Bioactive Element Biodistribution of Different Biological Substrates in Sheep and Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Preparation
2.3. Multielement Analysis by ICP-MS
2.4. Analytical Performances
2.5. Statistical Analysis
3. Results
3.1. Element Contents in Different Biological Substrates of Sheep and Goat
3.2. Principal Component Analysis
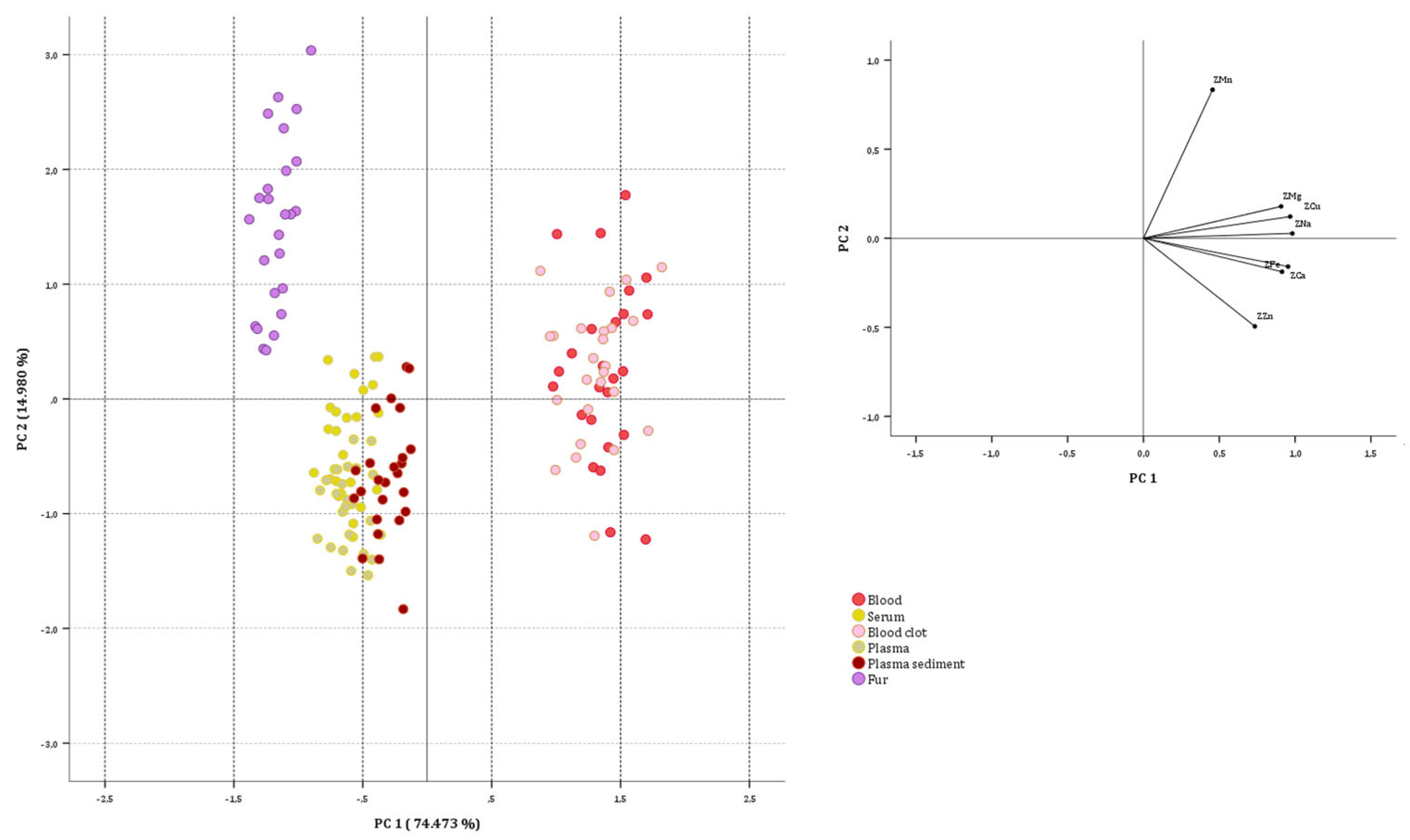
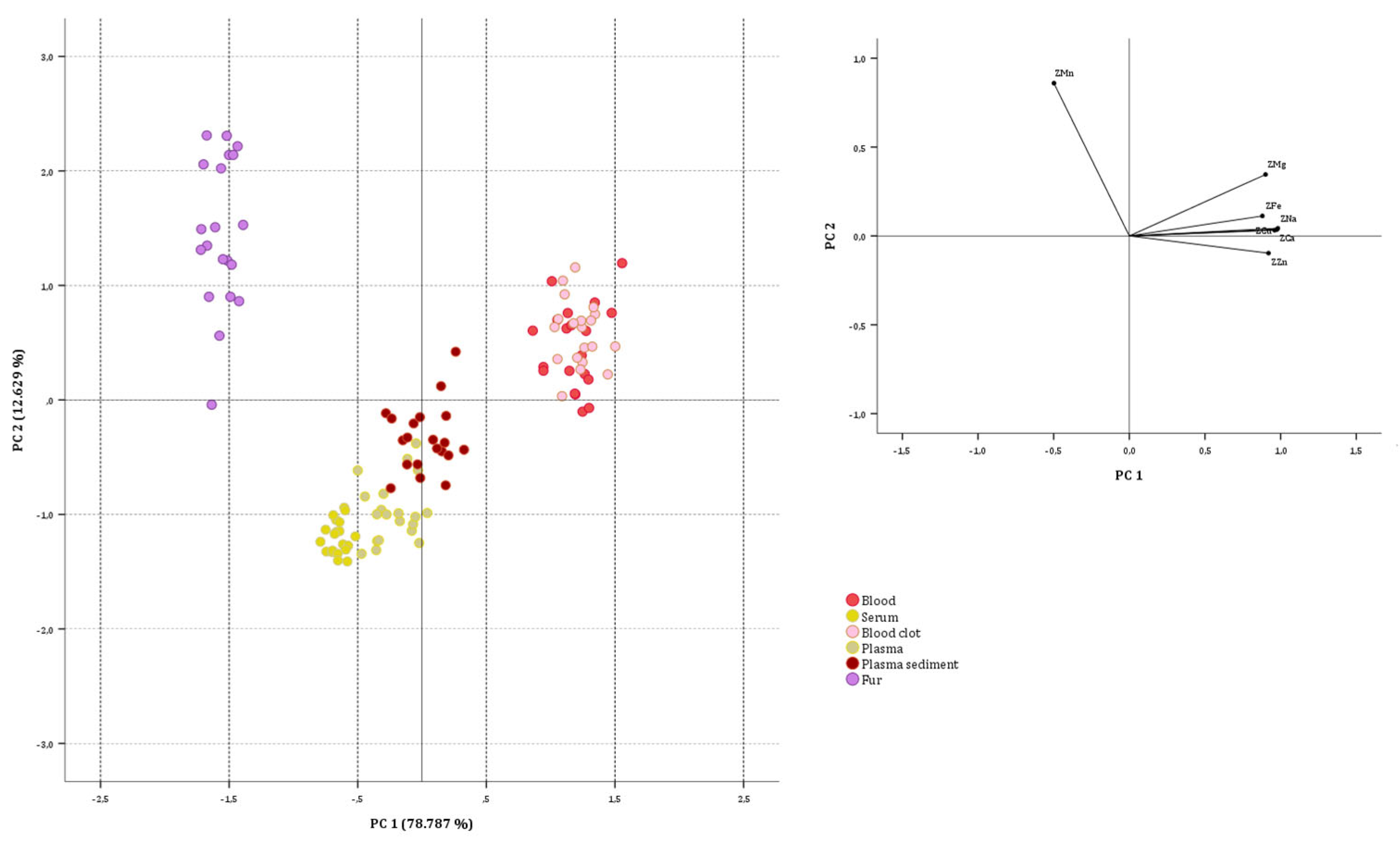
3.2.1. On Sheep Data Set
3.2.2. Goat Data Set
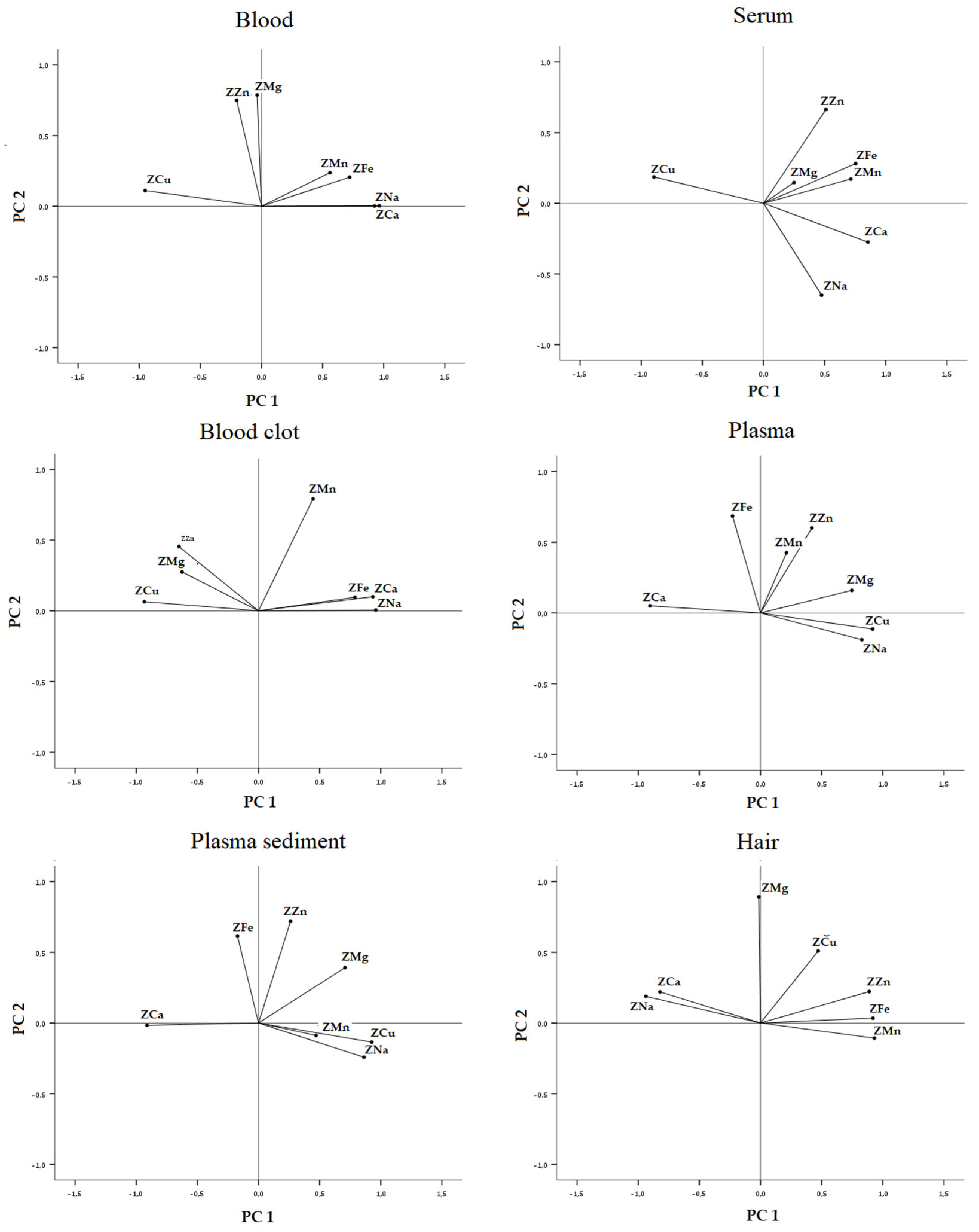
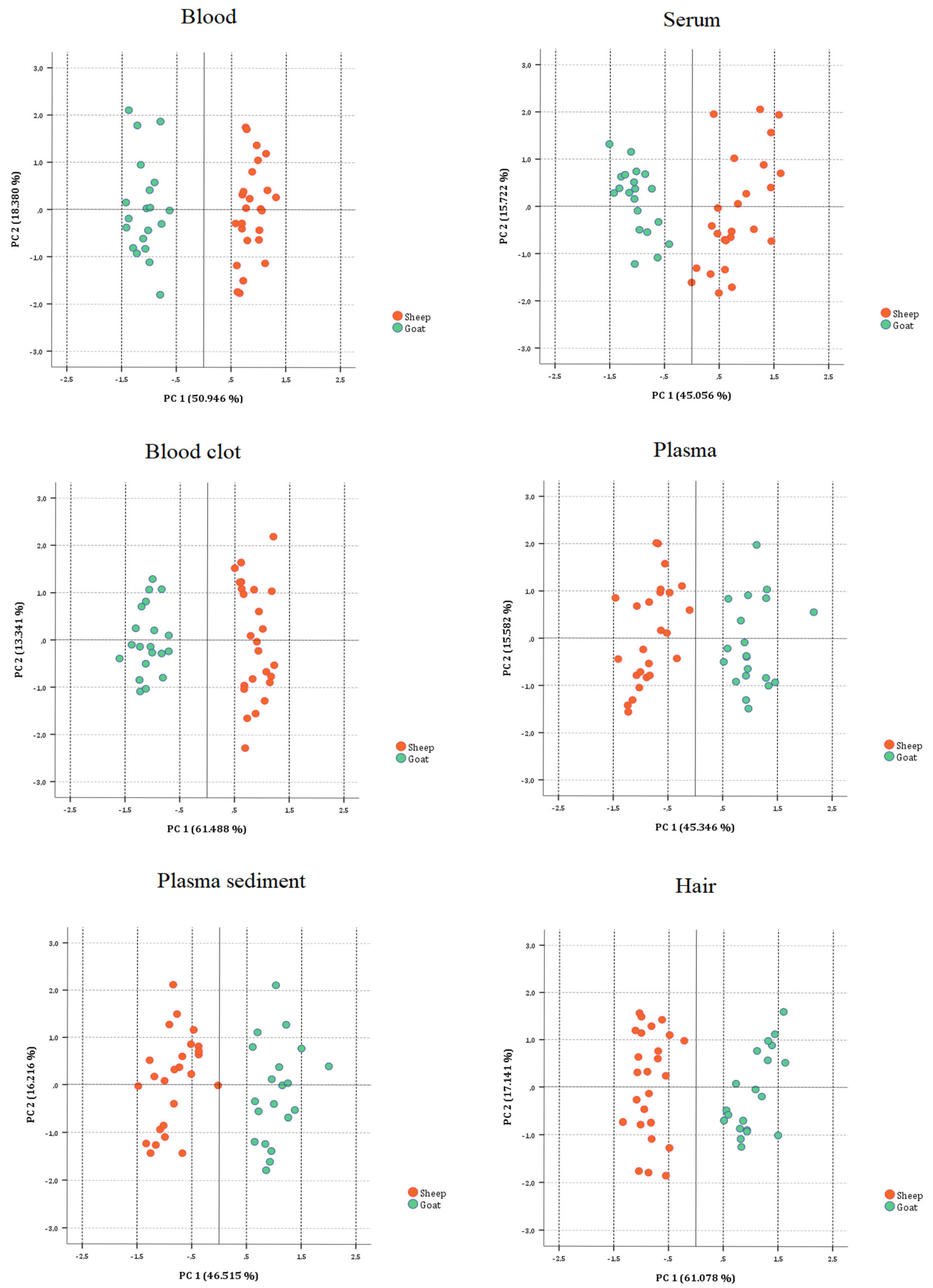
3.2.3. On Biological Substrate Data Sets
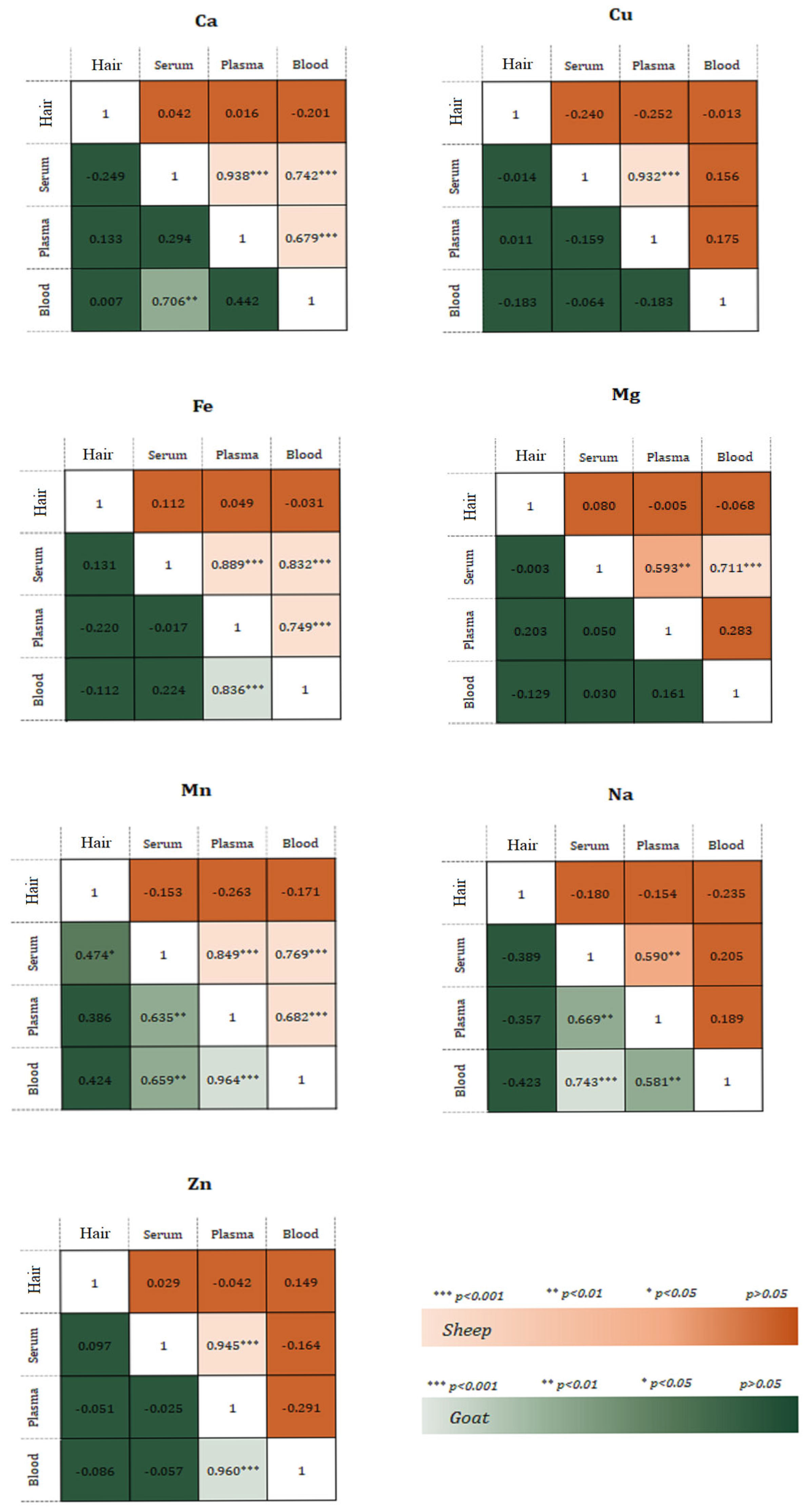
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herdt, T.H.; Hoff, B. The Use of Blood Analysis to Evaluate Trace Mineral Status in Ruminant Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 255–283. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.I.; Saxena, A.; Deepa, P.M.; Habeab, B.P.; Devi, S.; Jatav, R.S.; Dimri, U. Role of Trace Elements in Animals: A Review. Vet. World 2013, 6, 963–967. [Google Scholar] [CrossRef]
- Palomares, R.A. Trace Minerals Supplementation with Great Impact on Beef Cattle Immunity and Health. Animals 2022, 12, 2839. [Google Scholar] [CrossRef]
- Bobeck, E.A. Nutrition and Health: Companion Animal Applications: Functional Nutrition in Livestock and Companion Animals to Modulate the Immune Response. J. Anim. Sci. 2020, 98, skaa035. [Google Scholar] [CrossRef]
- Naz, S.; Arshad, M.; Majeed, S.; Maqaddas, S.; Habib, S.S.; Kesbiç, O.S.; Al-Rejaie, S.S.; Mohany, M.; Bottari, T.; Aragona, F.; et al. Assessing Heavy Metal Contamination in Commonly Used Fertilizers for Polyculture Fish Ponds and Its Implications for Human Health: A Comprehensive Investigation. