Essential Trace Elements in the Shells of Commercial Mollusk Species from the Black Sea and Their Biotechnological Potential
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
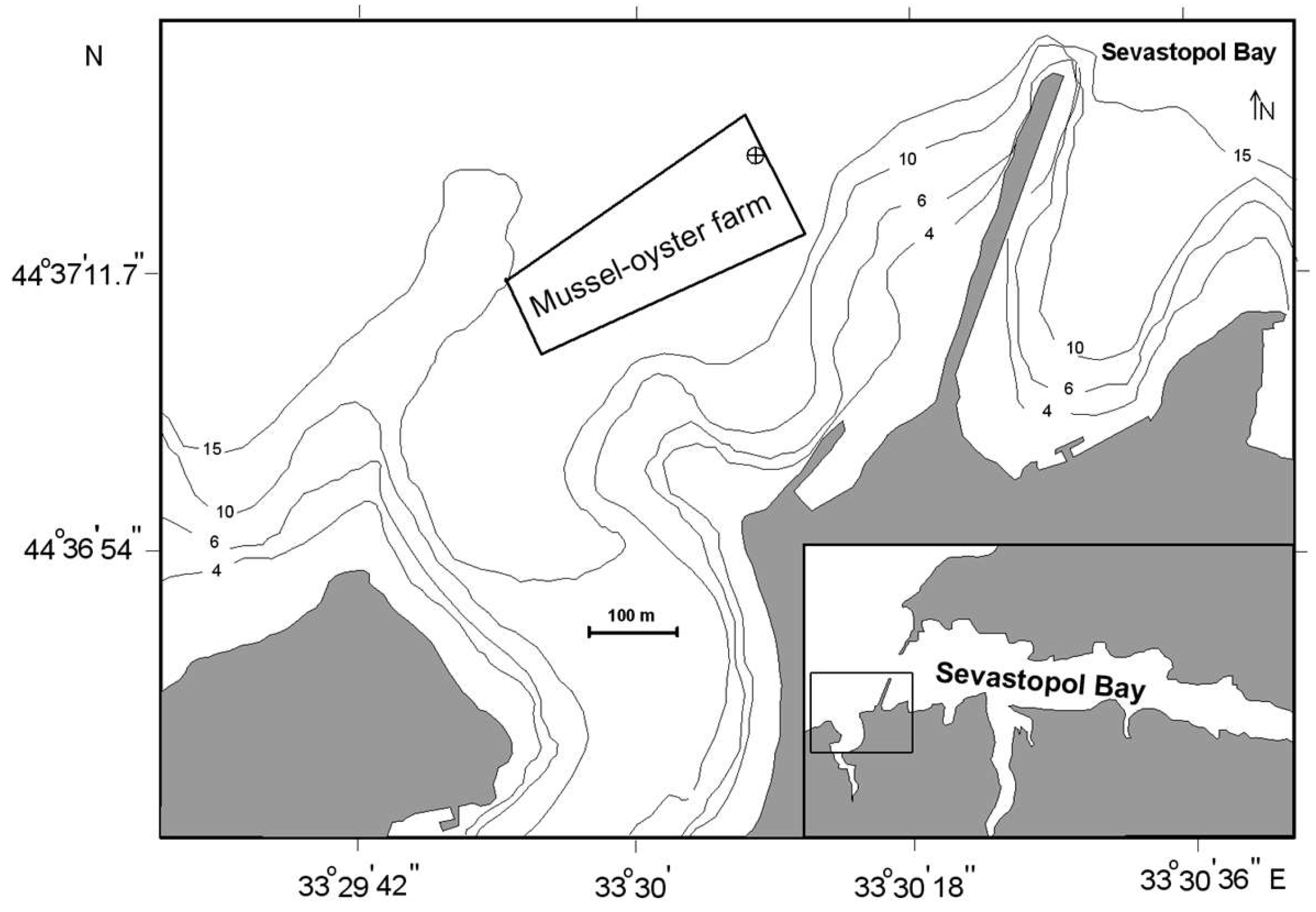
2.1. Sampling Site and Sampled Mollusks
2.2. Analytical Sample Preparation
2.3. ICP-MS Analysis
2.4. Quality Assurance/Quality Control
2.5. Statistical Analysis
3. Results
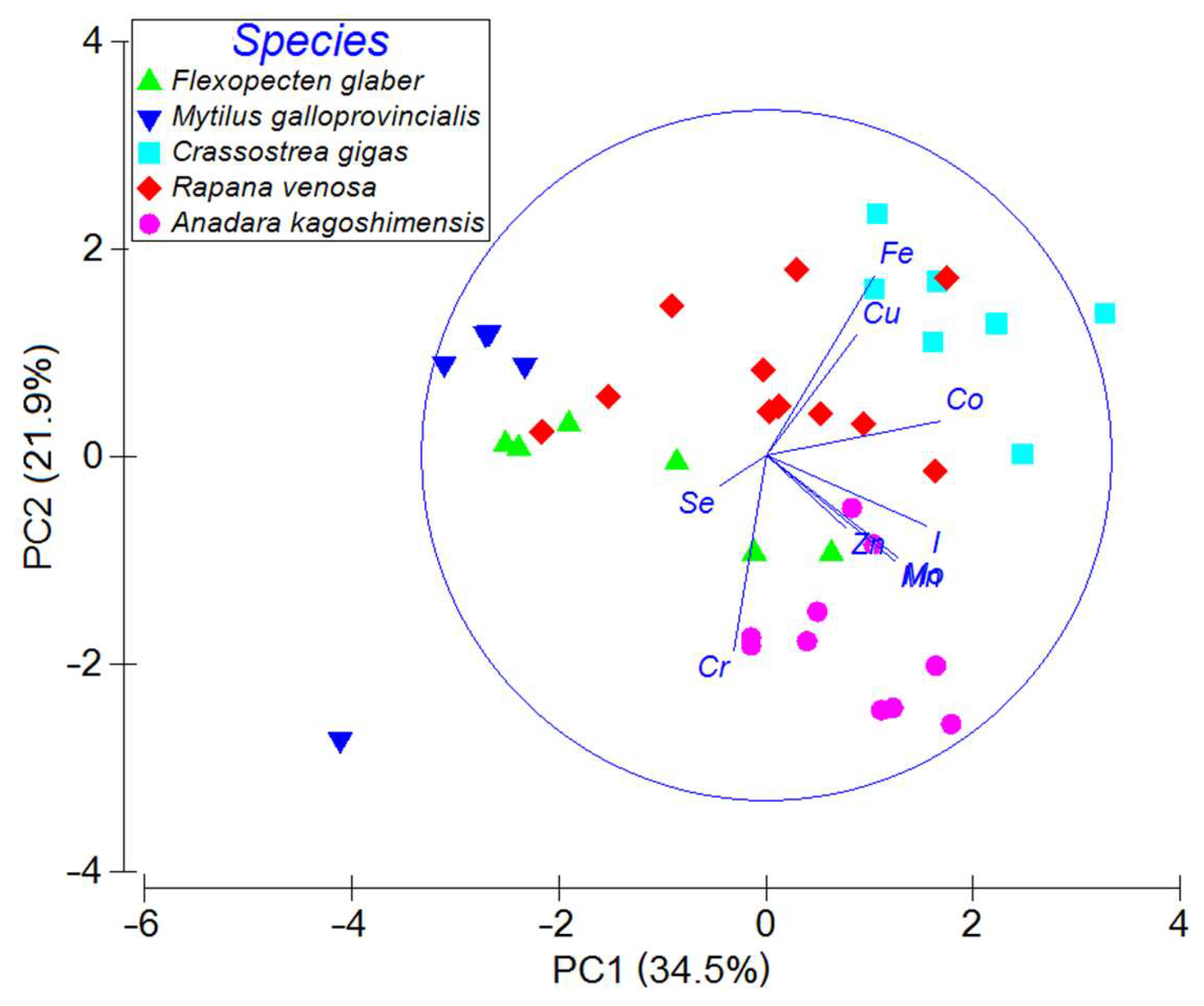
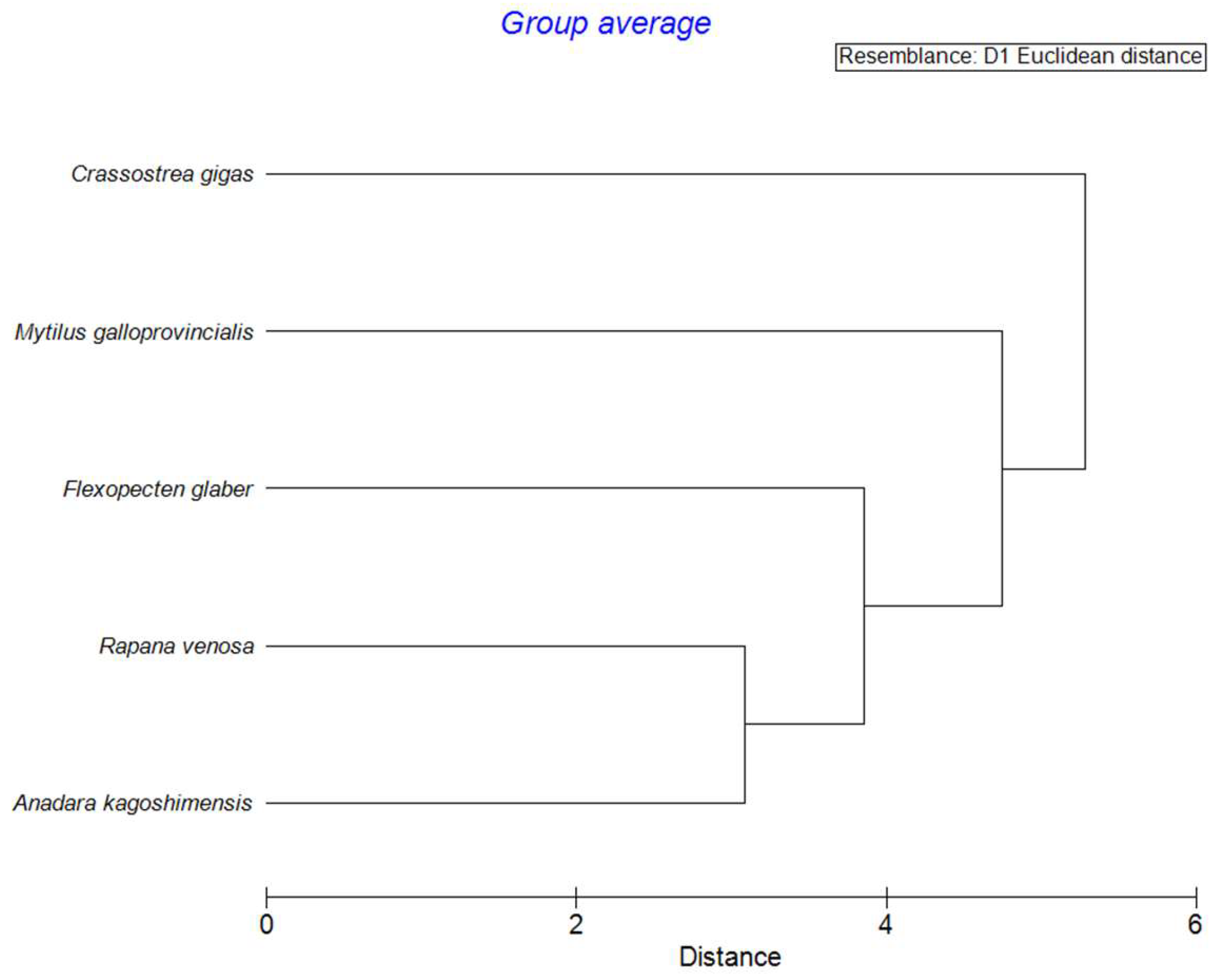
3.1. Element Concentrations and Multivariate Analyses
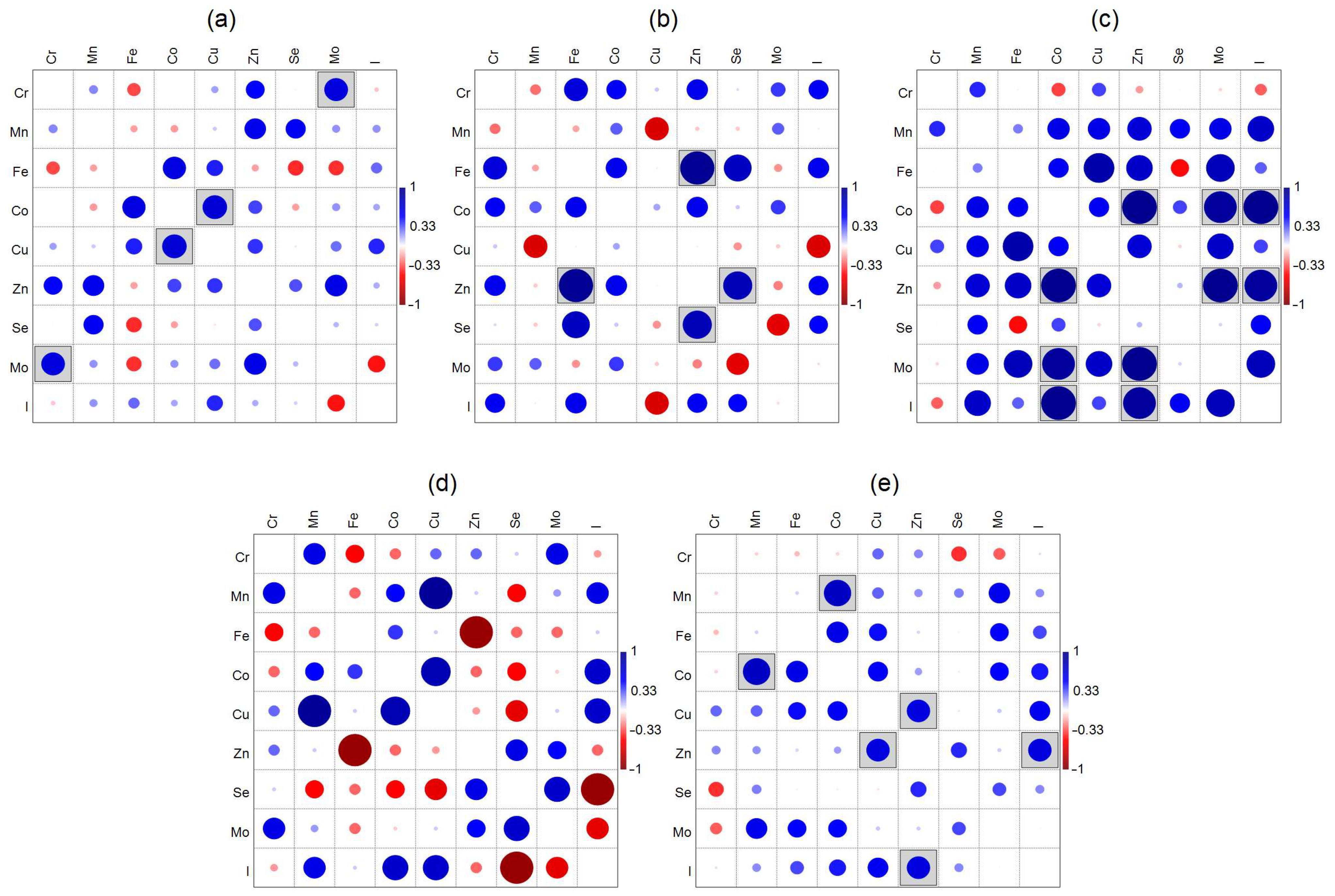
3.2. Correlations
4. Discussion
4.1. Factors Affecting the Element Accumulation in Mollusk Shells
4.2. The Comparison of the Results with the Literature Data
4.3. Role and Requirements for Essential Trace Elements in Humans
4.3.1. Iron
4.3.2. Manganese
4.3.3. Zinc
4.3.4. Copper
4.3.5. Cobalt
4.3.6. Iodine
4.3.7. Chromium
4.3.8. Molybdenum
4.3.9. Selenium
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ETEs | essential trace elements |
| ICP-MS | inductively coupled plasma mass spectrometry |
| PCA | principal component analysis |
| WHO | World Health Organization |
References
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2018, 11, 42–57. [Google Scholar] [CrossRef]
- Chelyadina, N.S.; Kapranov, S.V.; Popov, M.A.; Smirnova, L.L.; Bobko, N.I. The mussel Mytilus galloprovincialis (Crimea, Black Sea) as a source of essential trace elements in human nutrition. Biol. Trace Elem. Res. 2023, 201, 5415–5430. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.V.; Prabhu, G.N. Seafood. In Valorization of Food Processing By-Products; Chandrasekaran, M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 685–736. [Google Scholar]
- Demina, L.L.; Budko, D.M. Trace metals in carbonate biomineralization by the example of bivalvia Mytilus spp. from the Black Sea. Fundam. Res. 2014, 11, 2185–2189. (In Russian) [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology: IUPAC Recommendations, 2nd ed.; Blackwell Science: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, R. The Mineralization of Molluscan Shells: Some Unsolved Problems and Special Considerations. Front. Mar. Sci. 2022, 9, 874534. [Google Scholar] [CrossRef]
