Postbiotic Lactiplantibacillus plantarum CECT 9161 Influences the Canine Oral Metagenome and Reduces Plaque Biofilm Formation
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Preclinical Phase
2.1.1. Strain Preparation and Characterisation
2.1.2. Growth Inhibition of Canine Oral Early Colonizers
2.1.3. Inhibition of Oral Early Colonizer Biofilm Formation
2.1.4. Influence of HT L. Plantarum CECT 9161 on Growth and Microbial Composition of Biofilms from Canine Saliva
2.1.5. Statistical Analysis of In Vitro Microbiome Features
2.1.6. Modulation of Inflammatory Markers in a Buccal Epithelial Cell Model
2.1.7. Statistical Analysis of In Vitro Data
2.2. Clinical Phase
2.2.1. Animals
2.2.2. Assessment of Consumption
2.2.3. Dental Examinations
2.2.4. Supragingival Plaque Sample Collection
2.2.5. Statistical Analysis of Gingivitis, Halitosis, Plaque, and Calculus Scores
2.2.6. DNA Isolation and Metagenomic Next-Generation Sequencing of Supragingival Plaque Samples
2.2.7. Next-Generation Sequencing of Supragingival Plaque Samples
2.2.8. Preprocessing for Metagenome Analysis
2.2.9. Statistical Analysis of the Supragingival Plaque Microbiome
2.2.10. Identification of Taxonomic and Functional Features in the Microbiome of Supragingival Plaque
3. Results
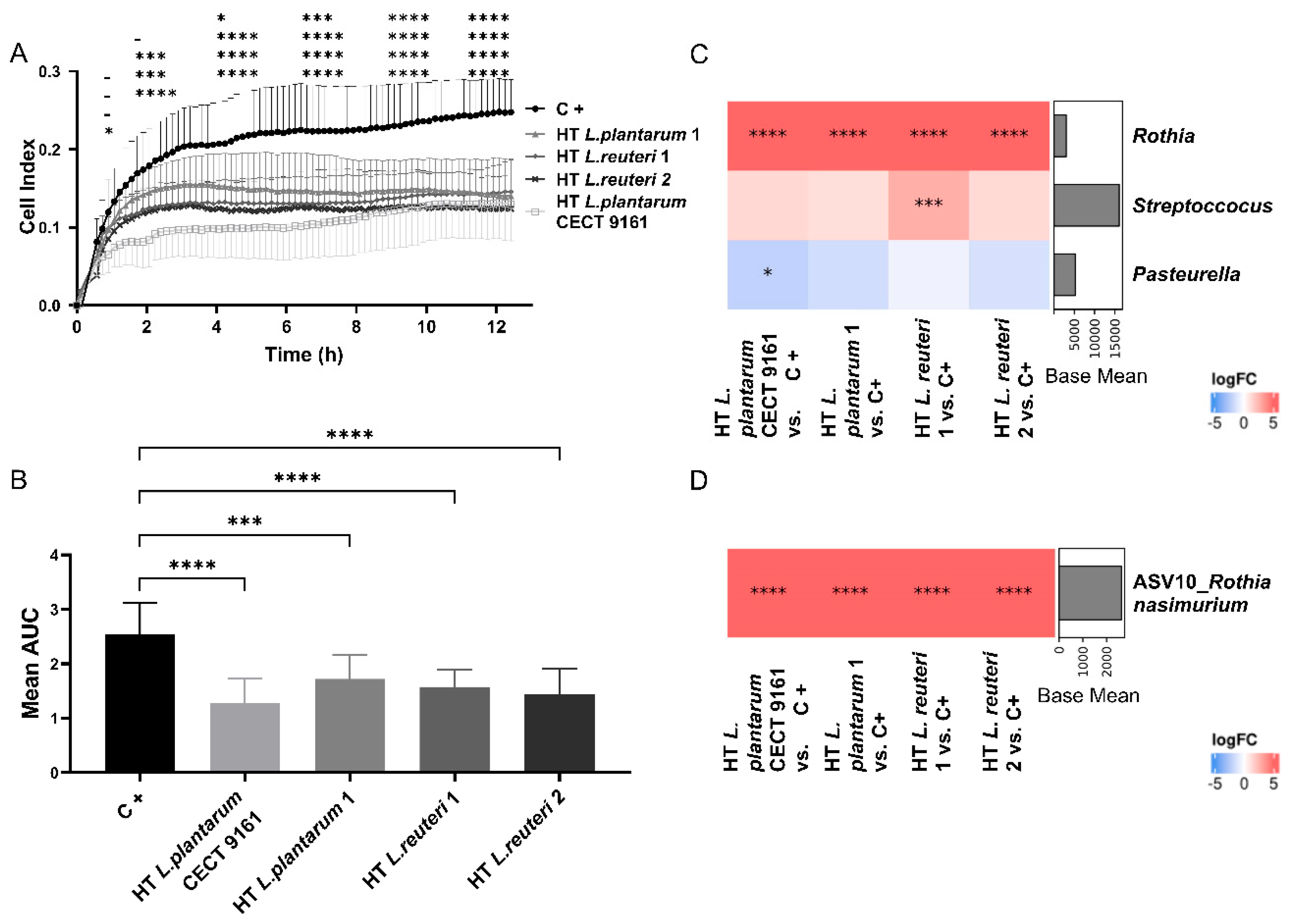
3.1. Growth Inhibition of Canine Oral Biofilm Early Colonizers
3.2. Influence of HT L. plantarum CECT 9161 on Growth and Microbial Composition of Biofilms from Canine Saliva
3.3. Modulation of Inflammatory Markers in a Buccal Epithelial Cell Model
3.4. Clinical Phase
3.5. Oral Health Markers
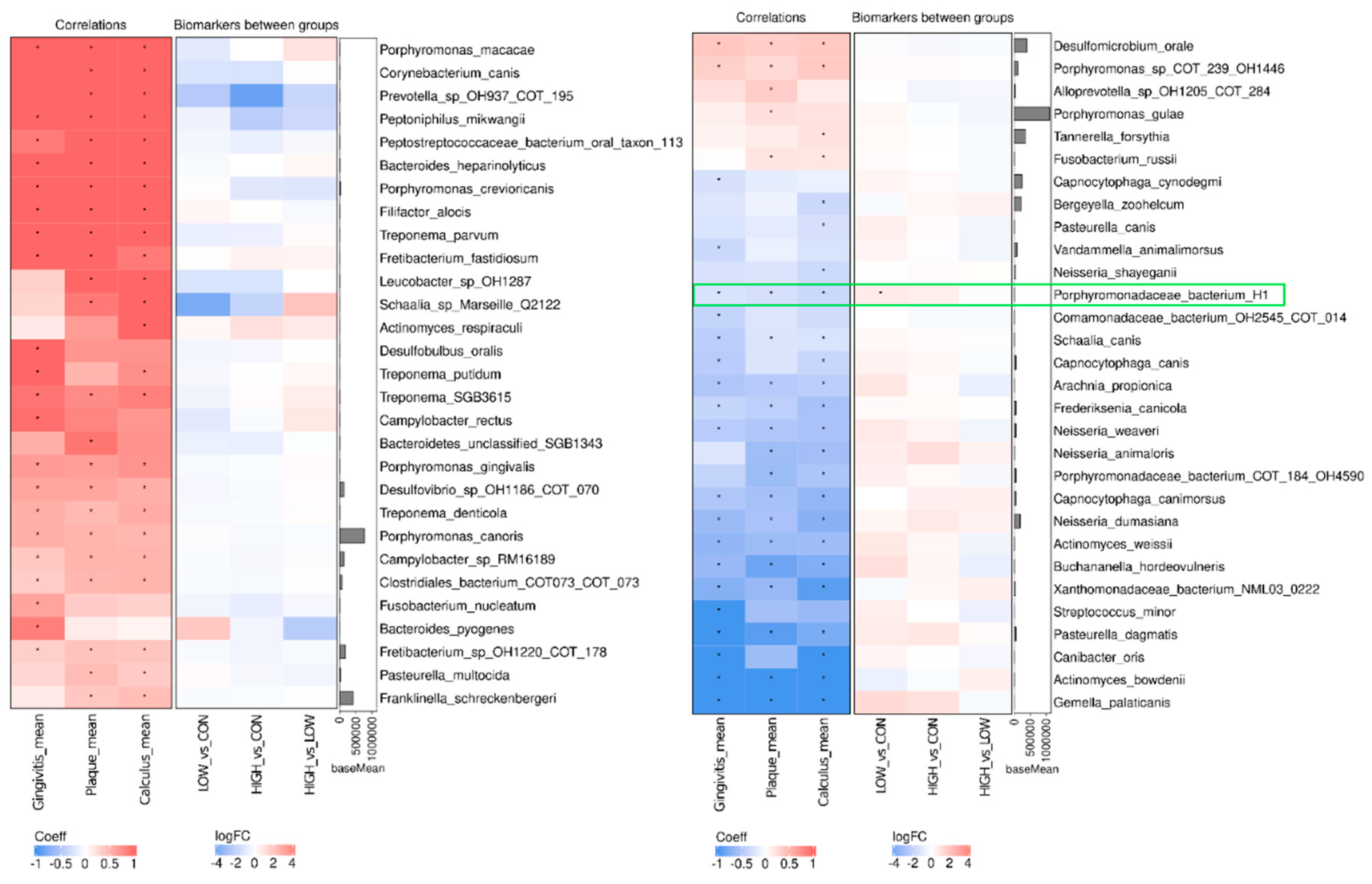
3.6. Supragingival Plaque Samples: Microbiota Diversity and Composition
3.7. Supragingival Plaque Samples: Microbiota Functional Gene Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral. Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Pereira Dos Santos, J.D.; Cunha, E.; Nunes, T.; Tavares, L.; Oliveira, M. Relation between periodontal disease and systemic diseases in dogs. Res. Vet. Sci. 2019, 125, 136–140. [Google Scholar] [CrossRef]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- Robinson, N.J.; Dean, R.S.; Cobb, M.; Brennan, M.L. Factors influencing common diagnoses made during first-opinion small-animal consultations in the United Kingdom. Prev. Vet. Med. 2016, 131, 87–94. [Google Scholar] [CrossRef]
- Kortegaard, H.E.; Eriksen, T.; Baelum, V. Periodontal disease in research beagle dogs—An epidemiological study. J. Small Anim. Pract. 2008, 49, 610–616. [Google Scholar] [CrossRef]