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Rind, K.H.; Arshad, M.; Majeed, S.; Habib, S.S.; Al-Rejaie, S.S.; Mohany, M.; Aragona, F.; Fazio, F. Impact of Heavy Metals on Health and Quality of Oreochromis Niloticus Cultured in Biofloc and Earthen Pond Systems. J. Environ. Sci. Health Part B 2025, 60, 129–137. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Wimalawansa, S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef]
- McDowell, L.R. Minerals in Animals and Human Nutrition, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 978-0-444-51367-0. [Google Scholar]
- Johansson, K. Salt to Ruminants and Horses; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2011; p. 26. [Google Scholar]
- Michell, A.R. Physiological Aspects of the Requirement for Sodium in Mammals. Nutr. Res. Rev. 1989, 2, 149–160. [Google Scholar] [CrossRef]
- Board on Agriculture and Natural Resources; Subcommittee on Dairy Cattle Nutrition; Committee on Animal Nutrition. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-06997-7. [Google Scholar]
- Sykes, A.R. Deficiency of Mineral Macro-Elements. Diseases of Sheep; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 363–377. ISBN 978-0-470-75331-6. [Google Scholar]
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givens, I. The Contribution of Dietary Magnesium in Farm Animals and Human Nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef]
- Rabiee, A.R.; Lean, I.J.; Stevenson, M.A.; Socha, M.T. Effects of Feeding Organic Trace Minerals on Milk Production and Reproductive Performance in Lactating Dairy Cows: A Meta-Analysis. J. Dairy. Sci. 2010, 93, 4239–4251. [Google Scholar] [CrossRef]
- Sales, J.N.S.; Pereira, R.V.V.; Bicalho, R.C.; Baruselli, P.S. Effect of Injectable Copper, Selenium, Zinc and Manganese on the Pregnancy Rate of Crossbred Heifers (Bos indicus × Bos taurus) Synchronized for Timed Embryo Transfer. Livest. Sci. 2011, 142, 59–62. [Google Scholar] [CrossRef]