- Nekhoroshkov, P.; Zinicovscaia, I.; Nikolayev, D.; Lychagina, T.; Pakhnevich, A.; Yushin, N.; Bezuidenhout, J. Effect of the elemental content of shells of the bivalve mollusks (Mytilus galloprovincialis) from Saldanha Bay (South Africa) on their crystallographic texture. Biology 2021, 10, 1093. [Google Scholar] [CrossRef]
- Mertz, W. Review of the scientific basis for establishing the essentiality of trace elements. Biol. Trace Elem. Res. 1998, 66, 185–191. [Google Scholar] [CrossRef]
- Nielsen, F.H. Importance of making dietary recommendations for elements designated as nutritionally beneficial, pharmacologically beneficial, or conditioinally essential. J. Trace Elem. Exp. Med. 2000, 13, 113–129. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V. Essential Trace Elements in Human Health: A Physician’s View. Tomsk State University Publishing House: Tomsk, Russia, 2018. [Google Scholar]
- Mozrzymas, R. Trace elements in human health. In Recent Advances in Trace Elements; Chojnacka, K., Saeid, A., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 373–402. [Google Scholar] [CrossRef]
- WHO. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.D.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Jović, M.; Mandić, M.; Šljivić-Ivanović, M.; Smičiklas, I. Recent trends in aplication of shell waste from mariculture. Stud. Mar. 2019, 32, 47–62. [Google Scholar] [CrossRef]
- Sanchez-Jerez, P.; Krüger, L.; Casado-Coy, N.; Valle, C.; Sanz-Lazaro, C. Mollusk shell debris accumulation in the seabed derived from coastal fish farming. J. Mar. Sci. Eng. 2019, 7, 335. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, R.; Han, T.; Liu, H.; Huang, H.; Qi, Z. Shell accumulation on seabed due to suspended coastal oyster farming and effects on burrowing capacity of the polychaete Perinereis aibuhitensis. Front. Mar. Sci. 2023, 10, 1219184. [Google Scholar] [CrossRef]
- Lolas, A.; Molla, A.; Georgiou, K.; Apostologamvrou, C.; Petrotou, A.; Skordas, K. Effect of mussel shells as soil pH amendment on the growth and productivity of rosemary (Rosmarinus officinalis L.) cultivation. Agriculture 2024, 14, 144. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Mu, T.; Pan, Y. Research and Application of Shell Powder. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 032031. [Google Scholar] [CrossRef]
- Topić Popović, N.; Lorencin, V.; Strunjak-Perović, I.; Čož-Rakovac, R. Shell Waste Management and Utilization: Mitigating Organic Pollution and Enhancing Sustainability. Appl. Sci. 2023, 13, 623. [Google Scholar] [CrossRef]
