Efficacy of Feeding Grape By-Products on Performance, Nutrient Digestibility, Gut Morphology, Gut Microbial Community, Oxidative Stress and Immune Response in Fast-Growing Broilers
Simple Summary
Abstract
1. Introduction
2. Methods
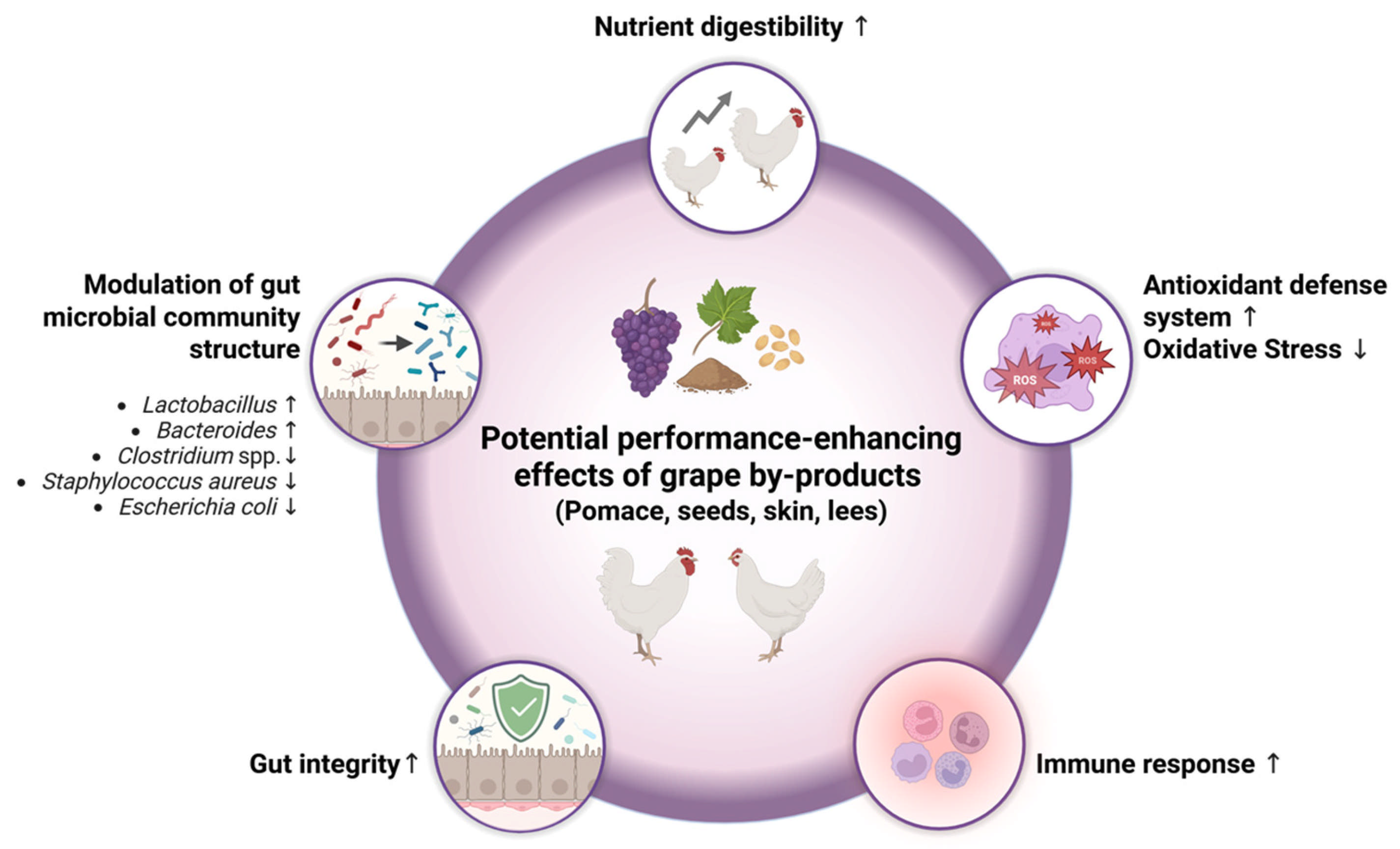
3. Effects of Grape By-Products on Fast-Growing Broilers
3.1. Effect of Grape By-Products on Growth Performance
3.2. Effect of Grape By-Products on Nutrient Digestibility
3.3. Effect of Grape By-Products on Gut Morphology and Integrity
3.4. Effect of Grape By-Products on Gut Microbial Community
3.5. Effect of Grape By-Products on Oxidative Stress and Antioxidant Status
3.6. Effect of Grape By-Products on Immune Response
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kazemi, M. Recycling Agricultural Waste: Sustainable Solutions for Enhancing Livestock Nutrition. Vet. Med. Sci. 2025, 11, e70321. [Google Scholar] [CrossRef] [PubMed]
- FAO. The future of food and agriculture—Alternative pathways to 2050|Global Perspectives Studies|Food and Agriculture Organization of the United Nations. 2018. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/e51e0cf0-4ece-428c-8227-ff6c51b06b16/content (accessed on 17 March 2025).