- Gry, J.; Black, L.; Eriksen, F.D.; Pilegaard, K.; Plumb, J.; Rhodes, M.; Sheehan, D.; Kiely, M.; Kroon, P.A. EuroFIR-BASIS—A Combined Composition and Biological Activity Database for Bioactive Compounds in Plant-Based Foods. Trends Food Sci. Technol. 2007, 18, 434–444. [Google Scholar] [CrossRef]
- Tuncer, S.; Sireli, H.; Dellal, G. Comparative analysis of various fleece characteristics of norduz and zom sheep. J. Anim. Plant Sci. 2017, 27, 763–770. [Google Scholar]
- Aragona, F.; Rizzo, M.; Arfuso, F.; Acri, G.; Fazio, F.; Piccione, G.; Giannetto, C. Eye Temperature Measured with Infrared Thermography to Assess Stress Responses to Road Transport in Horses. Animals 2024, 14, 1877. [Google Scholar] [CrossRef]
- Aragona, F.; Arfuso, F.; Rizzo, M.; Fazio, F.; Acri, G.; Piccione, G.; Giannetto, C. Using Infrared Thermography for the Evaluation of Road Transport Thermal Homeostasis in Athletic Horse. J. Equine Vet. Sci. 2024, 138, 105102. [Google Scholar] [CrossRef]
- Doğan, E.; Fazio, F.; Aragona, F.; Nava, V.; De Caro, S.; Zumbo, A. Toxic Element (As, Cd, Pb and Hg) Biodistribution and Blood Biomarkers in Barbaresca Sheep Raised in Sicily: One Health Preliminary Study. Environ. Sci. Pollut. Res. Int. 2024, 31, 43903–43912. [Google Scholar] [CrossRef]
- Nava, V.; Licata, P.; Biondi, V.; Catone, G.; Gugliandolo, E.; Pugliese, M.; Passantino, A.; Crupi, R.; Aragona, F. Horse Whole Blood Trace Elements from Different Sicily Areas: Biomonitoring of Environmental Risk. Biol. Trace Elem. Res. 2024, 202, 3086–3096. [Google Scholar] [CrossRef]
- Kincaid, R.L. Assessment of Trace Mineral Status of Ruminants: A Review. J. Anim. Sci. 2000, 77, 1–10. [Google Scholar] [CrossRef]