- Chung, W.H.; Tan, N.S.L.; Kim, M.; Pojtanabuntoeng, T.; Howieson, J. Exploring the functional properties and utilisation potential of mollusca shell by-products through an interdisciplinary approach. Sci. Rep. 2024, 14, 28274. [Google Scholar] [CrossRef]
- Potortì, A.G.; Messina, L.; Licata, P.; Gugliandolo, E.; Santini, A.; Di Bella, G. Snail Shell Waste Threat to Sustainability and Circular Economy: Novel Application in Food Industries. Sustainability 2024, 16, 706. [Google Scholar] [CrossRef]
- Ozuni, E.; Andoni, E.; Castrica, M.; Balzaretti, C.M.; Brecchia, G.; Agradi, S.; Curone, G.; Di Cesare, F.; Fehri, N.E.; Luke, B.; et al. Human exposure to heavy metals and possible public health risks via consumption of mussels M. galloprovincialis from the Albanian sea coast. Chemosphere 2024, 368, 143689. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chem. 2003, 80, 119–123. [Google Scholar] [CrossRef]
- Beldi, H.; Gimbert, F.; Maas, S.; Scheifler, R.; Soltani, N. Seasonal variations of Cd, Cu, Pb and Zn in the edible mollusc Donax trunculus (Mollusca, Bivalvia) from the gulf of Annaba, Algeria. Afr. J. Agric. Res. 2006, 1, 85–90. [Google Scholar]
- Keskin, Y.; Baskaya, R.; Özyaral, O.; Yurdun, T.; Lüleci, N.E.; Hayran, O. Cadmium, lead, mercury and copper in fish from the Marmara Sea, Turkey. Bull. Environ. Contam. Toxicol. 2007, 78, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.F.; Hamdan, S.; Rahman, M.R. Review on the risk assessment of heavy metals in Malaysian clams. Sci. World J. 2015, 2015, 905497. [Google Scholar] [CrossRef]
- Berik, N.; Çankırılıgil, E.C.; Gül, G. Mineral content of smooth scallop (Flexopecten glaber) caught Canakkale, Turkey and evaluation in terms of food safety. J. Trace Elem. Med. Biol. 2017, 42, 97–102. [Google Scholar] [CrossRef]
- Biandolino, F.; Parlapiano, I.; Grattagliano, A.; Fanelli, G.; Prato, E. Comparative characteristics of percentage edibility, condition index, biochemical constituents and lipids nutritional quality indices of wild and farmed scallops (Flexopecten glaber). Water 2020, 12, 1777. [Google Scholar] [CrossRef]
- Vural, P.; Acarlı, S. Monthly variation of micro-and macro-element composition in smooth scallop, Flexopecten glaber (Linnaeus,1758), from the Çardak Lagoon (Çanakkale Strait, Turkey). Ege J. Fish. Aquat. Sci. 2021, 38, 449–459. [Google Scholar] [CrossRef]
