Prevalence of ESBL-Resistant Genes in Birds in Italy—A Comprehensive Review
Simple Summary
Abstract
1. Introduction
- as reported by this study [13]. The blaTEM, blaCTX-M, blaSHV and blaOXA are the major groups that have been identified as ESBL resistance genes [14]. The blaTEM, the first β-lactamase found in ESBL bacteria, is thought to have originated in E. coli [15]. In poultry and humans, the most common ESBL gene is blaCTX-M and the variants of this ESBL gene vary in both species [15,16]. The transmission of an antibiotic-resistant plasmid, pSL222-6, in E. coli from hens to human handlers was described as far back as the early 1970s [17]. Furthermore, it has been reported that blaCTX-M, blaCTX-TEM and blaCTX-SHV families, which are poultry-associated genes mostly found on plasmids, are also present in humans [18,19]. The spread of pathogenic bacteria between poultry and humans was also suggested when the beta-lactamase blaCTXM-15 and its closely related ESBL genes were identified in E. coli isolates from poultry and humans [20,21]. In addition, the plasmids involved in ESBL production have the potential to cause resistance to other antimicrobials, including fluoroquinolones, trimethoprim and aminoglycosides, because they can carry their respective resistance genes. As a result, it would be difficult to treat diseases caused by ESBL-producing bacteria such as E. coli, Salmonella and Klebsiella. With one health prospective, the spread of ESBL plasmids in the bacterial population could exacerbate this problem in humans, animals and the environment [22,23].
Impact of Antibiotics Usage in Poultry Industry and One Health Approach
2. Materials and Methods
2.1. Data Inclusion Protocol
- Observational studies in which E. coli, Salmonella and Klebsiella spp. producing ESBL phenotypically and genotypically associated with Extended-Spectrum Beta-Lactamases were detected in cloacal swabs, caecal contents, faecal samples and some chicken and turkey meat and food samples, including samples from abattoirs, farms and food markets.
- All studies were carried out in Italian public institutes, universities and national reference centres for antimicrobial resistance. The data published in English between 2001 and 2024 were included, and data related to ESBL, where different gene resistance patterns, transfer patterns and plasmid and transfer patterns were discussed, were extracted.