- Humann-Ziehank, E.; Tegtmeyer, P.C.; Seelig, B.; Roehrig, P.; Ganter, M. Variation of Serum Selenium Concentrations in German Sheep Flocks and Implications for Herd Health Management Consultancy. Acta Vet. Scand. 2013, 55, 82. [Google Scholar] [CrossRef]
- Battini, M.; Peric, T.; Ajuda, I.; Vieira, A.; Grosso, L.; Barbieri, S.; Stilwell, G.; Prandi, A.; Comin, A.; Tubaro, F.; et al. Hair Coat Condition: A Valid and Reliable Indicator for on-Farm Welfare Assessment in Adult Dairy Goats. Small Rum. Res. 2015, 123, 197–203. [Google Scholar] [CrossRef]
- Agradi, S.; Munga, A.; Barbato, O.; Palme, R.; Tarhan, D.; Bilgiç, B.; Dokuzeylül, B.; Ercan, A.M.; Or, M.E.; Brecchia, G.; et al. Goat Hair as a Bioindicator of Environmental Contaminants and Adrenal Activation during Vertical Transhumance. Front. Vet. Sci. 2023, 10, 1274081. [Google Scholar] [CrossRef] [PubMed]
- Manuelian, C.L.; Maggiolino, A.; De Marchi, M.; Claps, S.; Esposito, L.; Rufrano, D.; Casalino, E.; Tateo, A.; Neglia, G.; De Palo, P. Comparison of Mineral, Metabolic, and Oxidative Profile of Saanen Goat during Lactation with Different Mediterranean Breed Clusters under the Same Environmental Conditions. Animals 2020, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Paksoy, N.; İriadam, M.; Bozkaya, F.; Yavuz, Ü.; Öztürk, E.E. Determination of Macro and Trace Element Levels of Serum, Tears, Saliva, and Hair Samples in Kilis Goats with ICP-MS. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 691–699. [Google Scholar] [CrossRef]
- Poppenga, R.H.; Ramsey, J.; Gonzales, B.J.; Johnson, C.K. Reference Intervals for Mineral Concentrations in Whole Blood and Serum of Bighorn Sheep (Ovis Canadensis) in California. J. Vet. Diagn. Investig. 2012, 24, 531–538. [Google Scholar] [CrossRef]