- Tepe, Y.; Süer, N. The levels of heavy metals in the Mediterranean mussel (Mytilus Galloprovincialis Lamarck, 1819); example of Giresun coasts of the Black Sea, Turkey. Indian J. Geo-Mar. Sci. 2016, 45, 283–289. [Google Scholar]
- Temerdashev, Z.A.; Eletskii, I.I.; Kaunova, A.A.; Korpakova, I.G. Determination of heavy metals in Mytilus galloprovincialis Lamarck mussels using the ICP-AES method. Anal. Control 2017, 21, 116–124. [Google Scholar] [CrossRef]
- Kapranov, S.V.; Karavantseva, N.V.; Bobko, N.I.; Ryabushko, V.I.; Kapranova, L.L. Element contents in three commercially important edible mollusks harvested off the southwestern coast of Crimea (Black Sea) and assessment of human health risks from their consumption. Foods 2021, 10, 2313. [Google Scholar] [CrossRef]
- Kapranov, S.V.; Kozintsev, A.F.; Bobko, N.I.; Ryabushko, V.I. Elements in soft tissues of the young mediterranean mussel Mytilus galloprovincialis Lam. 1819 collected in Sevastopol Bay (Crimea, Black Sea): Effects of age, sex, location, and principal morphometric parameters. Animals 2023, 13, 1950. [Google Scholar] [CrossRef]
- Ryabushko, V.I.; Toichkin, A.M.; Kapranov, S.V. Heavy metals and arsenic in soft tissues of the gastropod Rapana venosa (Valenciennes, 1846) collected on a mollusk farm off Sevastopol (Southwestern Crimea, Black Sea): Assessing human health risk and locating regional contamination areas. Bull. Environ. Contam. Toxicol. 2022, 108, 1039–1045. [Google Scholar] [CrossRef]
- Kapranov, S.V.; Kovrigina, N.P.; Troshchenko, O.A.; Rodionova, N.Y. Long-term variations of thermohaline and hydrochemical characteristics in the mussel farm area in the coastal waters off Sevastopol (Black Sea) in 2001–2018. Cont. Shelf Res. 2020, 206, 104185. [Google Scholar] [CrossRef]
- Pospelova, N.V.; Egorov, V.N.; Proskurnin, V.Y.; Priimak, A.S. Suspended Particulate Matter as a Biochemical Barrier to Heavy Metals in Marine Farm Areas (Sevastopol, the Black Sea). Mar. Biol. J. 2022, 7, 55–69. [Google Scholar]
- Schneider, A.; Domhoff, G.W. Corrections for the Statistical Significance of Multiple Comparisons. 2025. Available online: https://dreams.ucsc.edu/DreamSAT/multiple_comparisons.php (accessed on 28 April 2025).
