Non-Invasive Analyses of Altered Schaedler Flora in C57Bl/6J and Balb/c Mice to Monitor Hygiene Status of a Housing Facility
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feces from Cages Housing C57Bl/6J and Balb/c Mice Were Collected in Three Different Settings
2.1.1. Setting 1 (ASF Analysis in Balb/c and C57Bl/6J in Controlled Infection)
2.1.2. Setting 2 (ASF Analysis in Natural Infection Facility 1)
2.1.3. Setting 3 (ASF Analysis in Natural Infection in Facility 2)
2.1.4. qPCR and Statistics
- Statistic
- qPCR
- RNA Isolation
3. Results
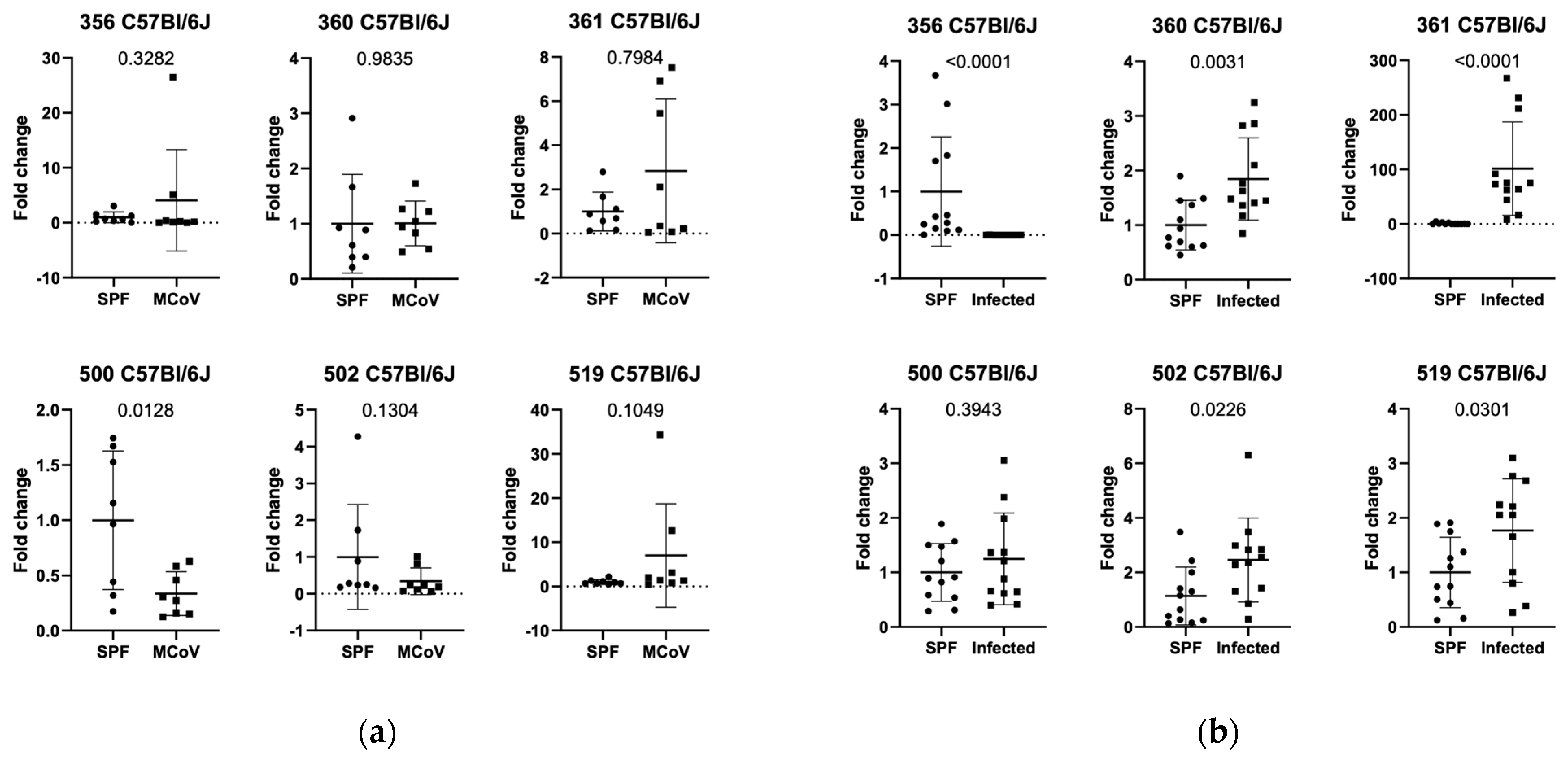
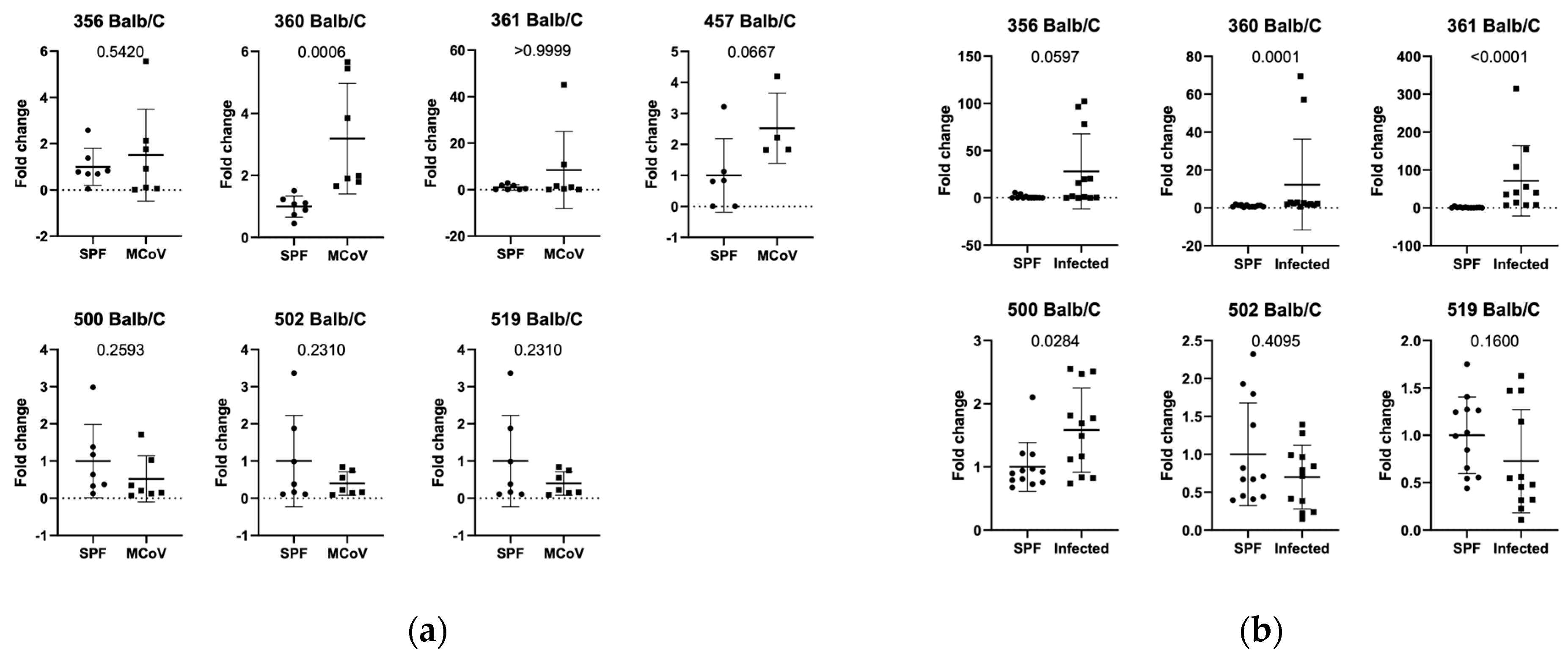
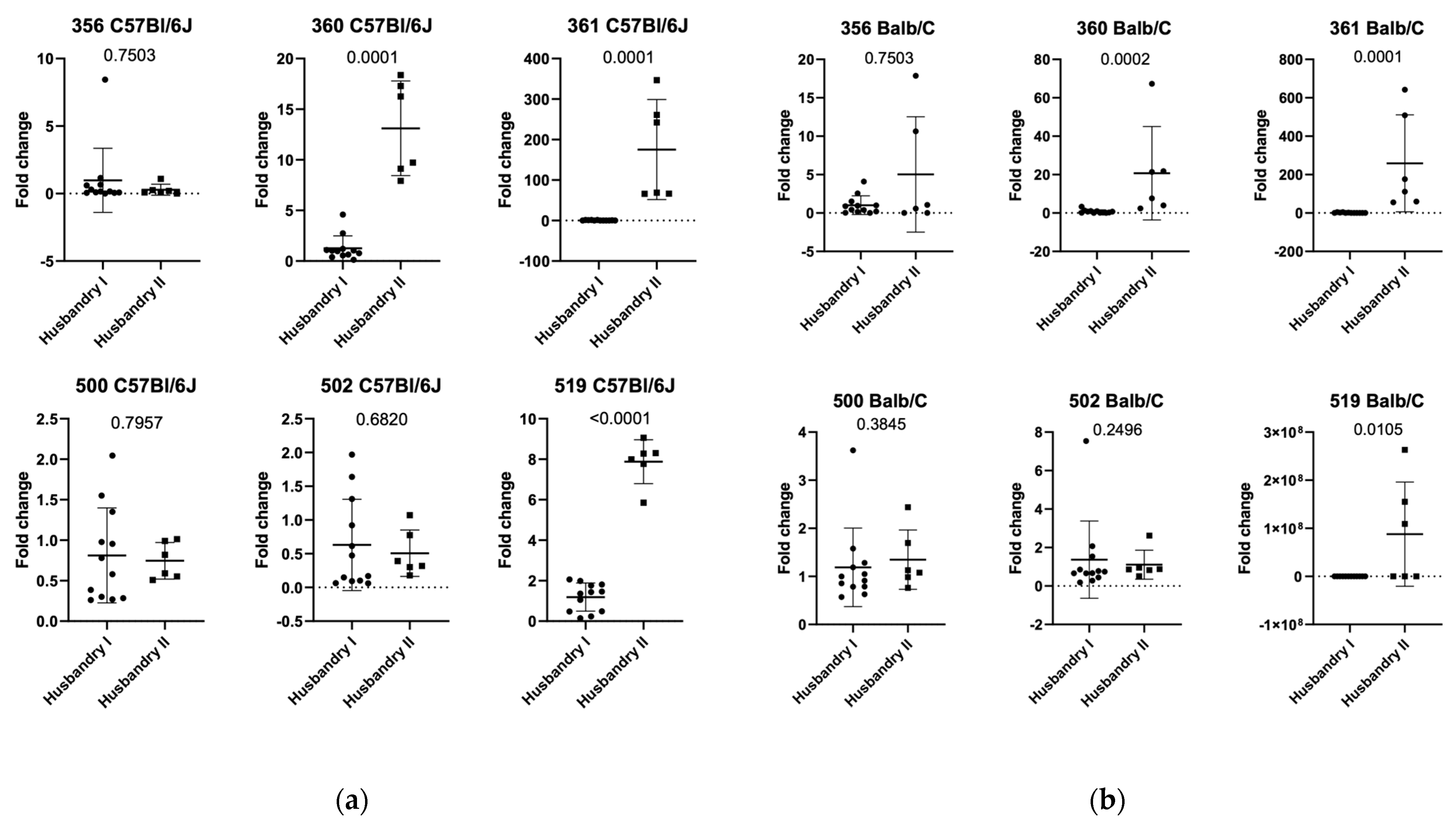
3.1. Setting 1 (ASF Analysis in Controlled Infection in C57Bl/6J and Balb/c Mice)
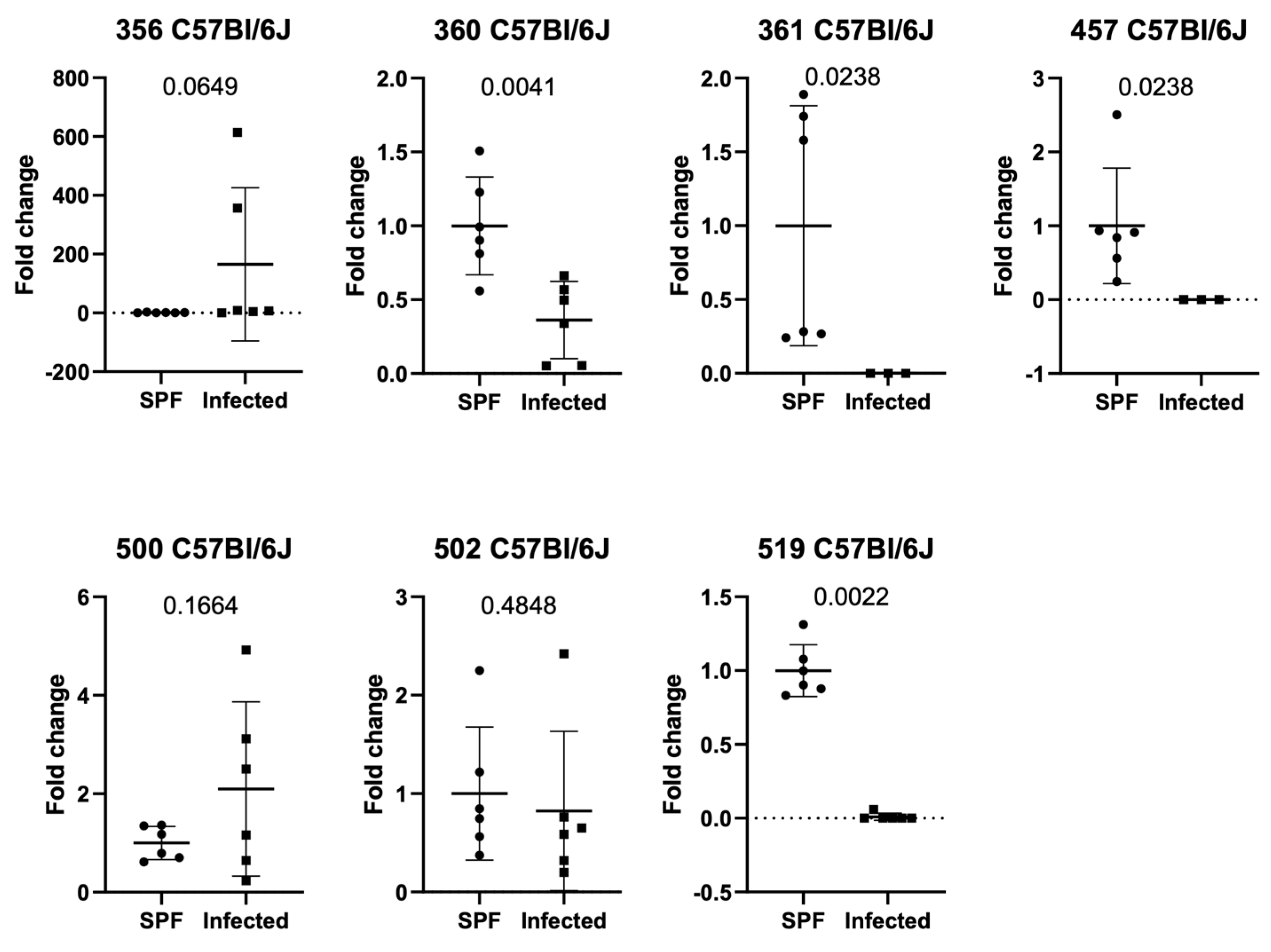
3.2. Setting 2a (ASF Analysis in Natural Infection in C57Bl/6J Mice in Facility 1)
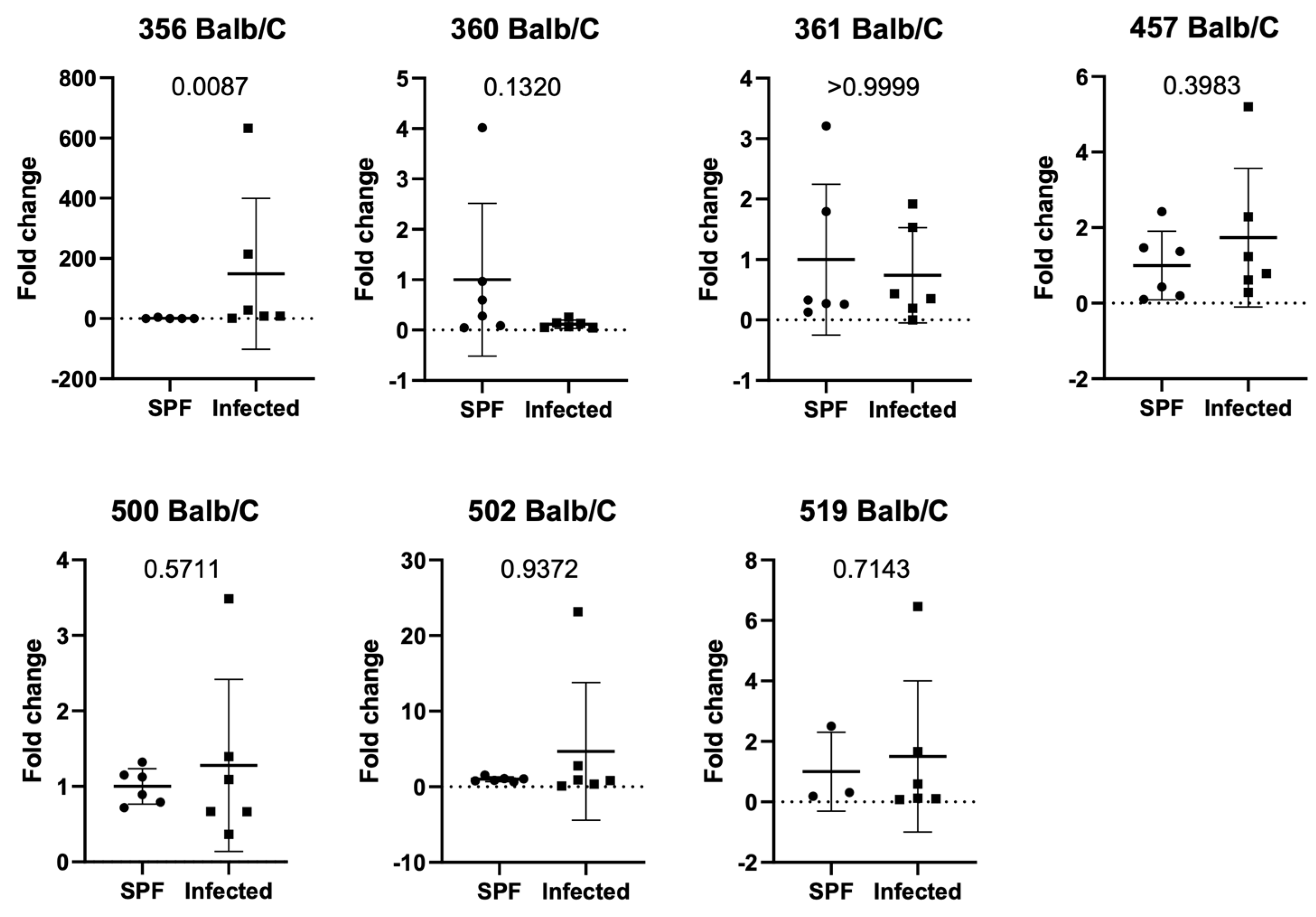
3.3. Setting 2b (ASF Analysis in Natural Infection in Balb/c Mice in Facility 1)
3.4. Setting 3 (ASF Analysis in Balb/c and C57Bl/6J in Natural Infection in Facility 2)