- OECD/FAO (2024) OECD-FAO Agricultural Outlook 2024–2033, Paris and Rome. Available online: https://www.oecd.org/en/publications/2024/07/oecd-fao-agricultural-outlook-2024-2033_e173f332.html (accessed on 17 March 2025).
- IOVW (International Organisation of Vine and Wine). State of the World Vine and Wine Sector in 2023; OIV: Dijon, France, 2024. [Google Scholar]
- Karastergiou, A.; Gancel, A.L.; Jourdes, M.; Teissedre, P.L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crop Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Costa, M.M.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A.M. Grape By-Products as Feedstuff for Pig and Poultry Production. Animals 2022, 12, 2239. [Google Scholar] [CrossRef]
- Kumar, H.; Guleria, S.; Kimta, N.; Nepovimova, E.; Dhalaria, R.; Dhanjal, D.S.; Sethi, N.; Alomar, S.Y.; Kuca, K. Selected fruit pomaces: Nutritional profile, health benefits, and applications in functional foods and feeds. Curr. Res. Food Sci. 2024, 9, 100791. [Google Scholar] [CrossRef]
- Peña-Portillo, G.C.; Acuña-Nelson, S.M.; Bastías-Montes, J.M. From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products-A Review. Antioxidants 2024, 13, 992. [Google Scholar] [CrossRef]
- Hegedüs, I.; Andreidesz, K.; Szentpéteri, J.L.; Kaleta, Z.; Szabó, L.; Szigeti, K.; Gulyás, B.; Padmanabhan, P.; Budan, F.; Máthé, D. The Utilization of Physiologically Active Molecular Components of Grape Seeds and Grape Marc. Int. J. Mol. Sci. 2022, 23, 11165. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic Compounds and Biopotential of Grape Pomace Extracts from Prokupac Red Grape Variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Korver, D.R.; Roura, E.; Klasing, K.C. Effect of dietary energy level and oil source on broiler performance and response to an inflammatory challenge. Poult. Sci. 1998, 77, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.H.; Merlot, E.; Damon, M.; Noblet, J.; Le Floc’h, N. High ambient temperature alleviates the inflammatory response and growth depression in pigs challenged with Escherichia coli lipopolysaccharide. Vet. J. 2014, 200, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Wyns, H.; Meyer, E.; Plessers, E.; Watteyn, A.; van Bergen, T.; Schauvliege, S.; De Baere, S.; Devreese, M.; De Backer, P.; Croubels, S. Modulation by gamithromycin and ketoprofen of in vitro and in vivo porcine lipopolysaccharide-induced inflammation. Vet. Immunol. Immunopathol. 2015, 168, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy. Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef]
- Pohl, C.S.; Medland, J.E.; Moeser, A.J. Early-life stress origins of gastrointestinal disease: Animal models, intestinal pathophysiology, and translational implications. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G927–G941. [Google Scholar] [CrossRef]
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J. Nutr. 1999, 129, 613–619. [Google Scholar] [CrossRef]
- Xing, T.; Luo, D.; Zhao, X.; Xu, X.; Li, J.; Zhang, L.; Gao, F. Enhanced cytokine expression and upregulation of inflammatory signaling pathways in broiler chickens affected by wooden breast myopathy. J. Sci. Food Agric. 2021, 101, 279–286. [Google Scholar] [CrossRef]
- Malila, Y.; Sanpinit, P.; Thongda, W.; Jandamook, A.; Srimarut, Y.; Phasuk, Y.; Kunhareang, S. Influences of Thermal Stress During Three Weeks Before Market Age on Histology and Expression of Genes Associated with Adipose Infiltration and Inflammation in Commercial Broilers, Native Chickens, and Crossbreeds. Front. Physiol. 2022, 13, 858735. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.E.; Kiepper, B.H.; Ritz, C.W.; McLendon, B.L.; Wilson, J.L. Growth, livability, feed consumption, and carcass composition of the Athens Canadian random bred 1955 meat-type chicken versus the 2012 high-yielding Cobb 500 broiler. Poult. Sci. 2014, 93, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, E.; Sosnicki, A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999, 78, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS ONE 2016, 11, e0153750. [Google Scholar] [CrossRef]