- Fowler, S.W. Critical review of selected heavy metal and chlorinated hydrocarbon concentrations in the marine environment. Mar. Environ. Res. 1990, 29, 1–64. [Google Scholar] [CrossRef]
- Rainbow, P.S.; Phillips, D.J.H. Cosmopolitan biomonitors of trace metals. Mar. Pollut. Bull. 1993, 26, 593–601. [Google Scholar] [CrossRef]
- Boran, M.; Altinok, I. A review of heavy matals in water, sediment and living organisms in the Black Sea. Turk. J. Fish. Aquat. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef]
- Casas, S.; Gonzalez, J.-L.; Andral, B.; Cossa, D. Relation between metal concentration in water and metal content of marine mussels (Mytilus galloprovincialis): Impact of physiology. Environ. Toxicol. Chem. 2008, 27, 1543–1552. [Google Scholar] [CrossRef]
- Vander Putten, E.; Dehairs, F.; Keppens, E.; Baeyens, W. High resolution distribution of trace elements in the calcite shell layer of modern Mytilus edulis: Environmental and biological controls. Geochim. Cosmochim. Acta 2000, 64, 997–1011. [Google Scholar] [CrossRef]
- Gillikin, D.P.; Dehairs, F.; Lorrain, A.; Steenmans, D.; Baeyens, W.; André, L. Barium uptake into the shells of the common mussel (Mytilus edulis) and the potential for estuarine paleo-chemistry reconstruction. Geochim. Cosmochim. Acta 2006, 70, 395–407. [Google Scholar] [CrossRef]
- Akagi, T.; Edanami, K. Sources of rare earth elements in shells and soft-tissues of bivalves from Tokyo Bay. Mar. Chem. 2017, 194, 55–62. [Google Scholar] [CrossRef]
- Lyubas, A.A.; Kuznetsova, I.A.; Bovykina, G.V.; Eliseeva, T.A.; Gofarov, M.Y.; Khrebtova, I.S.; Kondakov, A.V.; Malkov, A.V.; Mavromatis, V.; Shevchenko, A.R.; et al. Trace Element Patterns in Shells of Mussels (Bivalvia) Allow to Distinguish between Fresh- and Brackish-Water Coastal Environments of the Subarctic and Boreal Zone. Water 2023, 15, 3625. [Google Scholar] [CrossRef]
- Freitas, P.S.; Clarke, L.J.; Kennedy, H.; Richardson, C.A. Manganese in the shell of the bivalve Mytilus edulis: Seawater Mn or physiological control? Geochim. Cosmochim. Acta 2016, 194, 266–278. [Google Scholar] [CrossRef]
- Barrat, J.-A.; Chauvaud, L.; Olivier, F.; Poitevin, P.; Rouget, M.-L. Trace elements in bivalve shells: How “vital effects” can bias environmental studies. Chem. Geol. 2023, 638, 121695. [Google Scholar] [CrossRef]
- Khoo, H.W.; Mok, K.F.; Tang, S.M.; Yap, C.T. Strontium/calcium ratio analysis of molluscan shells in Singapore waters using the X-ray fluorescence technique. Environ. Monit. Assess. 1985, 5, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Piwoni-Piórewicz, A.; Strekopytov, S.; Humphreys-Williams, E.; Kukliński, P. The patterns of elemental concentration (Ca, Na, Sr, Mg, Mn, Ba, Cu, Pb, V, Y, U and Cd) in shells of invertebrates representing different CaCO3 polymorphs: A case study from the brackish Gulf of Gdańsk (the Baltic Sea). Biogeosciences 2021, 18, 707–728. [Google Scholar] [CrossRef]
- de Winter, N.J.; van Sikkeleras, S.; Goudsmit-Harzevoort, B.; Boer, W.; de Nooijer, L.; Reichart, G.-J.; Claeys, P.; Witbaard, R. Tracing timing of growth in cultured molluscs using strontium spiking. Front. Mar. Sci. 2023, 10, 1157929. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, S.Y.; Owens, V.N.; Park, S.; Kim, J.; Hong, C.O. How Does Oyster Shell Immobilize Cadmium? Arch. Environ. Contam. Toxicol. 2018, 74, 114–120. [Google Scholar] [CrossRef]
- Kwoczek, M.; Szefer, P.; Hać, E.; Grembecka, M. Essential and toxic elements in seafood available in Poland from different geographical regions. J. Agric. Food Chem. 2006, 54, 3015–3024. [Google Scholar] [CrossRef]
- Guérin, T.; Chekri, R.; Vastel, C.; Sirot, V.; Volatier, J.-L.; Leblanc, J.-C.; Noël, L. Determination of 20 trace elements in fish and other seafood from the French market. Food Chem. 2011, 127, 934–942. [Google Scholar] [CrossRef]
- Ergül, H.A.; Aksan, S. Evaluation of non-essential element and micronutrient concentrations in seafood from the Marmara and Black Seas. J. Black Sea/Mediterr. Environ. 2013, 19, 312–331. [Google Scholar]
- Lehel, J.; Magyar, M.; Palotás, P.; Abonyi-Tóth, Z.; Bartha, A.; Budai, P. To Eat or Not to Eat?—Food Safety Aspects of Essential Metals in Seafood. Foods 2023, 12, 4082. [Google Scholar] [CrossRef]
- He, M.; Wang, W.-X. Bioaccessibility of 12 trace elements in marine molluscs. Food Chem. Toxicol. 2013, 55, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Laird, B.D.; Chan, H.M. Bioaccessibility of metals in fish, shellfish, wild game, and seaweed harvested in British Columbia, Canada. Food Chem. Toxicol. 2013, 58, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Li, R.; Gong, Y.; Shen, X.; Yu, L. Bioaccessibility-corrected health risk of heavy metal exposure via shellfish consumption in coastal region of China. Environ. Pollut. 2021, 273, 116529. [Google Scholar] [CrossRef] [PubMed]
