TCCCATGAAGATTCCACGCA3′ | ||
| Sus scrofa occludin, mRNA (OCLN. GenBank: NM_001163647.2) | ||
| Fw 5′AGGCGTCAGGGTCTCTCTAC3′ | 347 | |
| Rv 5′CTCCGCATAGTCCGAAAGGG3′ | ||
| PREDICTED: Sus scrofa tight junction protein 1, transcript variant X1, mRNA (TJP1; GenBank: XM_021098827.1) | ||
| Fw 5′TGGTATGGGTTTCTGAGGGGA3′ | 251 | |
| Rv 5′AGGCTCAGAGGACCGTGTAA3′ | ||
| Sus scrofa cytoskeletal beta actin mRNA, partial cds (GenBank: AY550069.1) | ||
| Fw 5′ACAGCAGTCGGTTGGATGG3′ | 311 | |
| Rv 5′TGCCCACTCAAAATAAACCAAC3′ | ||
| Treatment | Contrast p-Value 1 | |||||
|---|---|---|---|---|---|---|
| Item | TN-C | HS-C | HS-PHY | SEM | AT | PHY |
| Essential AA | ||||||
| Arginine | 51.4 | 38.5 | 42.9 | 2.6 | 0.005 | 0.256 |
| Histidine | 11.0 | 7.0 | 11.9 | 0.4 | 0.001 | 0.001 |
| Isoleucine | 20.0 | 18.2 | 19.7 | 0.9 | 0.200 | 0.279 |
| Leucine | 28.8 | 23.4 | 30.6 | 1.6 | 0.039 | 0.010 |
| Lysine | 49.3 | 36.0 | 46.6 | 2.5 | 0.003 | 0.012 |
| Methionine | 8.5 | 4.0 | 8.5 | 0.6 | 0.001 | 0.001 |
| Phenylalanine | 19.9 | 11.7 | 22.4 | 0.7 | 0.001 | 0.001 |
| Threonine | 37.9 | 15.6 | 35.5 | 1.9 | 0.001 | 0.001 |
| Tryptophan | 12.0 | 9.0 | 12.6 | 0.6 | 0.004 | 0.001 |
| Valine | 39.9 | 29.1 | 42.2 | 1.6 | 0.001 | 0.001 |
| Non-essential AA | ||||||
| Alanine | 104.7 | 91.1 | 113.1 | 5.1 | 0.083 | 0.010 |
| Aspartate | 5.5 | 4.6 | 6.0 | 0.6 | 0.347 | 0.154 |
| Asparagine | 15.7 | 9.2 | 16.3 | 1.2 | 0.002 | 0.001 |
| Glutamate | 36.5 | 41.3 | 35.3 | 4.8 | 0.502 | 0.400 |
| Glutamine | 101.8 | 79.8 | 103.8 | 6.1 | 0.026 | 0.017 |
| Glycine | 53.2 | 61.3 | 61.3 | 3.5 | 0.124 | 0.994 |
| Proline | 84.7 | 53.8 | 83.1 | 5.4 | 0.001 | 0.002 |
| Serine | 23.4 | 15.8 | 25.5 | 1.8 | 0.010 | 0.002 |
| Tyrosine | 22.5 | 13.9 | 26.0 | 1.4 | 0.001 | 0.001 |
| Treatment 1 | Contrast p-Value 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | TN-C | TN-PHY | HS-C | HS-PHY | SEM | AT | PHY | AT-PHY |
| Crude protein | 93.18 | 93.45 | 91.38 | 90.44 | 0.78 | 0.020 | 0.709 | 0.512 |
| EAA | ||||||||
| Arginine | 90.31 | 90.36 | 87.60 | 85.23 | 1.16 | 0.025 | 0.337 | 0.320 |
| Histidine | 88.95 | 89.23 | 86.32 | 84.84 | 1.45 | 0.033 | 0.687 | 0.555 |
| Isoleucine | 85.23 | 86.66 | 82.12 | 80.15 | 1.74 | 0.033 | 0.896 | 0.412 |
| Leucine | 85.88 | 87.12 | 82.87 | 81.23 | 1.64 | 0.036 | 0.914 | 0.458 |
| Lysine | 87.30 | 88.61 | 85.56 | 83.36 | 1.57 | 0.074 | 0.808 | 0.344 |
