Development of a Multiplex Quantitative Polymerase Chain Reaction Assay for the Detection of Duck Enteritis Virus, Goose Parvovirus, and Muscovy Duck Parvovirus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Primers and Probes
2.2. Virus Strains and Clinic Samples
2.3. Construction, Culture, and Purification of Standard Plasmids
2.4. Optimization of Optimal Reaction Conditions for Multiplex qPCR
2.5. Construction of Standard Curves
2.6. Sensitivity of Singleplex and Multiplex qPCR
2.7. Specificity, and Repeatability of Multiplex qPCR
2.8. Sample Tested
3. Results
3.1. Optimization of Multiplex qPCR Reaction System
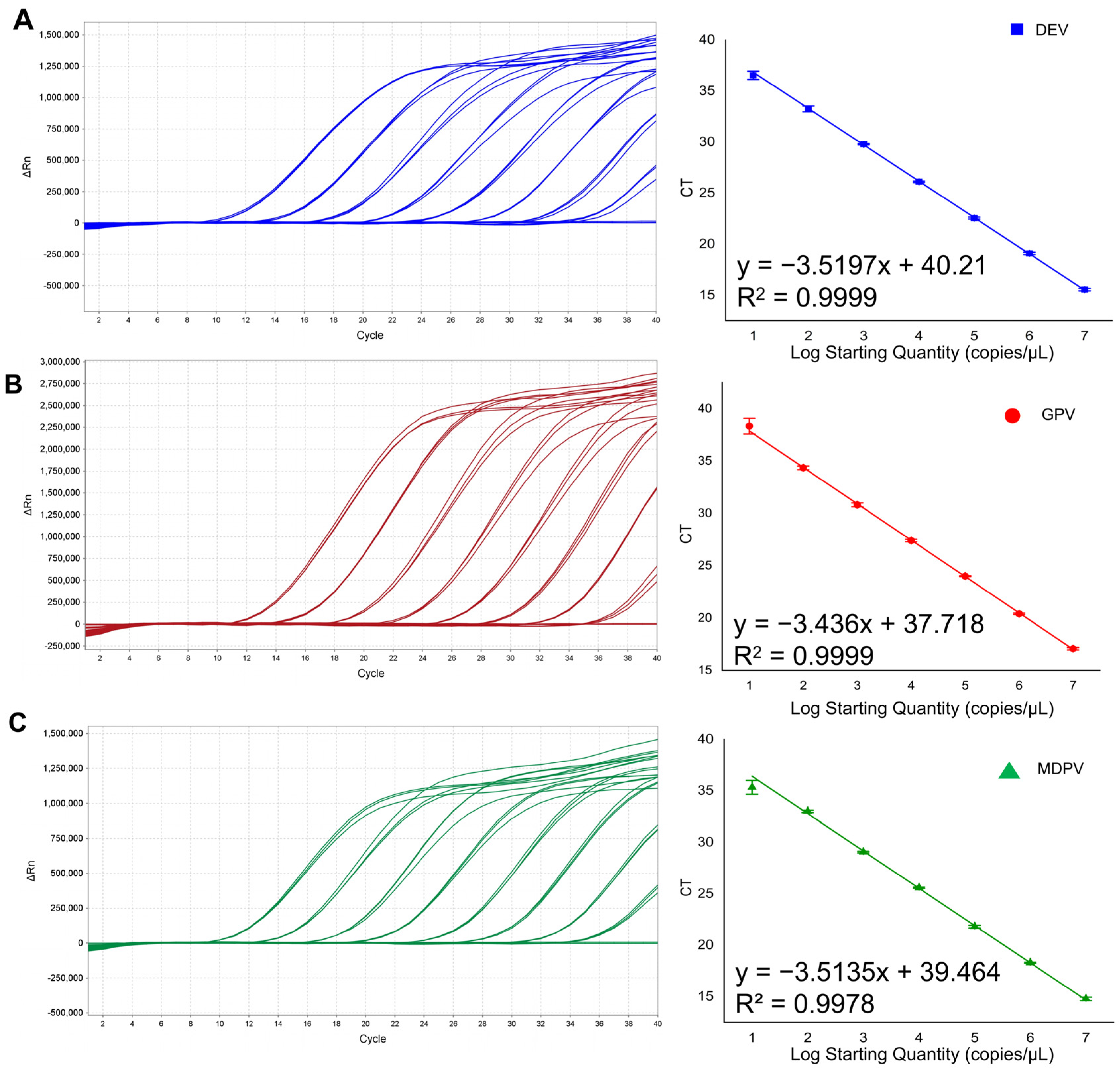
3.2. Singleplex qPCR Detection of a Single Virus
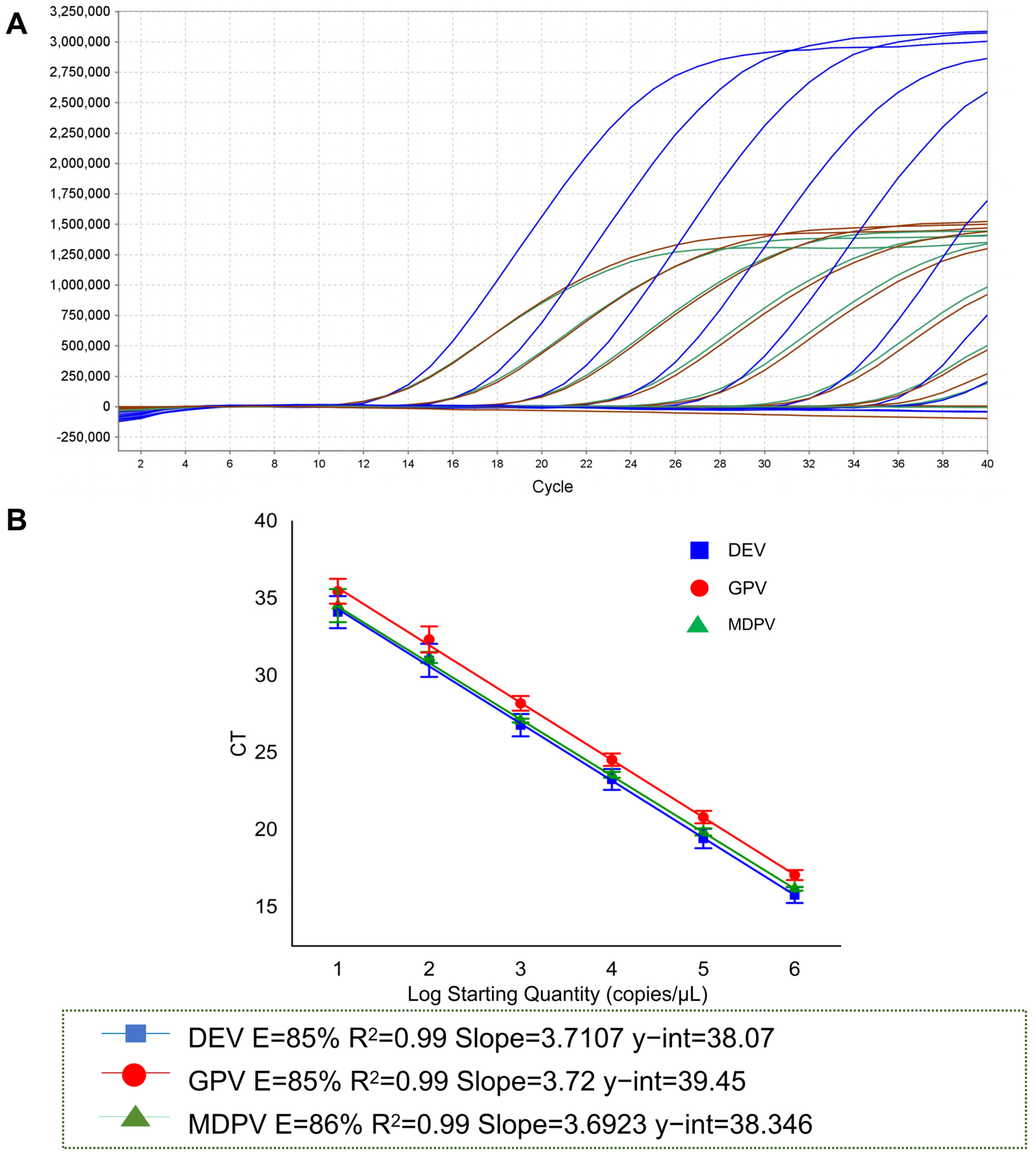
3.3. Establishment of a Multiplex qPCR Detection Assay
3.4. Sensitivity of Multiplex qPCR Detection
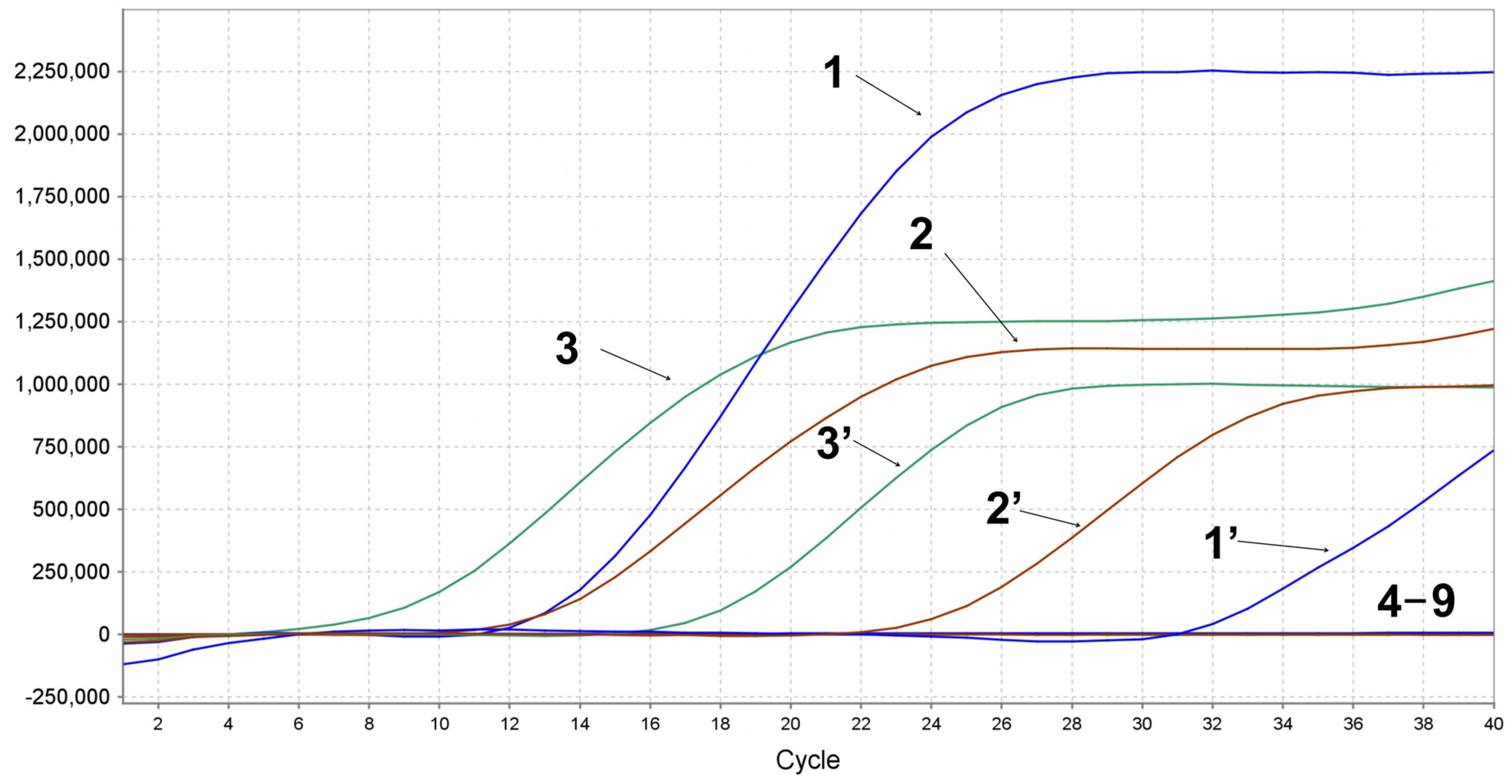
3.5. Specificity of Multiplex qPCR Detection
3.6. Repeatability of Multiplex qPCR Detection
3.7. Sample Tested
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Qu, Y.; Wang, F.; Hu, D.; Liu, L.; Li, N.; Yue, R.; Li, C.; Liu, S. The comprehensive diagnosis and prevention of duck plague in northwest Shandong province of China. Poult. Sci. 2013, 92, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Therapeutic vaccines for herpesviruses. J. Clin. Investig. 2024, 134, e179483. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, Z.; Huang, L.; Wang, S.; Huang, J.; Zhang, Y.; Zeng, T.; Luo, S. A polymerase chain reaction assay for detection of virulent and attenuated strains of duck plague virus. J. Virol. Methods 2017, 249, 66–68. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Han, Z.; Shao, Y.; Chen, S.; Kong, X. Comparative analysis of the genes UL1 through UL7 of the duck enteritis virus and other herpesviruses of the subfamily alphaherpesvirinae. Genet. Mol. Biol. 2009, 32, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Sun, Z.; Shen, T.; Xu, D.; Huang, K.; Zhou, J.; Song, S.; Yan, L. Analysis of evolutionary processes of species jump in waterfowl parvovirus. Front. Microbiol. 2017, 8, 421. [Google Scholar] [CrossRef]