- Kapranova, L.; Dikareva, J.; Kapranov, S.; Ryabushko, V. The element contents in soft tissues and shells of the bivalve Anadara kagoshimensis (Tokunaga, 1906) from the Black Sea and Sea of Azov. Mar. Biol. J. 2024, 9, 24–33. [Google Scholar] [CrossRef]
- Bharatham, H.; Zakaria, M.Z.A.B.; Perimal, E.K.; Yusof, L.M.; Hamid, M. Mineral and physiochemical evaluation of cockle shell (Anadara granosa) and other selected molluscan shell as potential biomaterials. Sains Malays. 2014, 43, 1023–1029. [Google Scholar]
- Yap, C.K.; Hatta, Y.; Edward, F.B.; Tan, S.G. Comparison of heavy metal concentrations (Cd, Cu, Fe, Ni and Zn) in the shells and different soft tissues of Anadara granosa collected from Jeram, Kuala Juru and Kuala Kurau, Peninsular Malaysia. Pertanika J. Trop. Agric. Sci. 2008, 31, 205–215. [Google Scholar]
- Chenet, T.; Schwarz, G.; Neff, C.; Hattendorf, B.; Günther, D.; Martucci, A.; Cescon, M.; Baldi, A.; Pasti, L. Scallop shells as biosorbents for water remediation from heavy metals: Contributions and mechanism of shell components in the adsorption of cadmium from aqueous matrix. Heliyon 2024, 10, e29296. [Google Scholar] [CrossRef]
- Cardoso da Silva, P.S.C.; de Moura Farias, W.; Gomez, M.R.B.P.; Torrecilha, J.K.; Rocha, F.R.; Scapin, M.A.; Garcia, R.H.L.; de Simone, L.R.L.; de Amaral, V.S.; Vincent, M.; et al. Oyster shell element composition as a proxy for environmental studies. J. S. Am. Earth Sci. 2024, 134, 104749. [Google Scholar] [CrossRef]
- Sereanu, V.; Meghea, I.; Vasile, G.G.; Mihai, M. Environmental influence on Rapana venosa shell morphotypes and phenotypes from the Romanian Black Sea Coast. Rev. Chim. 2018, 69, 50–56. [Google Scholar] [CrossRef]
- Mititelu, M.; Dogaru, E.; Nicolescu, T.O.; Hincu, L.; Băncescu, A.; Ioniţă, C. Heavy metals analysis in some mollusks shells from Black Sea. Sci. Study Res. 2008, 9, 195–198. [Google Scholar]
- Pospelova, N.V.; Priimak, A.S.; Ryabushko, V.I. Chemical Composition of Mussel Mytilus galloprovincialis Cultivated at the Seashore of Sevastopol (Black Sea). Ecol. Saf. Coast. Shelf Zones Sea 2021, 4, 67–80. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Statistics 2008; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Williams, S.T. Molluscan shell colour. Biol. Rev. 2016, 92, 1039–1058. [Google Scholar] [CrossRef]
- Nekhoroshev, M.V.; Kozintsev, A.F.; Gureeva, E.V.; Kapranov, S.V.; Kapranova, L.L.; Ryabushko, V.I. Element and carotenoid contents in the oyster Crassostrea gigas (Thunberg. 1793) with different shell pigmentation intensities. Aquac. Int. 2025, 33, 104. [Google Scholar] [CrossRef]
- Bonnard, M.; Cantel, S.; Boury, B.; Parrot, I. Chemical evidence of rare porphyrins in purple shells of Crassostrea gigas oyster. Sci. Rep. 2020, 10, 12150. [Google Scholar] [CrossRef]
- Bonnard, M.; Boury, B.; Parrot, I. Xanthurenic acid in the shell purple patterns of Crassostrea gigas: First evidence of an ommochrome metabolite in a mollusk shell. Molecules 2021, 26, 7263. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. The elements of life and medicines. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140182. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M.A. Manganese metabolism in humans. Front. Biosci. Landmark 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Uğurlu, E.; Kumruoğlu, L.C. Various Elements Levels in Four Freshwater Mussels Shells Obtained from Gölbaşı Lake, Turkey. Pollution 2024, 10, 73–89. [Google Scholar] [CrossRef]
- Swinehart, J.H.; Smith, K.W. Iron and manganese deposition in the periostraca of several bivalve molluscs. Biol. Bull. 1979, 156, 369–381. [Google Scholar] [CrossRef]
- Dikareva, Y.D.; Kapranova, L.L.; Kapranov, S.V.; Ryabushko, V.I.; Shchurov, S.V. Heavy metals in mollusk shells of the Black Sea. Biodivers. Sustain. Dev. 2025, 9, 50–61. (In Russian) [Google Scholar]
- Stanković, S.; Jović, M.D.; Milanov, R.; Joksimović, D. Trace elements concentrations (Zn, Cu, Pb, Cd, As and Hg) in the Mediterranean mussel (Mytilus galloprovincialis) and evaluation of mussel quality and possible human health risk from cultivated and wild sites of the southeastern Adriatic Sea, Montenegro. J. Serbian Chem. Soc. 2011, 76, 1725–1737. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, M.; Chang, L.; Liu, Q.; Wang, C.; Hu, L.; Zhang, Z.; Ding, W.; Chen, L.; Guo, S.; et al. Overview of structure, function and integrated utilization of marine shell. Sci. Total Environ. 2023, 870, 161950. [Google Scholar] [CrossRef]
- Dato-Cajegas, C.R.S.; Yakupitiyage, A. The need for dietary mineral suplementation for Nile tilapia, Oreochromis niloticus, cultured in a semi-intensive system. Aquaculture 1996, 144, 227–237. [Google Scholar] [CrossRef]
- Araújo, D.F.; Ponzevera, E.; Knoery, J.; Briant, N.; Bruzac, S.; Sireau, T.; Pellouin-Grouhel, A.; Brach-Papa, C. Can coper isotope composition in oysters improve marine biomonitoring and seafood traceability? J. Sea Res. 2023, 191, 102334. [Google Scholar] [CrossRef]