3.5. Additional Findings (ASF Analysis in SPF Balb/c and C57Bl/6J in Facility 1 and 2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, G.H.; Shwetha Reddy, R.V.; Yogesh, C. Management of Specific Pathogen-Free (SPF) Mice and Rats. In Essentials of Laboratory Animal Science: Principles and Practices; Springer Nature Singapore: Singapore, 2021; pp. 633–653. [Google Scholar] [CrossRef]
- Jansma, J.; El Aidy, S. Understanding the host-microbe interactions using metabolic modeling. Microbiome 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Food, Immunity, and the Microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Dickerson, F.; Severance, E.; Yolken, R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav. Immun. 2017, 62, 46–52. [Google Scholar] [CrossRef]
- Hoeppli, R.E.; Wu, D.; Cook, L.; Levings, M.K. The Environment of Regulatory T Cell Biology: Cytokines, Metabolites, and the Microbiome. Front. Immunol. 2015, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2007, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wannemuehler, M.J.; Overstreet, A.-M.; Ward, D.V.; Phillips, G.J. Draft Genome Sequences of the Altered Schaedler Flora, a Defined Bacterial Community from Gnotobiotic Mice. Genome Announc. 2014, 2, e00287-14. [Google Scholar] [CrossRef]
- Laukens, D.; Brinkman, B.M.; Raes, J.; De Vos, M.; Vandenabeele, P. Heterogeneity of the gut microbiome in mice: Guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016, 40, 117–132. [Google Scholar] [CrossRef]
- Parker, K.D.; Albeke, S.E.; Gigley, J.P.; Goldstein, A.M.; Ward, N.L. Microbiome Composition in Both Wild-Type and Disease Model Mice Is Heavily Influenced by Mouse Facility. Front. Microbiol. 2018, 9, 1598. [Google Scholar] [CrossRef]
- Orcutt, R.P.; Gianni, F.J.; Judge, R.J. Development of an “altered Schaedler flora” for NCI gnotobiotic rodents. Microecol. Ther. 1987, 17, 59. [Google Scholar]
- Schaedler, R.W.; Dubos, R.; Costello, R. Association of germfree mice with bacteria isolated from normal mice. J. Exp. Med. 1965, 122, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.A.; Orcutt, R.P.; Henry, J.C.; Baker, J., Jr.; Bissahoyo, A.C.; Threadgill, D.W. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm. Genome 2006, 17, 1093–1104. [Google Scholar] [CrossRef]