- Gough, R.E.; Spackman, D. Studies with a duck embryo adapted goose parvovirus vaccine. Avian Pathol. 1982, 11, 503–510. [Google Scholar] [CrossRef]
- Wozniakowski, G.; Samorek-Salamonowicz, E.; Kozdrun, W. Quantitative analysis of waterfowl parvoviruses in geese and muscovy ducks by real-time polymerase chain reaction: Correlation between age, clinical symptoms and DNA copy number of waterfowl parvoviruses. BMC Vet. Res. 2012, 8, 29. [Google Scholar] [CrossRef]
- Yu, J.; Zou, J.; Liu, X.; Pan, Y.; Mu, Y.; Li, S.; Wang, J.; Xu, F.; Wang, Y. TaqMan-probe-based multiplex real-time RT-qPCR for simultaneous detection of GoAstV, GPV, and GoCV. Poult. Sci. 2023, 102, 102396. [Google Scholar] [CrossRef]
- He, D.; Wang, F.; Zhao, L.; Jiang, X.; Zhang, S.; Wei, F.; Wu, B.; Wang, Y.; Diao, Y.; Tang, Y. Epidemiological investigation of infectious diseases in geese on mainland China during 2018–2021. Transbound. Emerg. Dis. 2022, 69, 3419–3432. [Google Scholar] [CrossRef]
- Chang, P.C.; Shien, J.H.; Wang, M.S.; Shieh, H.K. Phylogenetic analysis of parvoviruses isolated in taiwan from ducks and geese. Avian Pathol. 2000, 29, 45–49. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhou, M.; Hardwidge, P.R.; Zhu, G. Construction and sequencing of an infectious clone of the goose embryo-adapted muscovy duck parvovirus vaccine strain FZ91-30. Virol. J. 2016, 13, 104. [Google Scholar] [CrossRef]
- Dong, J.; Bingga, G.; Sun, M.; Li, L.; Liu, Z.; Zhang, C.; Guo, P.; Huang, Y.; Zhang, J. Application of high-resolution melting curve analysis for identification of muscovy duck parvovirus and goose parvovirus. J. Virol. Methods 2019, 266, 121–125. [Google Scholar] [CrossRef]