- Schweinzer, V.; Iwersen, M.; Drillich, M.; Wittek, T.; Tichy, A.; Mueller, A.; Krametter-Froetscher, R. Macromineral and Trace Element Supply in Sheep and Goats in Austria. Vet. Med. 2017, 62, 62–73. [Google Scholar] [CrossRef]
- Yazar, E.; Altunok, V.; Eroglu, T. Concentrations of Some Elements in Blood Serum of Angora Goats. Med. Weter. 2006, 62, 1249–1251. [Google Scholar]
- Haenlein, G.F.W.; Anke, M. Mineral and Trace Element Research in Goats: A Review. Small Rum. Res. 2011, 95, 2–19. [Google Scholar] [CrossRef]
- McDonald, P.; Greenhalgh, J.F.D.; Morgan, C.; Edwards, R.; Sinclair, L.; Wilkinson, R. Animal Nutrition; Pearson: London, UK, 2021; ISBN 978-1-292-25168-4. [Google Scholar]
- Aragona, F.; Cicero, N.; Nava, V.; Piccione, G.; Giannetto, C.; Fazio, F. Blood and Hoof Biodistibution of Some Trace Element (Lithium, Copper, Zinc, Strontium and, Lead) in Horse from Two Different Areas of Sicily. J. Trace Elem. Med. Biol. 2024, 82, 127378. [Google Scholar] [CrossRef]
- Aragona, F.; Giannetto, C.; Piccione, G.; Licata, P.; Deniz, Ö.; Fazio, F. Hair and Blood Trace Elements (Cadmium, Zinc, Chrome, Lead, Iron and Copper) Biomonitoring in the Athletic Horse: The Potential Role of Haematological Parameters as Biomarkers. Animals 2024, 14, 3206. [Google Scholar] [CrossRef]
- Fazio, F.; Aragona, F.; Piccione, G.; Arfuso, F.; Giannetto, C. Lithium Concentration in Biological Samples and Gender Difference in Athletic Horses. J. Equine Vet. Sci. 2022, 117, 104081. [Google Scholar] [CrossRef]
- Brummer-Holder, M.; Cassill, B.D.; Hayes, S.H. Interrelationships Between Age and Trace Element Concentration in Horse Mane Hair and Whole Blood. J. Equine Vet. Sci. 2020, 87, 102922. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.; Fontes, M.; Fernandes, R. Heavy Metals in Equine Biological Components. Rev. Bras. Zootecn. 2014, 43, 60–66. [Google Scholar] [CrossRef]