- The review included only data from studies on domestic and commercial poultry and their meat.
- Studies related to broiler, breeder, layers and turkey were our focus and included in our review
2.2. Data Exclusion Protocol
- Studies involving bacterial species other than Salmonella, E. coli and Klebsiella spp. or specific strains of Salmonella and E. coli that are unable to produce or carry genes for ESBL.
- Studies that focused on the environment and animal species except poultry.
- Studies that investigated only geese, ducks, parrots, pigeons and wild bird species.
- Studies which included samples from water and dust samples within poultry areas, litter material, samples from eggs in hatchers and setters and sampling from egg storage rooms because these samples mainly associated with the environment.
- Abstracts of conferences, chapters from textbooks and books and case studies where full text was not available. In Supplementary Materials, Table S3: list of the excluded studies and the reason for their exclusion is provided.
- This lack of early data before 2000 is in line with findings reported in the literature, which show that systematic monitoring of ESBLs in food animals in Europe began predominantly in the early 2000s [47].
2.3. Screening of Studies
2.4. Extraction of Data
2.5. Data Analysis
3. Results
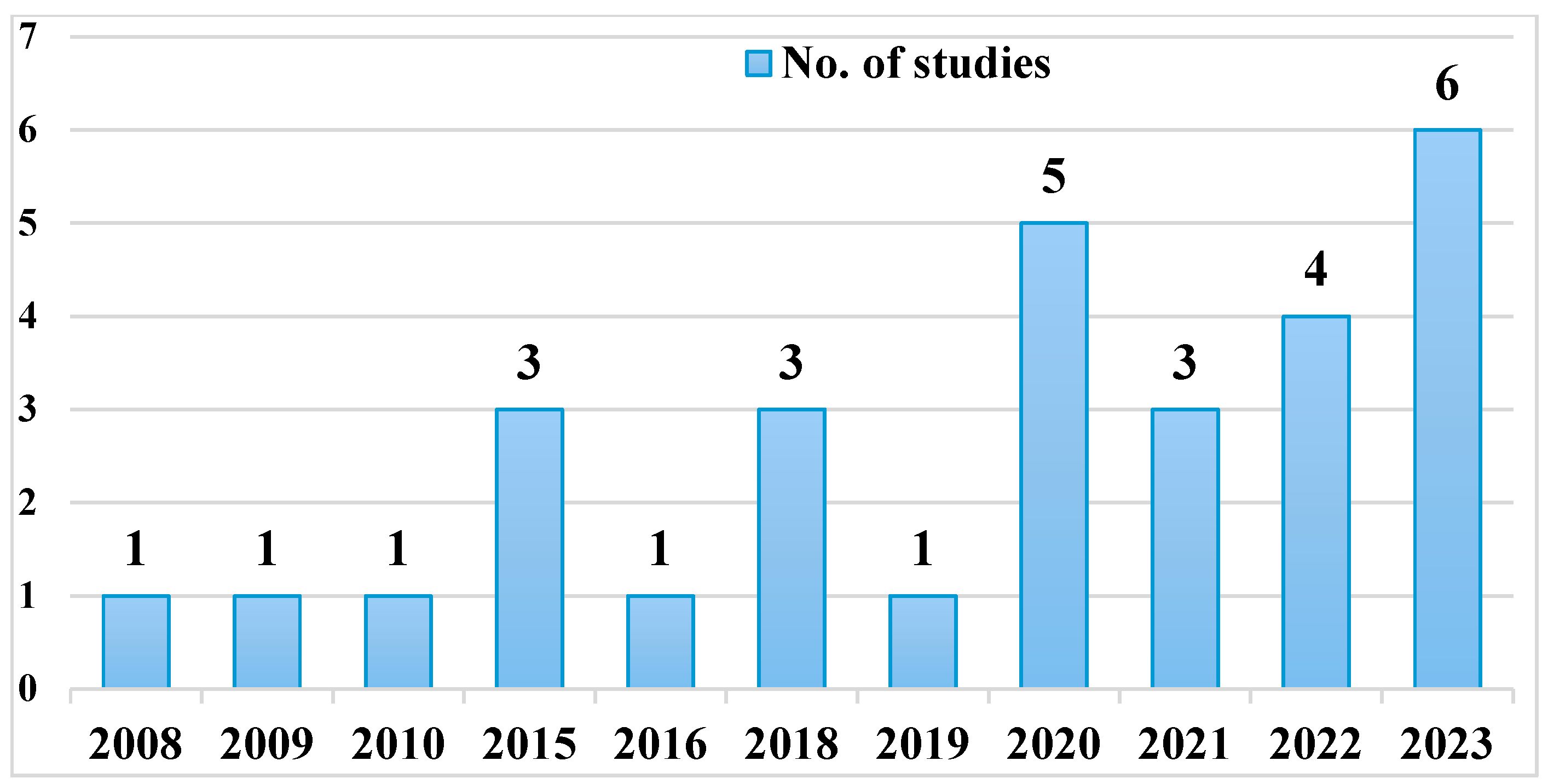
3.1. Study Quality
3.2. Sampling Strategy and Study Design
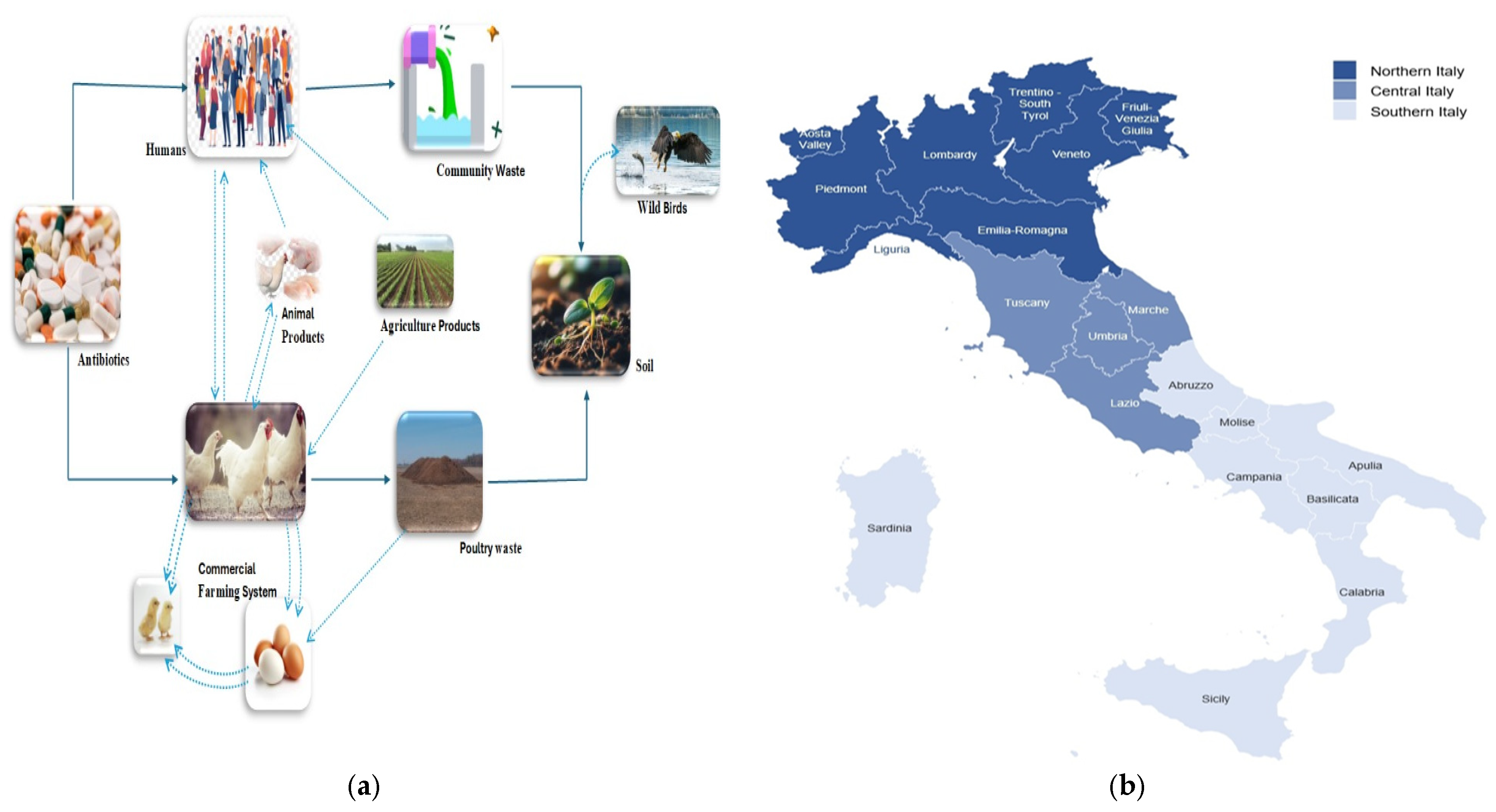
3.3. Nationwide Studies
3.4. Nothern Italy
3.5. Central Italy
3.6. Southern Italy
3.7. Extended-Spectrum Beta Lactamase Identification Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESBL | Extended-Spectrum Beta Lactamases |
| E. coli | Escherichia coli |
| blaCTX-M | Cefotaxime-Mediated Beta-Lactamase gene |
| RFLP | Restriction fragment length polymerase |
| PCR | Polymerase chain reaction |
| WGS | Whole genome sequence |
| CLSI | Clinical and Laboratory Standard Institute |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| ND | Not detected |
References