- Huber, K. Review: Welfare in farm animals from an animal-centred point of view. Animal 2024, 18, 101311. [Google Scholar] [CrossRef]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Velleman, S.G. Pectoralis Major (Breast) Muscle Extracellular Matrix Fibrillar Collagen Modifications Associated with the Wooden Breast Fibrotic Myopathy in Broilers. Front. Physiol. 2020, 11, 461. [Google Scholar] [CrossRef]
- Che, S.; Wang, C.; Varga, C.; Barbut, S.; Susta, L. Prevalence of breast muscle myopathies (spaghetti meat, woody breast, white striping) and associated risk factors in broiler chickens from Ontario Canada. PLoS ONE 2022, 17, e0267019. [Google Scholar] [CrossRef]
- de Almeida Mallmann, B.; Martin, E.M.; Soo Kim, K.; Calderon-Apodaca, N.L.; Baxter, M.F.A.; Latorre, J.D.; Hernandez-Velasco, X.; Paasch-Martinez, L.; Owens, C.M.; Dridi, S.; et al. Evaluation of Bone Marrow Adipose Tissue and Bone Mineralization on Broiler Chickens Affected by Wooden Breast Myopathy. Front. Physiol. 2019, 10, 674. [Google Scholar] [CrossRef]
- Wang, M.; He, Z.; Xiong, Z.; Liu, H.; Zhou, X.; He, J. Effects of dietary supplementation of grape seed extract in comparison with excessive level of vitamin E on growth performance and antioxidant function of broilers. Anim. Biotechnol. 2024, 35, 2331640. [Google Scholar] [CrossRef]
- Thema, K.K.; Mlambo, V.; Egbu, C.F.; Mnisi, C.M. Use of red grape pomace and Aloe vera gel as nutraceuticals to ameliorate stocking density-induced stress in commercial male broilers. Trop. Anim. Health Prod. 2024, 56, 107. [Google Scholar] [CrossRef] [PubMed]
- Shamkhi Noor, A.; Essa Al-Mashhdani, H.; Hasan Kadhim, A. Effects of Grape Seed Powder on Productive Performance, Lipid Profile and Total Bacteria in Duodenum and Ceca of Broiler Chickens. Arch. Razi Inst. 2022, 77, 2159–2164. [Google Scholar] [PubMed]
- Meng, W.S.; Zou, Q.; Xiao, Y.; Ma, W.; Zhang, J.; Wang, T.; Li, D. Growth performance and cecal microbiota of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation. Poult. Sci. 2023, 102, 102707. [Google Scholar] [CrossRef] [PubMed]
- de-Cara, A.; Saldaña, B.; Vázquez, P.; Rey, A.I. Dietary Protected Sodium Butyrate and/or Olive Leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers. Antioxidants 2023, 12, 201. [Google Scholar] [CrossRef]
- Pascual, A.; Pauletto, M.; Trocino, A.; Birolo, M.; Dacasto, M.; Giantin, M.; Bordignon, F.; Ballarin, C.; Bortoletti, M.; Pillan, G.; et al. Effect of the dietary supplementation with extracts of chestnut wood and grape pomace on performance and jejunum response in female and male broiler chickens at different ages. J. Anim. Sci. Biotechnol. 2022, 13, 102. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñi, I.; Centeno, C.; Sáyago-Ayerdy, S.G.; Arija, I.; Saura-Calixto, F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008, 87, 307–316. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Giamouri, E.; Myrtsi, E.D.; Evergetis, E.; Filippi, K.; Papapostolou, H.; Koulocheri, S.D.; Zoidis, E.; Pappas, A.C.; Koutinas, A.; et al. Antioxidant Status of Broiler Chickens Fed Diets Supplemented with Vinification By-Products: A Valorization Approach. Antioxidants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Romero, C.; Nardoia, M.; Arija, I.; Viveros, A.; Rey, A.I.; Prodanov, M.; Chamorro, S. Feeding Broiler Chickens with Grape Seed and Skin Meals to Enhance α- and γ-Tocopherol Content and Meat Oxidative Stability. Antioxidants 2021, 10, 699. [Google Scholar] [CrossRef]