- González, F. (Ed.) Doze Leituras Em Bioquímica Clínica Veterinária; Faculdade de Veterinária, Universidad Federal do Rio Grande do Sul: Portoalegre, Brazil, 2018; p. 166. ISBN 978-85-66094-39-8. [Google Scholar]
- Rashed, M.N.; Soltan, M.E. Animal Hair as Biological Indicator for Heavy Metal Pollution in Urban and Rural Areas. Environ. Monit. Assess. 2005, 110, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Visconti, V.V.; Gasperini, B.; Greggi, C.; Battistini, B.; Messina, A.; Renzi, M.; Bakhtafrouz, K.; Iundusi, R.; Botta, A.; Palombi, L.; et al. Plasma Heavy Metal Levels Correlate with Deregulated Gene Expression of Detoxifying Enzymes in Osteoporotic Patients. Sci. Rep. 2023, 13, 10641. [Google Scholar] [CrossRef]
- Van Zweden, E.; Tolsma, R.; Hung, V.; Awad, P.; Sawyer, R.; Li, Y. The Advances of Blood Clots Used as Biomaterials in Regenerative Medicine. Regen. Med. 2022, 17, 957–969. [Google Scholar] [CrossRef]
- Nicol, A.M.; Poppi, D.P.; Alam, M.R.; Collins, H.A. Dietary Differences between Goats and Sheep. Proc. N. Z. Grassl. Assoc. 1987, 48, 199–205. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmad, K.; Mukhtar, M.K.; Mirzaei, F.; Hussain, G. Assessment of Pasture and Plasma Minerals of Cows: A Case Study in Pakistan. Agricul. Sci. 2013, 4, 57–61. [Google Scholar] [CrossRef][Green Version]
- Khan, Z.; Ashraf, M.; Valeem, E.E.; Ahmad, K.; Danish, M. Pasture concentration of minerals in relation to the nutrient requirements of farm livestock. Pak. J. Bot. 2007, 39, 2183–2191. [Google Scholar]
| Whole Blood Metals Control Level 3 | ERM-DB001 (Human Hair) | |||||||
|---|---|---|---|---|---|---|---|---|
| Element | R2 | LOD (mg/Kg) | LOQ (mg/Kg) | Recovery (%) | R2 | LOD (mg/Kg) | LOQ (mg/Kg) | Recovery (%) |
| Na | 0.9980 | 1.255 | 4.142 | 90.17 ± 1.29 | 0.9982 | 1.167 | 3.851 | 91.12 ± 1.75 |