- Yang, Y.; Sui, N.; Zhang, R.; Lan, J.; Li, P.; Lian, C.; Li, H.; Xie, Z.; Jiang, S. Coinfection of novel goose parvovirus-associated virus and duck circovirus in feather sacs of cherry valley ducks with feather shedding syndrome. Poult. Sci. 2020, 99, 4227–4234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, F.; Shu, X.; Li, Q.; Ding, Q.; Hao, C.; Yan, X.; Xu, M.; Hu, H. Co-infection of porcine epidemic diarrhoea virus and porcine deltacoronavirus enhances the disease severity in piglets. Transbound. Emerg. Dis. 2022, 69, 1715–1726. [Google Scholar] [CrossRef]
- Hawkins, S.; Guest, P.C. Multiplex analyses using real-time quantitative PCR. Methods Mol. Biol. 2017, 1546, 125–133. [Google Scholar] [PubMed]
- Wan, C.; Chen, C.; Cheng, L.; Liu, R.; Shi, S.; Fu, G.; Chen, H.; Fu, Q.; Huang, Y. Specific detection and differentiation of classic goose parvovirus and novel goose parvovirus by TaqMan real-time PCR assay, coupled with host specificity. BMC Vet. Res. 2019, 15, 389. [Google Scholar] [CrossRef]
- Dai, Y.; Li, M.; Hu, X.; Zhao, R.; Xia, L. Development and application of a multiplex PCR method for simultaneous detection of waterfowl parvovirus, duck enteritis virus and goose astrovirus. 3 Biotech 2022, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; An, T.; Chen, H.; Wang, Y.; Zhu, L.; Yu, C.; Xia, C.; Zhang, H. Development and application of quadruplex real time quantitative PCR method for differentiation of muscovy duck parvovirus, goose parvovirus, duck circovirus, and duck adenovirus 3. Front. Cell. Infect. Microbiol. 2024, 14, 1448480. [Google Scholar] [CrossRef]
- Liang, Z.; Guo, J.; Yuan, S.; Cheng, Q.; Zhang, X.; Liu, Z.; Wang, C.; Li, Z.; Hou, B.; Huang, S.; et al. Pathological and molecular characterization of a duck plague outbreak in southern China in 2021. Animals 2022, 12, 3523. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Y.; Hu, D.; Huang, X.; Xiong, H.; Qi, K.; Liu, H. Clinical and histologic characterization of co-infection with astrovirus and goose parvovirus in goslings. Avian Dis. 2019, 63, 731–736. [Google Scholar] [CrossRef]