- Chand, N.; Ali, P.; Alhidary, I.A.; Abdelrahman, M.A.; Albadani, H.; Khan, M.A.; Seidavi, A.; Laudadio, V.; Tufarelli, V.; Khan, R.U. Protective Effect of Grape (Vitis vinifera) Seed Powder and Zinc-Glycine Complex on Growth Traits and Gut Health of Broilers Following Eimeria tenella Challenge. Antibiotics 2021, 10, 186. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of raw and fermented grape seed on growth performance, antioxidant capacity, and cecal microflora in broiler chickens. Animal 2021, 15, 100194. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of Raw and Fermented Grape Pomace on the Growth Performance, Antioxidant Status, Intestinal Morphology, and Selected Bacterial Species in Broiler Chicks. Animals 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zeng, X.; Liu, J.; Yan, F.; Xiang, Z.; Wang, Y.; Tao, F.; Yang, C. Change of Serum Metabolome and Cecal Microflora in Broiler Chickens Supplemented with Grape Seed Extracts. Front. Immunol. 2020, 11, 610934. [Google Scholar] [CrossRef] [PubMed]
- Nardoia, M.; Romero, C.; Brenes, A.; Arija, I.; Viveros, A.; Ruiz-Capillas, C.; Chamorro, S. Addition of fermented and unfermented grape skin in broilers’ diets: Effect on digestion, growth performance, intestinal microbiota and oxidative stability of meat. Animal 2020, 14, 1371–1381. [Google Scholar] [CrossRef]
- Kumanda, C.; Mlambo, V.; Mnisi, C.M. Valorization of Red Grape Pomace Waste Using Polyethylene Glycol and Fibrolytic Enzymes: Physiological and Meat Quality Responses in Broilers. Animals 2019, 9, 779. [Google Scholar] [CrossRef]
- Aditya, S.; Ohh, S.J.; Ahammed, M.; Lohakare, J. Supplementation of grape pomace (Vitis vinifera) in broiler diets and its effect on growth performance, apparent total tract digestibility of nutrients, blood profile, and meat quality. Anim. Nutr. 2018, 4, 210–214. [Google Scholar] [CrossRef]
- Rajput, A.S.; Sun, L.; Zhang, N.; Mohamed Khalil, M.; Gao, X.; Ling, Z.; Zhu, L.; Khan, F.A.; Zhang, J.; Qi, D. Ameliorative Effects of Grape Seed Proanthocyanidin Extract on Growth Performance, Immune Function, Antioxidant Capacity, Biochemical Constituents, Liver Histopathology and Aflatoxin Residues in Broilers Exposed to Aflatoxin B1. Toxins 2017, 9, 371. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Ibrahim, S.A. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2018, 102, 268–275. [Google Scholar] [CrossRef]
- Farahat, M.H.; Abdallah, F.M.; Ali, H.A.; Hernandez-Santana, A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal 2017, 11, 771–777. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, H.J.; Wang, J.; Wu, S.G.; Yue, H.Y.; Jiang, X.R.; Qi, G.H. Effects of dietary grape proanthocyanidins on the growth performance, jejunum morphology and plasma biochemical indices of broiler chicks. Animal 2017, 11, 762–770. [Google Scholar] [CrossRef]
- Fejerčáková, A.; Vašková, J.; Bača, M.; Vaško, L.; Marcinčák, S.; Hertelyová, Z.; Petrášová, D.; Guothová, L. Effect of dietary microbially produced gamma-linolenic acid and plant extracts on enzymatic and non-enzymatic antioxidants in various broiler chicken organs. J. Anim. Physiol. Anim. Nutr. 2014, 98, 860–866. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I.; Brenes, A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 2013, 7, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.E.; McDevitt, R.M.; Acamovic, T. Herbs, thyme essential oil and condensed tannin extracts as dietary supplements for broilers, and their effects on performance, digestibility, volatile fatty acids and organoleptic properties. Br. Poult. Sci. 2011, 52, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Suo, X.; Gu, J.H.; Zhang, W.W.; Fang, Q.; Wang, X. Influence of grape seed proanthocyanidin extract in broiler chickens: Effect on chicken coccidiosis and antioxidant status. Poult. Sci. 2008, 87, 2273–2280. [Google Scholar] [CrossRef]
- Erinle, T.J.; Oladokun, S.; MacIsaac, J.; Rathgeber, B.; Adewole, D. Dietary grape pomace—Effects on growth performance, intestinal health, blood parameters, and breast muscle myopathies of broiler chickens. Poult. Sci. 2022, 101, 101519. [Google Scholar] [CrossRef]