| Mg | 0.9993 | 0.051 | 0.168 | 96.34 ± 1.51 | 0.9993 | 0.045 | 0.149 | 96.34 ± 1.41 |
| Ca | 0.9989 | 0.616 | 2.033 | 90.56 ± 1.74 | 0.9988 | 0.873 | 2.881 | 91.57 ± 1.95 |
| Mn | 0.9996 | 0.001 | 0.003 | 98.85 ± 0.81 | 0.9998 | 0.003 | 0.010 | 99.67 ± 0.54 |
| Fe | 0.9998 | 0.010 | 0.033 | 98.27 ± 0.66 | 0.9995 | 0.018 | 0.059 | 98.47 ± 0.82 |
| Cu | 0.9985 | 0.013 | 0.043 | 97.68 ± 0.46 | 0.9985 | 0.010 | 0.033 | 97.25 ± 0.67 |
| Zn | 0.9994 | 0.050 | 0.165 | 97.75 ± 0.48 | 0.9990 | 0.068 | 0.224 | 97.69 ± 0.54 |
| Biological Substrate | Ca | Cu | Fe | Mg | Mn | Na | Zn |
|---|---|---|---|---|---|---|---|
| Whole Blood | |||||||
| Sheep | 130.92 ± 10.95 (c) * I ** | 1.21 ± 0.11 (b) | 30.57 ± 4.94 (c) I | 38.49 ± 4.65 (b) | 0.16 ± 0.05 (c) I | 1794.14 ± 57.06 (c) I | 19.65 ± 3.41 (c) |
| Goat | 84.26 ± 4.57 (C) | 2.29 ± 0.32 (D) I | 23.78 ± 2.44 (C) | 38.76 ± 4.85 (B) | 0.11 ± 0.03 (B) | 1208.39 ± 55.76 (C) | 21.05 ± 3.13 (D) |
| Serum | |||||||
| Sheep | 64.17 ± 5.95 (b) I | 0.30 ± 0.07 (a) | 18.99 ± 2.98 (b) I | 16.63 ± 3.82 (a) | 0.08 ± 0.03 (b) I | 444.16 ± 20.67 (b) I | 10.26 ± 3.40 (b) I |
| Goat | 42.07 ± 3.49 (B) | 0.72 ± 0.10 (B) I | 15.28 ± 0.94 (B) | 15.64 ± 1.69 (A) | 0.05 ± 0.02 (A) | 419.94 ± 32.67 (B) | 8.21 ± 0.43 (B) |
| Blood clot | |||||||
| Sheep | 135.62 ± 11.37 (c) I | 1.19 ± 0.11 (b) | 32.13 ± 4.90 (c) I | 34.89 ± 5.26 (b) | 0.15 ± 0.04 (c) | 1790.75 ± 51.82 (c) I | 17.34 ± 3.70 (c) |
| Goat | 87.78 ± 4.63 (C) | 2.35 ± 0.32 (D) I | 22.67 ± 2.48 (C) | 40.54 ± 3.60 (B) I | 0.12 ± 0.03 (B) | 1192.59 ± 48.70 (C) | 21.90 ± 2.17 (D) I |
| Plasma | |||||||
| Sheep | 74.05 ± 6.16 (b) I | 0.24 ± 0.07 (a) | 15.17 ± 3.12 (b) | 16.37 ± 2.96 (a) | 0.06 ± 0.02 (a) | 530.85 ± 29.97 (b) | 13.92 ± 3.67 (bc) |