- Wallis, C.; Ellerby, Z.; Amos, G.; Holcombe, L.J. Influence of wet and dry commercial diets on the oral microbiota of Yorkshire terriers. BMC Vet. Res. 2025, 21, 290. [Google Scholar] [CrossRef]
- Logan, E.I. Dietary influences on periodontal health in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1385–1401. [Google Scholar] [CrossRef]
- Gawor, J.P.; Reiter, A.M.; Jodkowska, K.; Kurski, G.; Wojtacki, M.P.; Kurek, A. Influence of diet on oral health in cats and dogs. J. Nutr. 2006, 136, 2021S–2023S. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral. Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Holcombe, L.J.; Patel, N.; Colyer, A.; Deusch, O.; O’Flynn, C.; Harris, S. Early canine plaque biofilms: Characterization of key bacterial interactions involved in initial colonization of enamel. PLoS ONE 2014, 9, e113744. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Klein, E.A.; Thompson, E.C.; Blanton, J.M.; Chen, T.; Milella, L.; Buckley, C.M.; Davis, I.J.; Bennett, M.L.; Marshall-Jones, Z.V. The canine oral microbiome. PLoS ONE 2012, 7, e36067. [Google Scholar] [CrossRef]
- Hardham, J.; Dreier, K.; Wong, J.; Sfintescu, C.; Evans, R.T. Pigmented-anaerobic bacteria associated with canine periodontitis. Vet. Microbiol. 2005, 106, 119–128. [Google Scholar] [CrossRef]
- Tavares, M.O.; Dos Reis, L.D.; Lopes, W.R.; Schwarz, L.V.; Rocha, R.K.M.; Scariot, F.J.; Echeverrigaray, S.; Delamare, A.P.L. Bacterial community associated with gingivitis and periodontitis in dogs. Res. Vet. Sci. 2023, 162, 104962. [Google Scholar] [CrossRef]
- Theilade, E. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J. Clin. Periodontol. 1986, 13, 905–911. [Google Scholar] [CrossRef]
- Gorrel, C.; Rawlings, J. The role of tooth-brushing and diet in the maintenance of periodontal health in dogs. J. Vet. Dent. 1996, 13, 139–143. [Google Scholar] [CrossRef]
- Harvey, C.E. Management of periodontal disease: Understanding the options. Vet. Clin. Small Anim. Pract. 2005, 35, 819–836. [Google Scholar] [CrossRef]
- Ray, J.D., Jr.; Eubanks, D.L. Dental homecare: Teaching your clients to care for their pet’s teeth. J. Vet. Dent. 2009, 26, 57–60. [Google Scholar] [CrossRef]
- Quest, B.W. Oral health benefits of a daily dental chew in dogs. J. Vet. Dent. 2013, 30, 84–87. [Google Scholar] [CrossRef]
- Gawor, J.; Jodkowska, K.; Jank, M. Effects of an Ascophyllum nodosum Formulation on Oral Health Index in Dogs and Cats. Vet. Pract. Dent. 2013, 10, 74–79. [Google Scholar]