- Goñi, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebolé, A.; Arija, I.; Estevez, R. Effect of dietary grape pomace and vitamin E on growth performance, nutrient digestibility, and susceptibility to meat lipid oxidation in chickens. Poult. Sci. 2007, 86, 508–516. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñi, I.; Centeno, C.; Saura-Calixto, F.; Arija, I. Effect of grape seed extract on growth performance, protein and polyphenol digestibilities, and antioxidant activity in chickens. Span. J. Agric. Res. 2010, 2, 326–333. [Google Scholar] [CrossRef]
- Ortiz, L.T.; Centeno, C.; Treviño, J. Tannin in faba bean seeds: Effects on the digestion of protein and amino acids in growing chicks. Anim. Feed. Sci. Technol. 1993, 41, 271–278. [Google Scholar] [CrossRef]
- Grala, W.; Verstegen, M.W.; Jansman, A.J.; Huisman, J.; Wasilewko, J. Nitrogen utilization in pigs fed diets with soybean and rapeseed products leading to different ileal endogenous nitrogen losses. J. Anim. Sci. 1998, 76, 569–577. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Rebolé, A.; Arija, I.; Romero, C.; Alvarez, I.; Rey, A.; Brenes, A. Addition of exogenous enzymes to diets containing grape pomace: Effects on intestinal utilization of catechins and antioxidant status of chickens. Food Res. Int. 2017, 96, 226–234. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvárez, I.; Vega, I.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Salinas, J.R.; Bulnes, P.; Zuñiga, M.C.; Perez-Jimenez, J.; Torres, J.L.; Mateos-Martin, M.L.; Agosin, E.; Perez-Correa, J.R. Effect of pressurized hot water extraction on antioxidants from grape pomace before and after enological fermentation. J. Agric. Food Chem. 2013, 61, 6929–6936. [Google Scholar] [CrossRef] [PubMed]
- Travaglia, F.; Bordiga, M.; Locatelli, M.; Coïson, J.D.; Arlorio, M. Polymeric proanthocyanidins in skins and seeds of 37 Vitis vinifera L. cultivars: A methodological comparative study. J. Food Sci. 2011, 76, C742–C749. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Abia, R.; Eastwood, M.A.; Saura-Calixto, F. Degradation of polyphenols (catechin and tannic acid) in the rat intestinal tract. Effect on colonic fermentation and faecal output. Br. J. Nutr. 1994, 71, 933–946. [Google Scholar] [CrossRef]
- Roy, D.M.; Schneeman, B.O. Effect of soy protein, casein and trypsin inhibitor on cholesterol, bile acids and pancreatic enzymes in mice. J. Nutr. 1981, 111, 878–885. [Google Scholar] [CrossRef]
- Chamorro, S.; Romero, C.; Brenes, A.; Sánchez-Patán, F.; Bartolomé, B.; Viveros, A.; Arija, I. Impact of a sustained consumption of grape extract on digestion, gut microbial metabolism and intestinal barrier in broiler chickens. Food Funct. 2019, 10, 1444–1454. [Google Scholar] [CrossRef]
- Duangnumsawang, Y.; Zentek, J.; Vahjen, W.; Tarradas, J.; Goodarzi Boroojeni, F. Alterations in bacterial metabolites, cytokines, and mucosal integrity in the caecum of broilers caused by feed additives and host-related factors. Front. Physiol. 2022, 13, 935870. [Google Scholar] [CrossRef]
- Duangnumsawang, Y.; Zentek, J.; Vahjen, W.; Tarradas, J.; Boroojeni, F.G. Impact of feed additives and host-related factors on bacterial metabolites, mucosal integrity and immune response in the ileum of broilers. Vet. Res. Commun. 2023, 47, 1861–1878. [Google Scholar] [CrossRef]
- Yvon, S.; Beaumont, M.; Dayonnet, A.; Eutamène, H.; Lambert, W.; Tondereau, V.; Chalvon-Demersay, T.; Belloir, P.; Paës, C. Effect of diet supplemented with functional amino acids and polyphenols on gut health in broilers subjected to a corticosterone-induced stress. Sci. Rep. 2024, 14, 1032. [Google Scholar] [CrossRef]