- Araújo, D.F.; Ponzevera, E.; Weiss, D.J.; Knoery, J.; Briant, N.; Yepez, S.; Bruzac, S.; Sireau, T.; Brach-Papa, C. Aplication of Zn isotope compositions in oysters to monitor and quantify anthropogenic Zn bioaccumulation in marine environments over four decades: A “Mussel Watch Program” upgrade. ACS EST Water 2021, 1, 1035–1046. [Google Scholar] [CrossRef]
- Barreira, J.; Araújo, D.F.; Machado, W.; Ponzevera, E. Coper and zinc isotope systematics in different bivalve mollusk species from the French coastline: Implications for biomonitoring. Mar. Pollut. Bull. 2024, 201, 116177. [Google Scholar] [CrossRef]
- Gondal, A.H.; Zafar, A.; Zainab, D.; Toor, M.D.; Sohail, S.; Ameen, S.; Ljaz, A.B.; Ch, B.I.; Hussain, I.; Haider, S.; et al. A detailed review study of zinc involvement in animal, plant and human nutrition. Indian J. Pure Appl. Biosci. 2021, 9, 262–271. [Google Scholar] [CrossRef]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Truong-Tran, A.Q.; Carter, J.; Ruffin, R.E.; Zalewski, P.D. The role of zinc in caspase activation and apoptotic cell death. Biometals 2001, 14, 129–144. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Altarelli, M.; Ben Hamouda, N.; Schneider, A.; Berger, M.M. Copper deficiency: Causes, manifestations, and treatment. Nutr. Clin. Pract. 2019, 34, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Binesh, A.; Venkatachalam, K. Copper in Human Health and Disease: A Comprehensive Review. J. Biochem. Mol. Toxicol. 2024, 38, e70052. [Google Scholar] [CrossRef]
- Karim, N. Copper and human health-a review. J. Bahria Univ. Med. Dent. Coll. 2018, 8, 117–122. [Google Scholar] [CrossRef]
- Klevay, L.M.; Reck, S.J.; Jacob, R.A.; Logan Jr, G.M.; Munoz, J.M.; Sandstead, H.H. The human requirement for copper I. Healthy men fed conventional, American diets. Am. J. Clin. Nutr. 1980, 33, 45–50. [Google Scholar] [CrossRef]
- Krampitz, G.; Drolshagen, H.; Häusle, J.; Hof-Irmscher, K. Organic matrices of mollusc shells. In Biomineralization and Biological Metal Accumulation. Biological and Geological Perspectives; Westbroek, P., Jong, E.W., Eds.; Reidel Publishing Company: Dordrecht, The Netherlands, 1983; pp. 231–247. [Google Scholar] [CrossRef]
- Lison, D. Chapter 9—Cobalt. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: London, UK, 2022; pp. 221–242. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of cobalt in the environment and its role in biological processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef]
- Finley, B.L.; Unice, K.M.; Kerger, B.D.; Otani, J.M.; Paustenbach, D.J.; Galbraith, D.A.; Tvermoes, B.E. 31-day study of cobalt (II) chloride ingestion in humans: Pharmacokinetics and clinical effects. J. Toxicol. Environ. Health Part A 2013, 76, 1210–1224. [Google Scholar] [CrossRef]
- Bost, M.; Martin, A.; Orgiazzi, J. Iodine deficiency: Epidemiology and nutritional prevention. Trace Elem. Med. 2014, 15, 3–7. [Google Scholar]
- Delange, F. Requirements of iodine in humans. In Iodine Deficiency in Europe: A Continuing Concern; Delange, F., Dunn, J.T., Glinoer, D., Eds.; NATO ASI Series; Plenum Press: New York, NY, USA, 1993; pp. 5–15. [Google Scholar] [CrossRef]
- Biban, B.G.; Lichiardopol, C. Iodine deficiency, still a global problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, A.; Brent, G.A. Chapter 12—Iodine and Thyroid Hormone Synthesis, Metabolism, and Action. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Academic Press: London, UK, 2017; pp. 143–150. [Google Scholar] [CrossRef]
- Frei, R.; Paulukat, C.; Bruggmann, S.; Klaebe, R.M. A systematic look at chromium isotopes in modern shells–implications for paleo-environmental reconstructions. Biogeosciences 2018, 15, 4905–4922. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Hu, F.B. Role of chromium in human health and in diabetes. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium and insulin resistance. Nutr. Res. Rev. 2003, 16, 267–275. [Google Scholar] [CrossRef]
- Reutina, S.V. The role of chromium in the person’s organism. Rudn. J. Ecol. Life Saf. 2009, 68, 50–55. (In Russian) [Google Scholar]
- Anderson, R.A. Chromium as an essential nutrient for humans. Regul. Toxicol. Pharmacol. 1997, 26, S35–S41. [Google Scholar] [CrossRef]
- Tallkvist, J.; Oskarsson, A. Chapter 47—Molybdenum. In Handbook on the Toxicology of Metals, 4th ed.; Gunnar, F.N., Bruce, A.F., Monica, N., Eds.; Academic Press: London, UK, 2015; pp. 1077–1089. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on dietary reference values for molybdenum. EFSA J. 2013, 11, 3333. [Google Scholar]
- WHO. Molybdenum in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Turnlund, J.R.; Weaver, C.M.; Kim, S.K.; Keyes, W.R.; Gizaw, Y.; Thompson, K.H.; Peiffer, G.L. Molybdenum absorption and utilization in humans from soy and kale intrinsically labeled with stable isotopes of molybdenum. Am. J. Clin. Nutr. 1999, 69, 1217–1223. [Google Scholar] [CrossRef]