- Darwich, L.; Vidal, A.; Seminati, C.; Albamonte, A.; Casado, A.; López, F.; Molina-López, R.A.; Migura-Garcia, L. High Prevalence and Diversity of Extended-Spectrum β-Lactamase and Emergence of OXA-48 Producing Enterobacterales in Wildlife in Catalonia. PLoS ONE 2019, 14, e0210686. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Chabou, S.; Daoud, Z.; Rolain, J.-M. Prevalence and Emergence of Extended-Spectrum Cephalosporin-, Carbapenem-and Colistin-Resistant Gram Negative Bacteria of Animal Origin in the Mediterranean Basin. Front. Microbiol. 2018, 9, 2299. [Google Scholar] [CrossRef] [PubMed]
- Scali, F.; Santucci, G.; Maisano, A.M.; Giudici, F.; Guadagno, F.; Tonni, M.; Amicabile, A.; Formenti, N.; Giacomini, E.; Lazzaro, M.; et al. The Use of Antimicrobials in Italian Heavy Pig Fattening Farms. Antibiotics 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Górska, A.; De Angelis, G.; Lammens, C.; Restuccia, G.; Schrenzel, J.; Huson, D.H.; Carević, B.; Preoţescu, L.; Carmeli, Y.; et al. Estimating the Association between Antibiotic Exposure and Colonization with Extended-Spectrum β-Lactamase-Producing Gram-Negative Bacteria Using Machine Learning Methods: A Multicentre, Prospective Cohort Study. Clin. Microbiol. Infect. 2020, 26, 87–94. [Google Scholar] [CrossRef]
- Zhu, F.H.; Rodado, M.P.; Asmar, B.I.; Salimnia, H.; Thomas, R.; Abdel-Haq, N. Risk Factors for Community Acquired Urinary Tract Infections Caused by Extended Spectrum β-Lactamase (ESBL) Producing Escherichia Coli in Children: A Case Control Study. Infect. Dis. 2019, 51, 802–809. [Google Scholar] [CrossRef]
- van den Bogaard, A.E.; London, N.; Driessen, C.; Stobberingh, E.E. Antibiotic Resistance of Faecal Escherichia coli in Poultry, Poultry Farmers and Poultry Slaughterers. J. Antimicrob. Chemother. 2001, 47, 763–771. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine: Categorization for the Development of Risk Management Strategies to contain Antimicrobial Resistance due to Non-Human Antimicrobial Use. In Proceedings of the Second WHO Expert Meeting, Copenhagen, Denmark, 29–31 May 2007. [Google Scholar]
- WHO. Critically Important Antimicrobials for Human Medicine: 6th Revision. Available online: https://iris.who.int/bitstream/handle/10665/312266/9789241515528-eng.pdf?sequence=1 (accessed on 4 September 2023).