- Nart, J.; Jiménez-Garrido, S.; Ramírez-Sebastià, A.; Astó, E.; Buj, D.; Huedo, P.; Espadaler, J. Oral colonization by Levilactobacillus brevis KABPTM-052 and Lactiplantibacillus plantarum KABPTM-051: A Randomized, Double-Blinded, Placebo-Controlled Trial (Pilot Study). J. Clin. Exp. Dent. 2021, 13, e433–e439. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hsieh, P.S.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Yang, S.F.; Lin, C.W. Antibacterial activity of viable and heat-killed probiotic strains against oral pathogens. Lett. Appl. Microbiol. 2020, 70, 310–317. [Google Scholar] [CrossRef]
- Jansen, P.M.; Abdelbary, M.M.H.; Conrads, G. A concerted probiotic activity to inhibit periodontitis-associated bacteria. PLoS ONE 2021, 16, e0248308. [Google Scholar] [CrossRef]
- Rosier, B.T.; Buetas, E.; Moya-Gonzalvez, E.M.; Artacho, A.; Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 2020, 10, 12895. [Google Scholar] [CrossRef]
- Heidrich, V.; Fackelmann, G.; Malesevic, M.; Armanini, F.; Dey, H.; Mengoni, C.; Stanisavljevic, N.; Vukotic, G.; Segata, N. Newly identified species from the dog dental plaque microbiome highlight little overlap with humans. Npj Biofilms Microbiomes 2025, 11, 30. [Google Scholar] [CrossRef]
- Pye, C.C.; Yu, A.A.; Weese, J.S. Evaluation of biofilm production by Pseudomonas aeruginosa from canine ears and the impact of biofilm on antimicrobial susceptibility in vitro. Vet. Dermatol. 2013, 24, 446-e99. [Google Scholar] [CrossRef]
- Mira, A.; Buetas, E.; Rosier, B.; Mazurel, D.; Villanueva-Castellote, Á.; Llena, C.; Ferrer, M.D. Development of an in vitro system to study oral biofilms in real time through impedance technology: Validation and potential applications. J. Oral. Microbiol. 2019, 11, 1609838. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.3-5. 2016. Available online: http://CRAN.R-project.org/package=vegan (accessed on 7 August 2024).
- Loe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Logan, E.I.; Boyce, E.N. Oral health assessment in dogs: Parameters and methods. J. Vet. Dent. 1994, 11, 58–63. [Google Scholar]
- Gorrel, C.; Warrick, J.; Bierer, T.L. Effect of a new dental hygiene chew on periodontal health in dogs. J. Vet. Dent. 1999, 16, 77–81. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Bushnell, B. Clumpify Guide. 2015. Available online: https://github.com/BioInfoTools/BBMap/blob/master/docs/guides/ClumpifyGuide.txt (accessed on 15 October 2024).