- Adámez, J.D.; Samino, E.G.; Sánchez, E.V.; González-Gómez, D. In vitro estimation of the antibacterial activity and antioxidant capacity of aqueous extracts from grapeseeds (Vitis vinifera L.). Food Control 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhi, F. Lower level of Bacteroides in the gut microbiota is associated with inflammatory bowel disease: A meta-analysis. Biomed. Res. Int. 2016, 2016, 5828959. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Mitomo, S.; Yuki, H.; Araki, M.; Seegers, L.M.; McNulty, I.; Lee, H.; Kuter, D.; Ishibashi, M.; Kobayashi, K.; et al. Gut Microbiota and Coronary Plaque Characteristics. J. Am. Heart Assoc. 2022, 11, e026036. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Koren, O.; Goodrich, J.K.; Johansson, M.E.; Nalbantoglu, I.; Aitken, J.D.; Su, Y.; Chassaing, B.; Walters, W.A.; González, A.; et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 2012, 12, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Hameed, I.H.; Hamza, L.F.; Kamal, S.A. Analysis of bioactive chemical compounds of Aspergillus niger by using gas chromatography-mass spectrometry and fouriertransform infrared spectroscopy. J. Pharmacog Phytother. 2015, 7, 132–163. [Google Scholar]
- Naidoo, V.; McGaw, L.J.; Bisschop, S.P.; Duncan, N.; Eloff, J.N. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008, 153, 214–219. [Google Scholar] [CrossRef]
- Makri, S.; Kafantaris, I.; Stagos, D.; Chamokeridou, T.; Petrotos, K.; Gerasopoulos, K.; Mpesios, A.; Goutzourelas, N.; Kokkas, S.; Goulas, P.; et al. Novel feed including bioactive compounds from winery wastes improved broilers’ redox status in blood and tissues of vital organs. Food Chem. Toxicol. 2017, 102, 24–31. [Google Scholar] [CrossRef]
- Rajput, S.A.; Sun, L.; Zhang, N.Y.; Khalil, M.M.; Ling, Z.; Chong, L.; Wang, S.; Rajput, I.R.; Bloch, D.M.; Khan, F.A.; et al. Grape Seed Proanthocyanidin Extract Alleviates AflatoxinB1;-Induced Immunotoxicity and Oxidative Stress via Modulation of NF-κB and Nrf2 Signaling Pathways in Broilers. Toxins 2019, 11, 23. [Google Scholar] [CrossRef]
- Vossen, E.; Ntawubizi, M.; Raes, K.; Smet, K.; Huyghebaert, G.; Arnouts, S.; De Smet, S. Effect of dietary antioxidant supplementation on the oxidative status of plasma in broilers. J. Anim. Physiol. Anim. Nutr. 2011, 95, 198–205. [Google Scholar] [CrossRef]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Șoica, C.; Iuga, M.; Mironeasa, S. Effects of Supplementing Grape Pomace to Broilers Fed Polyunsaturated Fatty Acids Enriched Diets on Meat Quality. Animals 2020, 10, 947. [Google Scholar] [CrossRef]
- Bennato, F.; Di Luca, A.; Martino, C.; Ianni, A.; Marone, E.; Grotta, L.; Ramazzotti, S.; Cichelli, A.; Martino, G. Influence of Grape Pomace Intake on Nutritional Value, Lipid Oxidation and Volatile Profile of Poultry Meat. Foods 2020, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, L.; Grundmann, S.M.; Friedrichs, S.; Lütjohann, D.; Höring, M.; Liebisch, G.; Most, E.; Ringseis, R.; Eder, K. Replacement of soybean oil by Hermetia illucens larvae fat in broiler diets alters the breast muscle lipidome and reduces lipid oxidation of the breast muscle during heat-processing. Arch. Anim. Nutr. 2023, 77, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Simitzis, P.E.; Kyriakaki, P.; Giamouri, E.; Myrtsi, E.D.; Evergetis, E.; Filippi, K.; Papapostolou, H.; Koulocheri, S.D.; Pappas, A.C.; et al. Immune-Related Gene Expression Profiling of Broiler Chickens Fed Diets Supplemented with Vinification Byproducts: A Valorization Approach II. Animals 2021, 11, 3038. [Google Scholar] [CrossRef]
- Chen, K.; Fang, J.; Peng, X.; Cui, H.; Chen, J.; Wang, F.; Chen, Z.; Zuo, Z.; Deng, J.; Lai, W. Effect of selenium supplementation on aflatoxin B1-induced histopathological lesions and apoptosis in bursa of fabricius in broilers. Food Chem. Toxicol. 2014, 74, 91–97. [Google Scholar] [CrossRef]