- Sarma-Rupavtarm, R.B.; Ge, Z.; Schauer, D.B.; Fox, J.G.; Polz, M.F. Spatial Distribution and Stability of the Eight Microbial Species of the Altered Schaedler Flora in the Mouse Gastrointestinal Tract. Appl. Environ. Microbiol. 2004, 70, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.B.; Medlock, G.L.; Moutinho, T.J.; Lees, H.J.; Swann, J.R.; Kolling, G.L.; Papin, J.A. Systems-level metabolism of the altered Schaedler flora, a complete gut microbiota. ISME J. 2017, 11, 426–438. [Google Scholar] [CrossRef]
- Wymore Brand, M.; Wannemuehler, M.J.; Phillips, G.J.; Proctor, A.; Overstreet, A.; Jergens, A.E.; Orcutt, R.P.; Fox, J.G. The Altered Schaedler Flora: Continued Applications of a Defined Murine Microbial Community. ILAR J. 2015, 56, 169–178. [Google Scholar] [CrossRef]
- Stehr, M.; Greweling, M.C.; Tischer, S.; Singh, M.; Blöcker, H.; Monner, D.A.; Müller, W. Charles River altered Schaedler flora (CRASF®) remained stable for four years in a mouse colony housed in individually ventilated cages. Lab. Anim. 2009, 43, 362–370. [Google Scholar] [CrossRef]
- Kornerup Hansen, A.; Franklin, C. Microbiota, laboratory animals, and research. Lab. Anim. 2019, 53, 229–231. [Google Scholar] [CrossRef]