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β -Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Biguenet, A.; Valot, B.; El Garch, F.; Bertrand, X.; Hocquet, D. Genomic Epidemiology of Third-Generation Cephalosporin-Resistant Escherichia coli from Companion Animals and Human Infections in Europe. One Health 2025, 20, 100971. [Google Scholar] [CrossRef]
- Cardozo, M.V.; Liakopoulos, A.; Brouwer, M.; Kant, A.; Pizauro, L.J.L.; Borzi, M.M.; Mevius, D.; de Ávila, F.A. Occurrence and Molecular Characteristics of Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Recovered from Chicken, Chicken Meat, and Human Infections in Sao Paulo State, Brazil. Front. Microbiol. 2021, 12, 628738. [Google Scholar] [CrossRef]
- World Health Organization. The Advanced HIV Disease Research Landscape; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Murtaza, A.; Ullah, S.; Tauseef, I.; Haleem, K.S.; Jamal, M.; Gu, J.; Asadullah; Ayaz, S.; Shafiq, M.; Khan, N.A.; et al. High Rate of ESBL Producing Escherichia coli from Retail Chicken Carrying BLACTX-M Gene on Plasmids Mainly Carrying FREPB Replicon. J. Anim. Plant Sci. 2021, 31, 698–707. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum β-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Falgenhauer, L.; Imirzalioglu, C.; Oppong, K.; Akenten, C.W.; Hogan, B.; Krumkamp, R.; Poppert, S.; Levermann, V.; Schwengers, O.; Sarpong, N.; et al. Detection and Characterization of ESBL-Producing Escherichia coli From Humans and Poultry in Ghana. Front. Microbiol. 2019, 9, 3358. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.; Jalal, S.; Omer, S.; Mawlood, A. Molecular Detection of SHV-Type ESBL in E. Coli and K.Pneumoniae and Their Antimicrobial Resistance Profile. Zanco J. Med. Sci. 2018, 22, 262–272. [Google Scholar] [CrossRef]
- Rehman, M.A.; Hasted, T.-L.; Persaud-Lachhman, M.G.; Yin, X.; Carrillo, C.; Diarra, M.S. Genome Analysis and Multiplex PCR Method for the Molecular Detection of Coresistance to Cephalosporins and Fosfomycin in Salmonella Enterica Serovar Heidelberg. J. Food Prot. 2019, 82, 1938–1949. [Google Scholar] [CrossRef]
- Levy, S.B.; Fitzgerald, G.B.; Macone, A.B. Spread of Antibiotic-Resistant Plasmids from Chicken to Chicken and from Chicken to Man. Nature 1976, 260, 40–42. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Dierikx, C.M.; Cohen Stuart, J.; Voets, G.M.; van den Munckhof, M.P.; van Essen-Zandbergen, A.; Platteel, T.; Fluit, A.C.; van de Sande-Bruinsma, N.; Scharinga, J.; et al. Dutch Patients, Retail Chicken Meat and Poultry Share the Same ESBL Genes, Plasmids and Strains. Clin. Microbiol. Infect. 2011, 17, 873–880. [Google Scholar] [CrossRef]
- Lemlem, M.; Aklilu, E.; Mohammed, M.; Kamaruzzaman, F.; Zakaria, Z.; Harun, A.; Devan, S.S. Molecular Detection and Antimicrobial Resistance Profiles of Extended-Spectrum Beta-Lactamase (ESBL) Producing Escherichia coli in Broiler Chicken Farms in Malaysia. PLoS ONE 2023, 18, e0285743. [Google Scholar] [CrossRef]
- Dierikx, C.M.; Van Der Goot, J.; Van Essen-Zandbergen, A.; Mevius, D.J. Dynamics of Cefotaxime Resistant Escherichia coli in Broilers in the First Week of Life. Vet. Microbiol. 2018, 222, 64–68. [Google Scholar] [CrossRef]
- Ramatla, T.; Mafokwane, T.; Lekota, K.; Monyama, M.; Khasapane, G.; Serage, N.; Nkhebenyane, J.; Bezuidenhout, C.; Thekisoe, O. “One Health” Perspective on Prevalence of Co-Existing Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli and Klebsiella Pneumoniae: A Comprehensive Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 88. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Ali, T.; Ali, I.; Khan, N.A.; Han, B.; Gao, J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. BioMed Res. Int. 2018, 2018, 9519718. [Google Scholar] [CrossRef]
- Tängdén, T.; Cars, O.; Melhus, Å.; Löwdin, E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum β-lactamases: A prospective study with Swedish volunteers. Antimicrob. Agents Chemother. 2010, 54, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Liu, C.-W.; Liu, P.-Y. Extended-Spectrum β-Lactamases (ESBL) Producing Bacteria in Animals. Antibiotics 2023, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2021; European Medicines Agency: Amsterdam, The Netherlands, 2021. [Google Scholar]
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Project Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018 (EMA/24309/2020). Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 6 November 2020).