- Coelho, L.P.; Alves, R.; Monteiro, P.; Huerta-Cepas, J.; Freitas, A.T.; Bork, P. NG-meta-profiler: Fast processing of metagenomes using NGLess, a domain-specific language. Microbiome 2019, 7, 84. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Beghini, F.; Cumbo, F.; McIver, L.J.; Thompson, K.N.; Zolfo, M.; Manghi, P.; Dubois, L.; Huang, K.D.; Thomas, A.M.; et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 2023, 41, 1633–1644. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot: An implementation of the Grammar of Graphics in R; Citeseer: Philadelphia, PA, USA, 2006. [Google Scholar]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 28 May 2025).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. iMeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Davis, I.J.; Wallis, C.; Deusch, O.; Colyer, A.; Milella, L.; Loman, N.; Harris, S. A Cross-Sectional Survey of Bacterial Species in Plaque from Client Owned Dogs with Healthy Gingiva, Gingivitis or Mild Periodontitis. PLoS ONE 2013, 8, e83158. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Dave, S. Risk factors for periodontitis. J. Int. Acad. Periodontol. 2005, 7, 3. [Google Scholar]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 72–122. [Google Scholar] [CrossRef]
- Smalley, J.W.; Olczak, T. Heme acquisition mechanisms of Porphyromonas gingivalis—Strategies used in a polymicrobial community in a heme-limited host environment. Mol. Oral. Microbiol. 2017, 32, 1–23. [Google Scholar] [CrossRef]
- O’Flynn, C.; Deusch, O.; Darling, A.E.; Eisen, J.A.; Wallis, C.; Davis, I.J.; Harris, S.J. Comparative Genomics of the Genus Porphyromonas Identifies Adaptations for Heme Synthesis within the Prevalent Canine Oral Species Porphyromonas cangingivalis. Genome Biol. Evol. 2015, 7, 3397–3413. [Google Scholar] [CrossRef]
- Li, X.; Yu, C.; Zhang, B.; Shan, X.; Mao, W.; Zhang, Z.; Wang, C.; Jin, X.; Wang, J.; Zhao, H. The recovery of the microbial community after plaque removal depends on periodontal health status. NPJ Biofilms Microbiomes 2023, 9, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moran, S.P.; Rosier, B.T.; Henriquez, F.L.; Burleigh, M.C. The effects of nitrate on the oral microbiome: A systematic review investigating prebiotic potential. J. Oral. Microbiol. 2024, 16, 2322228. [Google Scholar] [CrossRef]