- Mendel, R.R.; Kruse, T. Cell biology of molybdenum in plants and humans. Biochim. et Biophys. Acta (BBA)–Mol. Cell Res. 2012, 1823, 1568–1579. [Google Scholar] [CrossRef]
- Ireland, M.P. Interaction and effects of molybdenum compounds on growth and mineral content of Achatina fulica and Arion ater (Gastropoda: Pulmonata). Comp. Biochem. Physiol. Part C: Pharmacol. Toxicol. Endocrinol. 1994, 107, 441–446. [Google Scholar] [CrossRef]
- Barats, A.; Amouroux, D.; Pécheyran, C.; Chauvaud, L.; Thébault, J.; Donard, O.F. Spring molybdenum enrichment in scallop shells: A potential tracer of diatom productivity in temperate coastal environments (Brittany, NW France). Biogeosciences 2010, 7, 233–245. [Google Scholar] [CrossRef]
- Haque, I. Molybdenum in soils and plants and its potential importance to livestock nutrition, with special reference to sub-Saharan Africa. Ilca Bull. 1987, 26, 20–28. [Google Scholar]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Sobolev, O.; Gutyj, B.; Petryshak, R.; Pivtorak, J.; Kovalskyi, Y.; Naumyuk, A.; Petryshak, O.; Semchuk, I.; Mateusz, V.; Shcherbatyy, A.; et al. Biological role of selenium in the organism of animals and humans. Ukr. J. Ecol. 2018, 8, 654–665. (In Russian) [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.-J.; Meng, Q.; Han, H.; et al. Role of selenium and selenoproteins in male reproductive function: A review of past and present evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef]
- Rayman, M. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2004, 39, 112–120. [Google Scholar] [CrossRef]
- Kapranova, L.L.; Ryabushko, V.I.; Nekhoroshev, M.V.; Kapranov, S.V. Steroid hormones, selenium, and zinc in the gonads-gametes-larvae biological system of the mussel Mytilus galloprovincialis Lam. Mar. Biol. J. 2021, 6, 39–50. [Google Scholar] [CrossRef]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.H.; Iqbal, Z. Role of selenium in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef]
- Smrkolj, P.; Pograjc, L.; Hlastan-Ribič, C.; Stibilj, V. Selenium content in selected Slovenian foodstuffs and estimated daily intakes of selenium. Food Chem. 2005, 90, 691–697. [Google Scholar] [CrossRef]
- Vinceti, M.; Wei, E.T.; Malagoli, C.; Bergomi, M.; Vivoli, G. Adverse health effects of selenium in humans. Rev. Environ. Health 2001, 16, 233–251. [Google Scholar] [CrossRef]

| Anadara kagoshimensis | Crassostrea gigas | Flexopecten glaber ponticus | Mytilus galloprovincialis | Rapana venosa | |
|---|---|---|---|---|---|
| Cr | 7.81 ± 4.60 * (2.80–15.2) | 0.22 ± 0.28 AF (0.23–0.83) | 1.74 ± 0.65 AC (1.30–2.83) | 1.69 ± 1.45 A (0.98–4.30) | 0.77 ± 0.50 A (BDL–1.70) |
| Mn | 35.1 ± 18.2 (16.7–71.8) | 24.3 ± 12.0 (14.9–49.5) | 19.2 ± 5.3 (10.4–26.3) | 8.17 ± 1.56 (6.20–10.3) | 16.2 ± 10.9 (3.63–39.4) |
| Fe | 1564 ± 325 CRF (117–2304) | 13157 ± 4088 * (9391–21598) | 2285 ±185 ACR (2023–2536) | 1962 ± 1028 R (127–2490) | 3294 ± 665 ACF (2335–4972) |
| Co | 2.19 ± 0.14 F (1.91–2.44) | 2.73 ± 0.97 (2.03–4.78) | 1.29 ± 0.15 A (1.12–1.48) | 0.99 ± 0.39 (0.29–1.19) | 1.83 ± 0.63 (1.19–3.38) |
| Cu † | 75.0 ± 36.9 R (20.8–129) | 170 ±73 (87.3–295) | 89.4 ± 52.4 (46.2–178) | 77.1 ± 21.0 (44.5–97.8) | 190 ± 77A (22.2–303) |
| Zn | 8.85 ± 8.26 (3.24–32.9) | 21.1 ± 16.1 (4.71–63.3) | 4.61 ± 3.27 (1.06–8.96) | 30.0 ± 15.4 (1.26–69.1) | 6.81 ± 2.64 (3.03–11.0) |
| Se | 1.42 ± 0.95 (0.16–2.97) | 1.31 ± 0.66 (0.26–10.5) | 2.35 ± 0.26 (2.09–2.72) | 2.43 ± 1.45 (1.05–4.92) | 3.77 ± 3.41 (0.26–10.5) |
| Mo | 1.00 ± 0.70 (0.50–2.92) | 0.85 ± 0.41 (0.32–1.51) | 1.06 ± 0.96 (0.24–3.06) | 0.28 ± 0.12 (0.22–0.50) | 0.56 ± 0.14 (0.26–0.87) |
| I | 55.6 ± 17.3 FMR (39.0–82.1) | 35.1 ± 5.4FM (30.6–45.1) | 9.21 ± 4.83 AC (4.68–18.0) | 5.90 ± 0.45 ACR (5.18–6.36) | 26.0 ± 15.7 AM (10.7–67.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapranova, L.L.; Dikareva, J.D.; Kapranov, S.V.; Balycheva, D.S.; Ryabushko, V.I. Essential Trace Elements in the Shells of Commercial Mollusk Species from the Black Sea and Their Biotechnological Potential. Animals 2025, 15, 1637. https://doi.org/10.3390/ani15111637
Kapranova LL, Dikareva JD, Kapranov SV, Balycheva DS, Ryabushko VI. Essential Trace Elements in the Shells of Commercial Mollusk Species from the Black Sea and Their Biotechnological Potential. Animals. 2025; 15(11):1637. https://doi.org/10.3390/ani15111637
Chicago/Turabian StyleKapranova, Larisa L., Juliya D. Dikareva, Sergey V. Kapranov, Daria S. Balycheva, and Vitaliy I. Ryabushko. 2025. "Essential Trace Elements in the Shells of Commercial Mollusk Species from the Black Sea and Their Biotechnological Potential" Animals 15, no. 11: 1637. https://doi.org/10.3390/ani15111637
APA StyleKapranova, L. L., Dikareva, J. D., Kapranov, S. V., Balycheva, D. S., & Ryabushko, V. I. (2025). Essential Trace Elements in the Shells of Commercial Mollusk Species from the Black Sea and Their Biotechnological Potential. Animals, 15(11), 1637. https://doi.org/10.3390/ani15111637