| Methionine | 89.54 | 90.43 | 87.42 | 86.23 | 1.33 | 0.063 | 0.926 | 0.512 |
| Phenylalanine | 86.58 | 87.69 | 83.29 | 81.84 | 1.54 | 0.023 | 0.923 | 0.481 |
| Threonine | 81.32 | 83.08 | 77.83 | 75.57 | 2.21 | 0.045 | 0.921 | 0.440 |
| Tryptophan | 85.56 | 87.29 | 82.15 | 79.88 | 1.68 | 0.015 | 0.888 | 0.314 |
| Valine | 82.99 | 84.38 | 79.22 | 77.00 | 2.01 | 0.032 | 0.859 | 0.447 |
| NEAA | ||||||||
| Alanine | 82.6 | 88.2 | 86.7 | 85.5 | 2.1 | 0.745 | 0.317 | 0.125 |
| Aspartate | 51.8 | 31.9 | 26.0 | 16.3 | 12.1 | 0.113 | 0.246 | 0.680 |
| Cysteine | 92.0 | 98.9 | 98.5 | 98.4 | 2.0 | 0.135 | 0.110 | 0.100 |
| Glutamate | 75.5 | 55.6 | 50.7 | 45.6 | 7.4 | 0.037 | 0.384 | 0.632 |
| Glycine | 82.7 | 88.7 | 85.5 | 82.9 | 2.0 | 0.504 | 0.446 | 0.070 |
| Proline | 83.9 | 80.3 | 73.1 | 71.4 | 3.1 | 0.013 | 0.449 | 0.790 |
| Serine | 82.7 | 80.4 | 74.3 | 71.1 | 2.6 | 0.010 | 0.364 | 0.887 |
| Tyrosine | 93.2 | 99.7 | 99.6 | 99.6 | 1.9 | 0.127 | 0.123 | 0.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes, M.; Sakkas, P.; Valle, J.A.; Arce, N.; Avelar, E.; Quilichini, N.; Morales, A. Effects of a Capsaicin-Based Phytogenic Solution on Intestinal Permeability, Serum Amino Acid Concentrations, and Digestibility in Heat-Stressed Growing Pigs. Animals 2025, 15, 1757. https://doi.org/10.3390/ani15121757
Cervantes M, Sakkas P, Valle JA, Arce N, Avelar E, Quilichini N, Morales A. Effects of a Capsaicin-Based Phytogenic Solution on Intestinal Permeability, Serum Amino Acid Concentrations, and Digestibility in Heat-Stressed Growing Pigs. Animals. 2025; 15(12):1757. https://doi.org/10.3390/ani15121757
Chicago/Turabian StyleCervantes, Miguel, Panagiotis Sakkas, José A. Valle, Néstor Arce, Ernesto Avelar, Nicolas Quilichini, and Adriana Morales. 2025. "Effects of a Capsaicin-Based Phytogenic Solution on Intestinal Permeability, Serum Amino Acid Concentrations, and Digestibility in Heat-Stressed Growing Pigs" Animals 15, no. 12: 1757. https://doi.org/10.3390/ani15121757
APA StyleCervantes, M., Sakkas, P., Valle, J. A., Arce, N., Avelar, E., Quilichini, N., & Morales, A. (2025). Effects of a Capsaicin-Based Phytogenic Solution on Intestinal Permeability, Serum Amino Acid Concentrations, and Digestibility in Heat-Stressed Growing Pigs. Animals, 15(12), 1757. https://doi.org/10.3390/ani15121757