- Qi, X.; Yang, X.; Cheng, A.; Wang, M.; Guo, Y.; Jia, R. Replication kinetics of duck virus enteritis vaccine virus in ducklings immunized by the mucosal or systemic route using real-time quantitative PCR. Res. Vet. Sci. 2009, 86, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qi, X.; Cheng, A.; Wang, M.; Zhu, D.; Jia, R.; Chen, X. Intestinal mucosal immune response in ducklings following oral immunisation with an attenuated duck enteritis virus vaccine. Vet. J. 2010, 185, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, C.J.; Pan, L.; Wang, G.J.; Qi, K.Z.; Liu, H.M. Isolation and characterization of a goose parvovirus from yan goose. Acta Virol. 2016, 60, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Cheng, L.; Chen, C.; Liu, R.; Shi, S.; Fu, G.; Chen, H.; Fu, Q.; Huang, Y. A duplex PCR assay for the simultaneous detection and differentiation of muscovy duck parvovirus and goose parvovirus. Mol. Cell. Probes 2019, 47, 101439. [Google Scholar] [CrossRef]
- Gadkar, V.; Filion, M. New developments in quantitative real-time polymerase chain reaction technology. Curr. Issues Mol. Biol. 2014, 16, 1–6. [Google Scholar]
- Rychlik, W.; Spencer, W.J.; Rhoads, R.E. Optimization of the annealing temperature for DNA amplification in vitro. Nucleic Acids Res. 1990, 18, 6409–6412. [Google Scholar] [CrossRef]
- Wan, C.; Shi, S.; Chen, C.; Chen, H.; Cheng, L.; Fu, Q.; Fu, G.; Liu, R.; Huang, Y. Development of a PCR assay for detection and differentiation of muscovy duck and goose parvoviruses based on NS gene characterization. J. Vet. Med. Sci. 2018, 80, 1861–1866. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, X.; Gao, Y.; Song, S.; Xu, D.; Yan, L. Development and application of multiplex PCR method for simultaneous detection of seven viruses in ducks. BMC Vet. Res. 2019, 15, 103. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xie, Z.X.; Xie, L.J.; Deng, X.W.; Xie, Z.Q.; Luo, S.S.; Huang, L.; Huang, J.L.; Zeng, T.T. GeXP analyzer-based multiplex reverse-transcription PCR assay for the simultaneous detection and differentiation of eleven duck viruses. BMC Microbiol. 2015, 15, 247. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The ultimate qPCR experiment: Producing publication quality, reproducible data the first time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Lu, L.; Chen, Y.; Wang, Z.; Li, X.; Chen, W.; Tao, Z.; Shen, J.; Tian, Y.; Wang, D.; Li, G.; et al. The goose genome sequence leads to insights into the evolution of waterfowl and susceptibility to fatty liver. Genome Biol. 2015, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.M.; Koelle, K.; Bedford, T. Viral phylodynamics. PLoS Comput. Biol. 2013, 9, e1002947. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Feng, K.; Zhao, Q.; Chen, Y.; Wang, J.; Chen, S.; Shao, G.; Liao, L.; Li, Y.; Xie, Z.; et al. Pathogenicity and transmissibility studies on live attenuated duck enteritis virus vaccine in non-target species. Front. Microbiol. 2022, 13, 979368. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Chen, Y.; Zhu, J.; Wang, Y. Evolution, genetic recombination, and phylogeography of goose parvovirus. Comp. Immunol. Microbiol. Infect. Dis. 2023, 102, 102079. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, Z.; Xie, L.; Deng, X.; Xie, Z.; Luo, S.; Huang, L.; Huang, J.; Zeng, T. Molecular characterization of the full muscovy duck parvovirus, isolated in Guangxi, China. Genome Announc. 2014, 2, e01249-14. [Google Scholar] [CrossRef]

| Virus | Primer and Probe | Sequence (5′-3′) | Target Gene | Size (bp) |
|---|---|---|---|---|
| DEV | Forward | GGCCAGGGAGTTTATAATTCGG | UL6 | 161 |
| Reverse | GCTATATGTCGTGCATCTAACCC | |||
| Probe | HEX-CTGCCATACGACAAATCCAGGCGAC-BHQ1 | |||
| MDPV | Forward | AAGCTACAACAACCACATCTAC | VP1 | 119 |
| Reverse | GGCAGTGGAATCTGTTGAAAT | |||
| Probe | CY5-ATCACAAGCGGAACAAACCCAGAC-BHQ2 | |||
| GPV | Forward | AATTGTTCTCATCAGTCGCTC | REP | 153 |
| Reverse | AGTTTGCTTTCTCACATTCCATAC | |||
| Probe | FAM-CCTGTGACTCCTCAGAACTCCCCT-BHQ1 |
| Virus | Primer (μM) | 2 | 4 | 6 | 8 | 10 | |
|---|---|---|---|---|---|---|---|
| Probe (μM) | |||||||
| DEV | 2 | 24.49 | 24.30 | 25.28 | 24.80 | 24.78 | |
| 4 | 25.38 | 24.44 | 24.56 | 27.54 | 24.85 | ||
| 6 | 23.96 | 24.50 | 23.86 | 24.66 | 24.64 | ||
| 8 | 24.10 | 25.01 | 23.44 | 24.37 | 23.59 | ||