- Guillen, J. FELASA Guidelines and Recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar]
- Salzmann, M.; Haider, P.; Kaun, C.; Brekalo, M.; Hartmann, B.; Lengheimer, T.; Pichler, R.; Filip, T.; Derdak, S.; Podesser, B.; et al. Innate Immune Training with Bacterial Extracts Enhances Lung Macrophage Recruitment to Protect from Betacoronavirus Infection. J. Innate Immun. 2021, 14, 293–305. [Google Scholar] [CrossRef]
- Bolsega, S.; Basic, M.; Smoczek, A.; Buettner, M.; Eberl, C.; Ahrens, D.; Odum, K.A.; Stecher, B.; Bleich, A. Composition of the Intestinal Microbiota Determines the Outcome of Virus-Triggered Colitis in Mice. Front. Immunol. 2019, 10, 1708. [Google Scholar] [CrossRef] [PubMed]
- Hentges, D.J.; Stein, A.J.; Casey, S.W.; Que, J.U. Protective Role of Intestinal Flora Against Infection with Pseudomonas aeruginosa in Mice: Influence of Antibiotics on Colonization Resistance. Infect. Immun. 1985, 47, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.R.; Sperandio, V. Enteric Pathogens Exploit the Microbiota-generated Nutritional Environment of the Gut. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Singh, S.; Koo, O.K. A Comprehensive Review Exploring the Protective Role of Specific Commensal Gut Bacteria against Salmonella. Pathog. 2024, 13, 642. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Veiga, P.; Wardwell-Scott, L.H.; Tickle, T.; Segata, N.; Michaud, M.; Gallini, C.A.; Beal, C.; van Hylckama-Vlieg, J.E.; Ballal, S.A.; et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014, 8, 1403–1417. [Google Scholar] [CrossRef]
- LaFollette, M.R.; Clement, C.S.; Luchins, K.R.; Manuel, C.A.; Foley, P.L.; Hanson, W.H.; Pettan-Brewer, C.; Winn, C.B.; Garner, J.P. Do We Still Need a Canary in the Coal Mine for Laboratory Animal Facilities? A Systematic Review of Environmental Health Monitoring versus Soiled Bedding Sentinels. PLoS ONE 2024, 19, e0311840. [Google Scholar] [CrossRef]
- Mahabir, E.; Bleich, A.; van den Brandt, J.; Nicklas, W. Use of Environmental Samples for Health Monitoring of Rodents—A Recommendation from the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Health Monitoring of Rodents. GV-SOLAS 2022. Available online: https://www.gv-solas.de/wp-content/uploads/2022/11/Use-of-environmental-samples_2022.pdf (accessed on 24 March 2025).