- UNAITALIA. Best Practice Del Settore Avicolo in Italia; UNAITALIA: Rome, Italy, 2024; Available online: https://www.unaitalia.com/best-practice/ (accessed on 21 May 2025).
- Caucci, C.; Di Martino, G.; Dalla Costa, A.; Santagiuliana, M.; Lorenzetto, M.; Capello, K.; Mughini-Gras, L.; Gavazzi, L.; Bonfanti, L. Trends and Correlates of Antimicrobial Use in Broiler and Turkey Farms: A Poultry Company Registry-Based Study in Italy. J. Antimicrob. Chemother. 2019, 74, 2784–2787. [Google Scholar] [CrossRef]
- EFSA. Dashboard on Indicators of Antimicrobial Resistance; EFSA: Parma, Italy, 2022; Available online: https://www.efsa.europa.eu/en/microstrategy/dashboard-indicators-antimicrobial-resistance (accessed on 21 May 2025).
- EFSA. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- Urumova, V.; Stefanova, R.; Lyutskanov, M. Prevalence of Some Genetic Factors Determining Antimicrobial Resistance in Commensal Escherichia coli Isolated from Broilers and Laying Hens. Bulg. J. Vet. Med. 2024, 27, 143–151. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J. Antibiotic Resistance in the Food Supply Chain: Where Can Sequencing and Metagenomics Aid Risk Assessment? Curr. Opin. Food Sci. 2017, 14, 66–71. [Google Scholar] [CrossRef]
- Olsen, R.; Kudirkiene, E.; Thøfner, I.; Pors, S.; Karlskov-Mortensen, P.; Li, L.; Papasolomontos, S.; Angastiniotou, C.; Christensen, J. Impact of Egg Disinfection of Hatching Eggs on the Eggshell Microbiome and Bacterial Load. Poult. Sci. 2017, 96, 3901–3911. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of Egg Contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef]
- Aun, E.; Kisand, V.; Laht, M.; Telling, K.; Kalmus, P.; Väli, Ü.; Brauer, A.; Remm, M.; Tenson, T. Molecular Characterization of Enterococcus Isolates from Different Sources in Estonia Reveals Potential Transmission of Resistance Genes Among Different Reservoirs. Front. Microbiol. 2021, 12, 601490. [Google Scholar] [CrossRef]
- Saliu, E.M.; Vahjen, W.; Zentek, J. Types and Prevalence of Extended–Spectrum Beta–Lactamase Producing Enterobacteriaceae in Poultry. Anim. Health Res. Rev. 2017, 18, 46–57. [Google Scholar] [CrossRef]
- Leinweber, H.; Alotaibi, S.M.I.; Overballe-Petersen, S.; Hansen, F.; Hasman, H.; Bortolaia, V.; Hammerum, A.M.; Ingmer, H. Vancomycin Resistance in Enterococcus Faecium Isolated from Danish Chicken Meat Is Located on a PVEF4-like Plasmid Persisting in Poultry for 18 Years. Int. J. Antimicrob. Agents 2018, 52, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, P.; den Bergh, M.K.; Rossen, J.W.; Willemsen, I.; Verhulst, C.; Savelkoul, P.H.M.; Friedrich, A.W.; García-Cobos, S.; Kluytmans, J. Decreasing Prevalence of Contamination with Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae (ESBL-E) in Retail Chicken Meat in the Netherlands. PLoS ONE 2019, 14, e0226828. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Vinué, L.; Poeta, P.; Coelho, A.C.; Matos, M.; Sáenz, Y.; Somalo, S.; Zarazaga, M.; Rodrigues, J.; Torres, C. Prevalence of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Isolates in Faecal Samples of Broilers. Vet. Microbiol. 2009, 138, 339–344. [Google Scholar] [CrossRef]
- Clemente, L.; Leão, C.; Moura, L.; Albuquerque, T.; Amaro, A. Prevalence and Characterization of ESBL/AmpC Producing Escherichia coli from Fresh Meat in Portugal. Antibiotics 2021, 10, 1333. [Google Scholar] [CrossRef]
- Almeida, A.; Duarte, S.; Nunes, R.; Rocha, H.; Pena, A.; Meisel, L. Human and Veterinary Antibiotics Used in Portugal—A Ranking for Ecosurveillance. Toxics 2014, 2, 188–225. [Google Scholar] [CrossRef]
- Hu, F.; Guo, Y.; Yang, Y.; Zheng, Y.; Wu, S.; Jiang, X.; Zhu, D.; Wang, F.; on behalf of the China Antimicrobial Surveillance Network (CHINET) Study Group. Resistance Reported from China Antimicrobial Surveillance Network (CHINET) in 2018. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2275–2281. [Google Scholar] [CrossRef]
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian Poultry Productions and Anticipated Alternatives. Front. Microbiol. 2014, 5, 282. [Google Scholar] [CrossRef]
- Subirats, J.; Murray, R.; Scott, A.; Lau, C.H.-F.; Topp, E. Composting of Chicken Litter from Commercial Broiler Farms Reduces the Abundance of Viable Enteric Bacteria, Firmicutes, and Selected Antibiotic Resistance Genes. Sci. Total Environ. 2020, 746, 141113. [Google Scholar] [CrossRef]
- Piano Nazionale Di Contrasto All’Antibiotico-Resistenza (PNCAR) 2022–2025. Available online: https://www.epicentro.iss.it/antibiotico-resistenza/pncar-2022 (accessed on 25 May 2025).