| 10 | 24.41 | 24.58 | 24.12 | 24.83 | 24.57 | ||
| MDPV | 2 | 24.99 | 25.28 | 25.68 | 25.88 | 25.71 | |
| 4 | 24.30 | 24.08 | 25.17 | 25.15 | 25.42 | ||
| 6 | 24.81 | 25.13 | 25.20 | 25.20 | 25.15 | ||
| 8 | 24.96 | 25.01 | 24.12 | 24.37 | 23.59 | ||
| 10 | 24.41 | 24.58 | 24.26 | 24.83 | 24.36 | ||
| GPV | 2 | 26.71 | 26.34 | 26.92 | 27.52 | 27.44 | |
| 4 | 24.86 | 24.82 | 26.97 | 26.24 | 27.50 | ||
| 6 | 25.77 | 25.79 | 26.55 | 27.04 | 27.60 | ||
| 8 | 25.23 | 25.80 | 25.82 | 25.84 | 25.38 | ||
| 10 | 24.64 | 22.73 | 25.33 | 26.13 | 24.93 | ||
| Primer and Probe | Molarity (μM) | ||
|---|---|---|---|
| DEV | MDPV | GPV | |
| Forward (μM) | 6 | 6 | 8 |
| Reverse (μM) | 6 | 6 | 8 |
| Probe (μM) | 8 | 8 | 10 |
| Templates (Copies/Gene) | Ct Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DEV | MDPV | GPV | |||||||
| 108 | 12.65 | 12.63 | 11.88 | 12.88 | 12.69 | 12.88 | 13.95 | 13.75 | 13.33 |
| 107 | 16.10 | 16.08 | 15.20 | 16.34 | 16.12 | 16.15 | 17.39 | 17.13 | 16.75 |
| 106 | 19.83 | 19.81 | 18.73 | 20.14 | 19.85 | 19.68 | 21.21 | 20.89 | 20.42 |
| 105 | 23.52 | 23.81 | 22.52 | 23.65 | 23.74 | 23.37 | 24.81 | 24.78 | 24.10 |
| 104 | 27.12 | 27.31 | 25.97 | 27.19 | 27.16 | 26.97 | 28.50 | 28.48 | 27.67 |
| 103 | 31.41 | 31.82 | 29.78 | 31.37 | 31.44 | 30.79 | 32.86 | 32.84 | 31.38 |
| 102 | 33.14 | 35.22 | 34.03 | 33.32 | 35.13 | 35.23 | 34.84 | 36.38 | 35.23 |
| 101 | ND | 38.86 | 38.90 | 38.83 | 37.41 | 38.91 | 38.77 | ||
| NTC | ND | ND | ND | ||||||
| Plasmid Standards | Concentration (Copies/μL) | Intra-Assay Ct Values | Inter-Assay Ct Values | ||||
|---|---|---|---|---|---|---|---|
| SD | CV (%) | SD | CV (%) | ||||
| pMD19-DEV | 107 | 16.08 | 0.087 | 0.54 | 15.79 | 0.085 | 0.54 |
| 105 | 23.81 | 0.543 | 2.28 | 23.28 | 0.26 | 1.12 | |
| 103 | 31.82 | 0.161 | 0.51 | 31.01 | 0.087 | 0.28 | |
| pMD19-MDPV | 107 | 16.11 | 0.09 | 0.56 | 16.20 | 0.133 | 0.82 |
| 105 | 23.70 | 0.535 | 2.26 | 23.58 | 0.260 | 1.10 | |
| 103 | 31.43 | 0.132 | 0.42 | 31.19 | 0.172 | 0.55 | |
| pMD19-GPV | 107 | 17.131 | 0.136 | 0.79 | 17.091 | 0.142 | 0.83 |