| Goat | 48.56 ± 4.18 (B) | 1.26 ± 0.27 (C) I | 13.97 ± 3.58 (B) | 20.67 ± 3.00 (A) I | 0.06 ± 0.03 (A) | 597.51 ± 17.67 (B) I | 15.46 ± 2.96 (C) |
| Plasma sediment | |||||||
| Sheep | 77.97 ± 6.30 (b) I | 0.30 ± 0.07 (a) | 18.21 ± 3.39 (b) | 19.94 ± 3.37 (a) | 0.09 ± 0.03 (b) | 541.59 ± 29.81 (b) | 17.36 ± 3.43 (c) |
| Goat | 52.47 ± 4.38 (B) | 1.45 ± 0.27 (C) I | 17.08 ± 3.61 (B) | 24.44 ± 3.66 (A) I | 0.11 ± 0.03 (B) | 617.70 ± 17.33 (B) I | 18.25 ± 3.47 (CD) |
| Hair | |||||||
| Sheep | 34.95 ± 7.09 (a) I | 0.17 ± 0.05 (a) | 6.42 ± 0.87 (a) | 17.45 ± 5.08 (a) | 0.15 ± 0.05 (c) | 170.66 ± 13.77(a) I | 1.85 ± 0.25 (a) |
| Goat | 19.54 ± 4.56 (A) | 0.21 ± 0.05 (A) I | 10.83 ± 1.68 (A) I | 16.66 ± 3.59 (A) | 0.36 ± 0.07 (C) I | 97.99 ± 4.87 (A) | 2.87 ± 0.44 (A) I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava, V.; Aragona, F.; Potortì, A.G.; De Caro, S.; Di Bella, B.; Litrenta, F.; Fazio, F. Bioactive Element Biodistribution of Different Biological Substrates in Sheep and Goats. Animals 2025, 15, 1686. https://doi.org/10.3390/ani15121686
Nava V, Aragona F, Potortì AG, De Caro S, Di Bella B, Litrenta F, Fazio F. Bioactive Element Biodistribution of Different Biological Substrates in Sheep and Goats. Animals. 2025; 15(12):1686. https://doi.org/10.3390/ani15121686
Chicago/Turabian StyleNava, Vincenzo, Francesca Aragona, Angela Giorgia Potortì, Salvatore De Caro, Beatrice Di Bella, Federica Litrenta, and Francesco Fazio. 2025. "Bioactive Element Biodistribution of Different Biological Substrates in Sheep and Goats" Animals 15, no. 12: 1686. https://doi.org/10.3390/ani15121686
APA StyleNava, V., Aragona, F., Potortì, A. G., De Caro, S., Di Bella, B., Litrenta, F., & Fazio, F. (2025). Bioactive Element Biodistribution of Different Biological Substrates in Sheep and Goats. Animals, 15(12), 1686. https://doi.org/10.3390/ani15121686