- Stępień-Pyśniak, D.; Hauschild, T.; Kosikowska, U.; Dec, M.; Urban-Chmiel, R. Biofilm Formation Capacity and Presence of Virulence Factors among Commensal Enterococcus Spp. from Wild Birds. Sci. Rep. 2019, 9, 11204. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Proietti, P.C.; Musa, L.; Stefanetti, V.; Orsini, M.; Toppi, V.; Branciari, R.; Blasi, F.; Magistrali, C.F.; Capomaccio, S.; Kika, T.S.; et al. Mcr-1-Mediated Colistin Resistance and Genomic Characterization of Antimicrobial Resistance in ESBL-Producing Salmonella Infantis Strains from a Broiler Meat Production Chain in Italy. Antibiotics 2022, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P.C.; Stefanetti, V.; Musa, L.; Zicavo, A.; Dionisi, A.M.; Bellucci, S.; La Mensa, A.; Menchetti, L.; Branciari, R.; Ortenzi, R.; et al. Genetic Profiles and Antimicrobial Resistance Patterns of Salmonella Infantis Strains Isolated in Italy in the Food Chain of Broiler Meat Production. Antibiotics 2020, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Giufrè, M.; Mazzolini, E.; Cerquetti, M.; Brusaferro, S.; Accogli, M.; Agnoletti, F.; Agodi, A.; Alborali, G.L.; Arghittu, M.; Auxilia, F.; et al. Extended-Spectrum β-Lactamase-Producing Escherichia coli from Extraintestinal Infections in Humans and from Food-Producing Animals in Italy: A ‘One Health’ Study. Int. J. Antimicrob. Agents 2021, 58, 106433. [Google Scholar] [CrossRef]
- Niero, G.; Bortolaia, V.; Vanni, M.; Intorre, L.; Guardabassi, L.; Piccirillo, A. High Diversity of Genes and Plasmids Encoding Resistance to Third-Generation Cephalosporins and Quinolones in Clinical Escherichia coli from Commercial Poultry Flocks in Italy. Vet. Microbiol. 2018, 216, 93–98. [Google Scholar] [CrossRef]
- Bortolaia, V.; Trevisani, M.; Guardabassi, L.; Bisgaard, M.; Venturi, L.; Delle Donne, G.; Bojesen, A.M. Quinolone-and Eta-Lactam-Resistance in Escherichia coli from Danish and Italian Broiler Flocks. Ital. J. Food Saf. 2009, 3, 33–36. [Google Scholar] [CrossRef]
- Apostolakos, I.; Mughini-Gras, L.; Fasolato, L.; Piccirillo, A. Assessing the Occurrence and Transfer Dynamics of ESBL/PAmpC-Producing Escherichia coli across the Broiler Production Pyramid. PLoS ONE 2019, 14, e0217174. [Google Scholar] [CrossRef]
- Bortolaia, V.; Guardabassi, L.; Trevisani, M.; Bisgaard, M.; Venturi, L.; Bojesen, A.M. High Diversity of Extended-Spectrum β-Lactamases in Escherichia coli Isolates from Italian Broiler Flocks. Antimicrob. Agents Chemother. 2010, 54, 1623–1626. [Google Scholar] [CrossRef]
- Chiaretto, G.; Zavagnin, P.; Bettini, F.; Mancin, M.; Minorello, C.; Saccardin, C.; Ricci, A. Extended Spectrum β-Lactamase SHV-12-Producing Salmonella from Poultry. Vet. Microbiol. 2008, 128, 406–413. [Google Scholar] [CrossRef]
- Massella, E.; Giacometti, F.; Bonilauri, P.; Reid, C.J.; Djordjevic, S.P.; Merialdi, G.; Bacci, C.; Fiorentini, L.; Massi, P.; Bardasi, L.; et al. Antimicrobial Resistance Profile and ExPEC Virulence Potential in Commensal Escherichia coli of Multiple Sources. Antibiotics 2021, 10, 351. [Google Scholar] [CrossRef]
- Ferraresso, J.; Apostolakos, I.; Fasolato, L.; Piccirillo, A. Third-Generation Cephalosporin (3GC) Resistance and Its Association with Extra-Intestinal Pathogenic Escherichia coli (ExPEC). Focus on Broiler Carcasses. Food Microbiol. 2022, 103, 103936. [Google Scholar] [CrossRef]