| Target | Species | Fwd-Seq. | Rev-Seq. |
|---|---|---|---|
| 356 | Clostridium sp. | AAA ATA ATT AGG AGC TTG CTT TTA A | TTA GAA GAT GCC TCC TAA GAA CC |
| 360 | Lactobacillus intestinalis | GGT GAT GAC GCT GGG AAC | AAG CAA TAG CCA TGC AGC |
| 316 | Lactobacillus murinus | GAA CGA AAC TTC TTT ATC ACC | TAG CAT AGC CAC CTT TTA CA |
| 457 | Mucispirillum schaedleri | TCT CTT CGG GGA TGA TTA AAC | AAC TTT TCC TAT ATA AAC ATG CAC |
| 492 | Eubacterium plexicaudatum | AAT TCC TTC GGG GAG GAA GC | TAA AAC CAT GCG GTT TTA AAA AC |
| 500 | Pseudoflavonifractor sp. | ACG GAG GAC CCC TGA AGG | AGC GAT AAA TCT TTG ATG TCC |
| 502 | Clostridium sp. | GAG CGA AGC ACT TTT TTA GAA C | TTA CAC CAC CTC AGT TTT TAC C |
| 519 | Parabacteroides goldsteinii | GCA GCA CGA TGT AGC AAT ACA | TTA ACA AAT ATT TCC ATG TGG AAC |
| 515R | Universal 16S | TTACCGCGGCTGCTGGCAC | |
| 8F | Universal 16S | CTC CTA CGG GAG GCA GCA G |
| ASF Group | Group Name |
|---|---|
| 356 | Clostridium sp. |
| 360 | Lactobacillus intestinalis |
| 361 | Lactobacillus murinus |
| 457 | Mucispirillum schaedleri |
| 492 | Eubacterium plexicaudatum |
| 500 | Pseudoflavonifractor sp. |
| 502 | Clostridium sp. |
| 519 | Parabacteroides goldsteinii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistelberger, R.; Gibler, P.; Barones, L.; Absenger, A.; Kral-Pointner, J.B.; Salzmann, M.; Hartmann, B.; Podesser, B.K.; Hohensinner, P.J.; Plasenzotti, R. Non-Invasive Analyses of Altered Schaedler Flora in C57Bl/6J and Balb/c Mice to Monitor Hygiene Status of a Housing Facility. Animals 2025, 15, 1725. https://doi.org/10.3390/ani15121725
Nistelberger R, Gibler P, Barones L, Absenger A, Kral-Pointner JB, Salzmann M, Hartmann B, Podesser BK, Hohensinner PJ, Plasenzotti R. Non-Invasive Analyses of Altered Schaedler Flora in C57Bl/6J and Balb/c Mice to Monitor Hygiene Status of a Housing Facility. Animals. 2025; 15(12):1725. https://doi.org/10.3390/ani15121725
Chicago/Turabian StyleNistelberger, Rebecca, Patrizia Gibler, Lisa Barones, Arno Absenger, Julia B. Kral-Pointner, Manuel Salzmann, Boris Hartmann, Bruno K. Podesser, Phillip J. Hohensinner, and Roberto Plasenzotti. 2025. "Non-Invasive Analyses of Altered Schaedler Flora in C57Bl/6J and Balb/c Mice to Monitor Hygiene Status of a Housing Facility" Animals 15, no. 12: 1725. https://doi.org/10.3390/ani15121725
APA StyleNistelberger, R., Gibler, P., Barones, L., Absenger, A., Kral-Pointner, J. B., Salzmann, M., Hartmann, B., Podesser, B. K., Hohensinner, P. J., & Plasenzotti, R. (2025). Non-Invasive Analyses of Altered Schaedler Flora in C57Bl/6J and Balb/c Mice to Monitor Hygiene Status of a Housing Facility. Animals, 15(12), 1725. https://doi.org/10.3390/ani15121725