- Virden, W.S.; Lilburn, M.S.; Thaxton, J.P.; Corzo, A.; Hoehler, D.; Kidd, M.T. The effect of corticosterone-induced stress on amino acid digestibility in Ross broilers. Poult. Sci. 2007, 86, 338–342. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Eder, K. The Gut-Liver Axis in the Control of Energy Metabolism and Food Intake in Animals. Annu. Rev. Anim. Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Z.; Wang, G.; Li, Y.; Qi, Y. Effects of feed supplemented with fermented pine needles (Pinus ponderosa) on growth performance and antioxidant status in broilers. Poult. Sci. 2015, 94, 1138–1144. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Cao, F.; Ahmad, H.; Yang, X.; Zhao, L.; Wang, T. Effects of dietary supplementation with fermented ginkgo leaves on antioxidant capacity, intestinal morphology and microbial ecology in broiler chicks. Br. Poult. Sci. 2015, 56, 370–380. [Google Scholar] [CrossRef]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. Biomed. Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef]
- Eder, K.; Ringseis, R.; Gessner, D.K. Effects of Grape By-Products on Oxidative Stress and Inflammation in Farm Animals: An Overview of Studies Performed in Pigs, Chickens, and Cattle. Animals 2025, 15, 1536. [Google Scholar] [CrossRef]

| Broiler Breed and Sex | Grape By-Product | Dose (g/kg Diet) | Polyphenol Content (per kg Diet) | Duration (Days) | Main Effects | Ref. |
|---|---|---|---|---|---|---|
| Ross 308, male | GS extract | 0.1 | - | 42 | 1–42 d of age: FI: ↑, BW gain: ↑, F:G ratio: ↓ | [33] |
| Ross 308, male | GP | 30 | - | 28 | 14–42 d of age: FI: -, BW gain: ↑, F:G ratio: ↓ | [34] |
| Ross 308, unsexed | GS | 10, 20 or 30 | - | 42 | 22–42 d of age: FI: ↑ (20 and 30 g/kg diet), BW gain: ↑ (20 and 30 g/kg diet), F:G ratio: - | [35] |
| Arbor acres, unsexed | Herbal extract blend | 1.5 | - | 42 | 1–42 d of age: FI: -, BW gain: ↑, F:G ratio: ↓ | [36] |
| Ross 308, male | Olive leaf (62%) and grape- based by-product (24%) | 2 | GAE: 14 mg | 40 | 1–40 d of age: FI: -, BW gain: -, F:G ratio: - | [37] |
| Ross 308, mixed sex | GP extract | 2 | GAE: 154 mg | 44 | 1–44 d of age: FI: -, BW gain: ↑, F:G ratio: - | [38] |
| Cobb-500, mixed sex | GP | 25 | GAE: 2.18, 1.95 and 2.08 g in starter, grower and finisher diets | 42 | 1–42 d of age: FI: -, BW gain: ↑, F:G ratio: - | [39] |
| Ross 308, unsexed | GP, wine lees extract (WLE) or grape stem extract (GSE) | 25 (GP), 2 (WLE) or 0.1 (GSE) | GAE: 193 mg (GP), 7.3 mg (WLE) and 10 mg (GSE) | 42 | GP, WLE, and GSE: 1–42 d of age: FI: -, BW gain: -, F:G ratio: - | [40] |
| Cobb 500, male | GS or GK | 30 (GS) or 110 (GK) | TEP: 4.09 g (GS) and 4.18 g (GK) | 21 | GS: 1–21 d of age: FI: -, BW gain: -, F:G ratio: -; GK: 1–21 d of age: FI: -, BW gain: ↓, F:G ratio: ↑ | [41] |
| Hubbard, male | GS | 2.5 or 5 (together with organic zinc) | - | 35 | 8–35 d of age: FI: ↑, BW gain: ↓, F:G ratio: ↓ | [42] |
| Ross 308, female | Fermented GP (FGP) or unfermented GP (GP) | 5 | Total phenolic compounds: 142.5 µg (FGP) and 117 µg (GP) | 42 | FGP and GP: 1–42 d of age: FI: -, BW gain: ↑, F:G ratio: - | [43] |
| Ross 308, male | Fermented GP (FGP) or unfermented GP (GP) | 15 | - | 42 | FGP: 1–42 d of age: FI: -, BW gain: ↑, F:G ratio: -; GP: 1–42 d of age: FI: -, BW gain: -, F:G ratio: - | [44] |
| Arbor Acres Plus | GS extract | 0.2 or 0.4 | - | 21 | 0.2 g/kg diet: 1–21 d of age: FI: ↓, BW gain: ↑, F:G ratio: ↓; 0.4 g/kg diet: 1–21 d of age: FI: ↓, BW gain: -, F:G ratio: ↓ | [45] |
| Cobb 500, male | Fermented (FS) or unfermented (UF) GK | 30 or 60 | TEP: 1.98 g (FS30), 2.27 g (FS60), 2.42 g (UF30) and 3.13 g (UF60) | 21 | FS30: 1–21 d of age: FI: -, BW gain: -, F:G ratio: -; FS60: 1–21 d of age: FI: -, BW gain: ↓, F:G ratio: ↑; UF30: 1–21 d of age: FI: -, BW gain: -, F:G ratio: ↑; UF60: 1–21 d of age: FI: -, BW gain: ↓, F:G ratio: ↑ | [46] |
| Cobb 500, mixed sex | GP | 100 | - | 42 | 1–42 d of age: FI: -, BW gain: ↓, F:G ratio: - | [47] |
| Ross 308, male | GP | 5, 7.5 or 10 | - | 28 | 1–28 d of age: FI: -, BW gain: -, F:G ratio: - | [48] |
| Cobb 500, unsexed | GS extract | 0.25 or 0.5 | - | 28 | 0.25: 1–28 d of age: FI: ↑, BW gain: ↑, F:G ratio: ↓; 0.5: 1–28 d of age: FI: ↑, BW gain: ↑, F:G ratio: - | [49] |
| Cobb 500, mixed sex | GS | 10, 20 or 40 | GAE: 0.5, 1 or 2 g | 42 | 10: 1–42 d of age: FI: -, BW gain: ↑, F:G ratio: - 20: 1–42 d of age: FI: -, BW gain: ↑, F:G ratio: ↓ 40: 1–42 d of age: FI: -, BW gain: ↓, F:G ratio: ↑ | [50] |