- Thorpe, H.A.; Booton, R.; Kallonen, T.; Gibbon, M.J.; Couto, N.; Passet, V.; López-Fernández, S.; Rodrigues, C.; Matthews, L.; Mitchell, S.; et al. A Large-Scale Genomic Snapshot of Klebsiella Spp. Isolates in Northern Italy Reveals Limited Transmission between Clinical and Non-Clinical Settings. Nat. Microbiol. 2022, 7, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, I.; Feudi, C.; Eichhorn, I.; Palmieri, N.; Fasolato, L.; Schwarz, S.; Piccirillo, A. High-Resolution Characterisation of ESBL/PAmpC-Producing Escherichia coli Isolated from the Broiler Production Pyramid. Sci. Rep. 2020, 10, 11123. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E.; et al. Emergence of a Clonal Lineage of Multidrug-Resistant ESBL-Producing Salmonella Infantis Transmitted from Broilers and Broiler Meat to Humans in Italy between 2011 and 2014. PLoS ONE 2015, 10, e0144802. [Google Scholar] [CrossRef]
- Musa, L.; Casagrande Proietti, P.; Branciari, R.; Menchetti, L.; Bellucci, S.; Ranucci, D.; Marenzoni, M.L.; Franciosini, M.P. Antimicrobial Susceptibility of Escherichia coli and ESBL-Producing Escherichia coli Diffusion in Conventional, Organic and Antibiotic-Free Meat Chickens at Slaughter. Animals 2020, 10, 1215. [Google Scholar] [CrossRef]
- Alba, P.; Leekitcharoenphon, P.; Franco, A.; Feltrin, F.; Ianzano, A.; Caprioli, A.; Stravino, F.; Hendriksen, R.S.; Bortolaia, V.; Battisti, A. Molecular Epidemiology of Mcr-Encoded Colistin Resistance in Enterobacteriaceae from Food-Producing Animals in Italy Revealed Through the EU Harmonized Antimicrobial Resistance Monitoring. Front. Microbiol. 2018, 9, 1217. [Google Scholar] [CrossRef]
- Carfora, V.; Alba, P.; Leekitcharoenphon, P.; Ballarò, D.; Cordaro, G.; Di Matteo, P.; Donati, V.; Ianzano, A.; Iurescia, M.; Stravino, F.; et al. Colistin Resistance Mediated by Mcr-1 in ESBL-Producing, Multidrug Resistant Salmonella Infantis in Broiler Chicken Industry, Italy (2016–2017). Front. Microbiol. 2018, 9, 1880. [Google Scholar] [CrossRef]
- Alba, P.; Leekitcharoenphon, P.; Carfora, V.; Amoruso, R.; Cordaro, G.; Di Matteo, P.; Ianzano, A.; Iurescia, M.; Diaconu, E.L.; Pedersen, S.K.; et al. Molecular Epidemiology of Salmonella Infantis in Europe: Insights into the Success of the Bacterial Host and Its Parasitic PESI-like Megaplasmid. Microb. Genom. 2020, 6, e000365. [Google Scholar] [CrossRef]
- Bertelloni, F.; Bresciani, F.; Cagnoli, G.; Scotti, B.; Lazzerini, L.; Marcucci, M.; Colombani, G.; Bilei, S.; Bossù, T.; De Marchis, M.L.; et al. House Flies (Musca Domestica) from Swine and Poultry Farms Carrying Antimicrobial Resistant Enterobacteriaceae and Salmonella. Vet. Sci. 2023, 10, 118. [Google Scholar] [CrossRef]
- Alba, P.; Carfora, V.; Feltrin, F.; Diaconu, E.L.; Sorbara, L.; Dell’Aira, E.; Cerci, T.; Ianzano, A.; Donati, V.; Franco, A.; et al. Evidence of Structural Rearrangements in ESBL-Positive PESI(like) Megaplasmids of S.Infantis. FEMS Microbiol. Lett. 2023, 370, fnad014. [Google Scholar] [CrossRef]
- Ferri, G.; Buonavoglia, A.; Farooq, M.; Festino, A.R.; Ruffini, F.; Paludi, D.; Di Francesco, C.E.; Vergara, A.; Smoglica, C. Antibiotic Resistance in Italian Poultry Meat Production Chain: A One-Health Perspective Comparing Antibiotic Free and Conventional Systems from the Farming to the Slaughterhouse. Front. Food Sci. Technol. 2023, 3, 1168896. [Google Scholar] [CrossRef]