| 105 | 24.784 | 0.538 | 2.17 | 24.567 | 0.274 | 1.12 | |
| 103 | 32.849 | 0.111 | 0.34 | 32.364 | 0.078 | 0.24 | |
| Virus | Total Clinical Samples | Positive Rate (%) |
|---|---|---|
| qPCR | ||
| DEV | 215 | 33/215 (15.3%) |
| GPV | 215 | 25/215 (11.6%) |
| MDPV | 215 | 24/215 (11.2%) |
| DEV and MDPV | 215 | 6/215 (2.8%) |
| GPV and MDPV | 215 | 3/215 (1.4%) |
| GPV and DEV | 215 | 8/215 (3.7%) |
| DEV, MDPV and GPV | 215 | 4/215 (1.9%) |
| Province | Number | Number of Positive Samples | ||
|---|---|---|---|---|
| DEV | GPV | MDPV | ||
| Anhui | 12 | 0 | 3 | 3 |
| Fujian | 71 | 7 | 11 | 15 |
| Guangdong | 23 | 0 | 2 | 0 |
| Guangxi | 33 | 15 | 1 | 6 |
| Shandong | 30 | 0 | 6 | 0 |
| Jiangxi | 43 | 10 | 1 | 0 |
| Sichuan | 3 | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Hu, R.; Liu, Z.; Yan, L.; Yang, F.; Dai, X.; Xing, C.; Cao, H. Development of a Multiplex Quantitative Polymerase Chain Reaction Assay for the Detection of Duck Enteritis Virus, Goose Parvovirus, and Muscovy Duck Parvovirus. Animals 2025, 15, 1599. https://doi.org/10.3390/ani15111599
Qiu Q, Hu R, Liu Z, Yan L, Yang F, Dai X, Xing C, Cao H. Development of a Multiplex Quantitative Polymerase Chain Reaction Assay for the Detection of Duck Enteritis Virus, Goose Parvovirus, and Muscovy Duck Parvovirus. Animals. 2025; 15(11):1599. https://doi.org/10.3390/ani15111599
Chicago/Turabian StyleQiu, Qian, Ruiming Hu, Zirui Liu, Linjie Yan, Fan Yang, Xueyan Dai, Chenghong Xing, and Huabin Cao. 2025. "Development of a Multiplex Quantitative Polymerase Chain Reaction Assay for the Detection of Duck Enteritis Virus, Goose Parvovirus, and Muscovy Duck Parvovirus" Animals 15, no. 11: 1599. https://doi.org/10.3390/ani15111599
APA StyleQiu, Q., Hu, R., Liu, Z., Yan, L., Yang, F., Dai, X., Xing, C., & Cao, H. (2025). Development of a Multiplex Quantitative Polymerase Chain Reaction Assay for the Detection of Duck Enteritis Virus, Goose Parvovirus, and Muscovy Duck Parvovirus. Animals, 15(11), 1599. https://doi.org/10.3390/ani15111599