| Ross 308, unsexed | GS extract | 0.125, 0.25, 0.5, 1 or 2 | - | 42 | 0.125, 0.25, 0.5, 1 and 2: 1–42 d of age: FI: -, BW gain: -, F:G ratio: - | [51] |
| Cobb 500, male | Grape proanthocyanidins | 0.0075, 0.015 or 0.03 | - | 42 | 0.0075, 0.015 and 0.03: 1–42 d of age: FI: ↓, BW gain: -, F:G ratio: ↓ | [52] |
| Cobb 500, unsexed | GP | 1 g/L drinking water | - | 42 | 1–42 d of age: FI: -, BW gain: -, F:G ratio: - | [53] |
| Cobb 500, male | GS extract | 0.025, 0.25, 2.5 or 5 | TEP: 6.6, 66, 660 or 1320 mg | 21 | 0.025, 0.25, 2.5: 1–21 d of age: FI: -, BW gain: -, F:G ratio: -; 5: 1–21 d of age: FI: -, BW gain: ↓, F:G ratio: ↑ | [54] |
| Ross 308, female | GS extract | 1 | - | 42 | 22–42 d of age: FI: -, BW gain: -, F:G ratio: - | [55] |
| Cobb 500, male | GS extract (GSE) or GP concentrate (GPC) | 7.2 (GSE) or 60 (GPC) | TEP: 5.4 g (GSE) and 4.3 g (GPC) | 21 | GSE: 1–21 d of age: FI: -, BW gain: ↓, F:G ratio: -; GPC: 1–21 d of age: FI: -, BW gain: -, F:G ratio: ↓ | [56] |
| Hubbard, male | GS extract | 0.005, 0.01, 0.02, 0.04 or 0.08 | - | 15 | 1–15 d of age: FI: -, BW gain: ↑, F:G ratio: - | [57] |
| Cobb, male | GP concentrate | 15, 30 or 60 | TEP + THP: 16.5, 19.2 and 22.1 g in groups 15, 30 and 60 | 21 | 21–42 d of age: FI: -, BW gain: -, F:G ratio: - | [58] |
| Cobb, male | GP | 5, 15 or 30 | GAE: 4.1 g (GP30) | 21 | 1–21 d of age: FI: -, BW gain: -, F:G ratio: - | [59] |
| Broiler Breed and Sex | Grape By-Product | Dose (g/kg Diet) | Main Effects | Ref. |
|---|---|---|---|---|
| Cobb 500, male | GS or GK | 30 (GS) or 110 (GK) | AID of protein: GS: -, GK: ↓ | [41] |
| Cobb 500, male | Fermented or unfermented GK | 30 or 60 | AID of protein: fermented 30, fermented 60 and unfermented 30: -; unfermented 60: ↓ | [46] |
| Ross 308, male | GP | 5, 7.5 or 10 | ATTD of crude protein: 5, 7.5 and 10: - | [48] |
| Cobb, male | GP | 50 or 100 | AID of protein: 50: -, 100: ↓ | [63] |
| Cobb, male | GS extract | 0.025, 0.25, 2.5 or 5 | AID of protein: 0.025: ↑, 0.25 and 2.5: -, 5: ↓, AID of arginine, histidine, phenylalanine, glutamic acid, cysteine, proline: 0.025–2.5: -; 5: ↓, AID of other amino acids: 0.025–5: - | [54] |
| Ross 308, female | GS extract | 1 | AID of nitrogen: - | [55] |
| Cobb, male | GP concentrate | 15, 30 or 60 | 15, 30 and 60: AID of protein: -; AID fat: - | [58] |
| Cobb, male | GP | 5, 15 or 30 | 5, 15 and 30: AID of amino acids: - | [59] |
| Grape By-Product | Dosage Range for Performance Enhancement (g/kg Diet) | Dosage Range for Performance Inhibition (g/kg Diet) |
|---|---|---|
| Grape pomace | 5–30 | ≥60 |
| Grape pomace extract | 2 | - * |
| Grape seeds | 5–30 | ≥40 |
| Grape seed extract | 0.01–0.5 | ≥5 |
| Grape skins | - * | ≥60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringseis, R.; Eder, K.; Gessner, D.K. Efficacy of Feeding Grape By-Products on Performance, Nutrient Digestibility, Gut Morphology, Gut Microbial Community, Oxidative Stress and Immune Response in Fast-Growing Broilers. Animals 2025, 15, 1943. https://doi.org/10.3390/ani15131943
Ringseis R, Eder K, Gessner DK. Efficacy of Feeding Grape By-Products on Performance, Nutrient Digestibility, Gut Morphology, Gut Microbial Community, Oxidative Stress and Immune Response in Fast-Growing Broilers. Animals. 2025; 15(13):1943. https://doi.org/10.3390/ani15131943
Chicago/Turabian StyleRingseis, Robert, Klaus Eder, and Denise K. Gessner. 2025. "Efficacy of Feeding Grape By-Products on Performance, Nutrient Digestibility, Gut Morphology, Gut Microbial Community, Oxidative Stress and Immune Response in Fast-Growing Broilers" Animals 15, no. 13: 1943. https://doi.org/10.3390/ani15131943
APA StyleRingseis, R., Eder, K., & Gessner, D. K. (2025). Efficacy of Feeding Grape By-Products on Performance, Nutrient Digestibility, Gut Morphology, Gut Microbial Community, Oxidative Stress and Immune Response in Fast-Growing Broilers. Animals, 15(13), 1943. https://doi.org/10.3390/ani15131943