- Rosier, B.T.; Takahashi, N.; Zaura, E.; Krom, B.P.; MartÍnez-Espinosa, R.M.; van Breda, S.G.J.; Marsh, P.D.; Mira, A. The Importance of Nitrate Reduction for Oral Health. J. Dent. Res. 2022, 101, 887–897. [Google Scholar] [CrossRef]
- Mazurel, D.; Carda-Diéguez, M.; Langenburg, T.; Žiemytė, M.; Johnston, W.; Martínez, C.P.; Albalat, F.; Llena, C.; Al-Hebshi, N.; Culshaw, S.; et al. Nitrate and a nitrate-reducing Rothia aeria strain as potential prebiotic or synbiotic treatments for periodontitis. npj Biofilms Microbiomes 2023, 9, 40. [Google Scholar] [CrossRef]
- Fritsch, P.; Klein, D.; de Saint Blanquat, G. Salivary and biliary excretion of nitrates in the dog. Ann. Nutr. Aliment. 1980, 34, 1089–1096. [Google Scholar]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Tomofuji, T.; Irie, K.; Sanbe, T.; Azuma, T.; Ekuni, D.; Tamaki, N.; Yamamoto, T.; Morita, M. Periodontitis and increase in circulating oxidative stress. Jpn. Dent. Sci. Rev. 2009, 45, 46–51. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florit-Ruiz, A.; Rago, L.; Rojas, A.; Guzelkhanova, B.; Pont-Beltran, A.; Lamelas, A.; Solaz-Fuster, M.C.; Martinez-Blanch, J.F.; López, M.E.; García-Lainez, G.; et al. Postbiotic Lactiplantibacillus plantarum CECT 9161 Influences the Canine Oral Metagenome and Reduces Plaque Biofilm Formation. Animals 2025, 15, 1615. https://doi.org/10.3390/ani15111615
Florit-Ruiz A, Rago L, Rojas A, Guzelkhanova B, Pont-Beltran A, Lamelas A, Solaz-Fuster MC, Martinez-Blanch JF, López ME, García-Lainez G, et al. Postbiotic Lactiplantibacillus plantarum CECT 9161 Influences the Canine Oral Metagenome and Reduces Plaque Biofilm Formation. Animals. 2025; 15(11):1615. https://doi.org/10.3390/ani15111615
Chicago/Turabian StyleFlorit-Ruiz, Adrián, Laura Rago, Antonia Rojas, Bellahanum Guzelkhanova, Adrià Pont-Beltran, Araceli Lamelas, María Carmen Solaz-Fuster, Juan F. Martinez-Blanch, María Enrique López, Guillermo García-Lainez, and et al. 2025. "Postbiotic Lactiplantibacillus plantarum CECT 9161 Influences the Canine Oral Metagenome and Reduces Plaque Biofilm Formation" Animals 15, no. 11: 1615. https://doi.org/10.3390/ani15111615
APA StyleFlorit-Ruiz, A., Rago, L., Rojas, A., Guzelkhanova, B., Pont-Beltran, A., Lamelas, A., Solaz-Fuster, M. C., Martinez-Blanch, J. F., López, M. E., García-Lainez, G., Rosier, B. T., Day, R., Rubio, T., Batchelor, R., & Nixon, S. L. (2025). Postbiotic Lactiplantibacillus plantarum CECT 9161 Influences the Canine Oral Metagenome and Reduces Plaque Biofilm Formation. Animals, 15(11), 1615. https://doi.org/10.3390/ani15111615