- Cornacchia, A.; Janowicz, A.; Centorotola, G.; Saletti, M.A.; Ranieri, S.C.; Ancora, M.; Ripà, P.; Cammà, C.; Pomilio, F.; Chiaverini, A. Multi-Approach Methods to Predict Cryptic Carbapenem Resistance Mechanisms in Klebsiella Pneumoniae Detected in Central Italy. Front. Microbiol. 2023, 14, 1242693. [Google Scholar] [CrossRef] [PubMed]
- Tofani, S.; Orsini, S.; Pesciaroli, M.; Lovito, C.; Scoccia, E.; Maresca, C.; Pezzotti, G.; Magistrali, C.F. Characterization of Escherichia coli ESBL Producers Circulating in Three Different Production Systems of Broilers in Italy [Conference Poster]. In Proceedings of the XVIII Congresso Nazionale S.I.Di.L.V., Perugia, Italy, 7–9 November 2018; pp. 161–163. [Google Scholar]
- Pesciaroli, M.; Magistrali, C.F.; Filippini, G.; Epifanio, E.M.; Lovito, C.; Marchi, L.; Maresca, C.; Massacci, F.R.; Orsini, S.; Scoccia, E.; et al. Antibiotic-Resistant Commensal Escherichia coli Are Less Frequently Isolated from Poultry Raised Using Non-Conventional Management Systems than from Conventional Broiler. Int. J. Food Microbiol. 2020, 314, 108391. [Google Scholar] [CrossRef] [PubMed]
- Beninati, C.; Reich, F.; Muscolino, D.; Giarratana, F.; Panebianco, A.; Klein, G.; Atanassova, V. ESBL-Producing Bacteria and MRSA Isolated from Poultry and Turkey Products Imported from Italy. Czech J. Food Sci. 2015, 33, 97–102. [Google Scholar] [CrossRef]
- Diaconu, E.L.; Alba, P.; Feltrin, F.; Di Matteo, P.; Iurescia, M.; Chelli, E.; Donati, V.; Marani, I.; Giacomi, A.; Franco, A.; et al. Emergence of IncHI2 Plasmids with Mobilized Colistin Resistance (Mcr)-9 Gene in ESBL-Producing, Multidrug-Resistant Salmonella typhimurium and Its Monophasic Variant ST34 From Food-Producing Animals in Italy. Front. Microbiol. 2021, 12, 705230. [Google Scholar] [CrossRef]
- Ghodousi, A.; Bonura, C.; Di Carlo, P.; van Leeuwen, W.B.; Mammina, C. Extraintestinal Pathogenic Escherichia coli Sequence Type 131 H30-R and H30-Rx Subclones in Retail Chicken Meat, Italy. Int. J. Food Microbiol. 2016, 228, 10–13. [Google Scholar] [CrossRef]
- Ghodousi, A.; Bonura, C.; Di Noto, A.M.; Mammina, C. Extended-Spectrum ß-Lactamase, AmpC-Producing, and Fluoroquinolone-Resistant Escherichia coli in Retail Broiler Chicken Meat, Italy. Foodborne Pathog. Dis. 2015, 12, 619–625. [Google Scholar] [CrossRef]
- Castello, A.; Piraino, C.; Butera, G.; Alio, V.; Cardamone, C.; Oliveri, G.; Cascone, G.; Ciravolo, C.; Costa, A. Prevalence and Antimicrobial Resistance Profiles of Salmonella spp. in Poultry Meat. Ital. J. Food Saf. 2023, 12, 11135. [Google Scholar] [CrossRef]
- Di Marcantonio, L.; Romantini, R.; Marotta, F.; Chiaverini, A.; Zilli, K.; Abass, A.; Di Giannatale, E.; Garofolo, G.; Janowicz, A. The Current Landscape of Antibiotic Resistance of Salmonella infantis in Italy: The Expansion of Extended-Spectrum Beta-Lactamase Producers on a Local Scale. Front. Microbiol. 2022, 13, 812481. [Google Scholar] [CrossRef]
- Bortolaia, V.; Bisgaard, M.; Bojesen, A.M. Distribution and Possible Transmission of Ampicillin-and Nalidixic Acid-Resistant Escherichia coli within the Broiler Industry. Vet. Microbiol. 2010, 142, 379–386. [Google Scholar] [CrossRef]
- Farooq, M.; Smoglica, C.; Ruffini, F.; Soldati, L.; Marsilio, F.; Di Francesco, C.E. Antibiotic Resistance Genes Occurrence in Conventional and Antibiotic-Free Poultry Farming, Italy. Animals 2022, 12, 2310. [Google Scholar] [CrossRef]
- Di Francesco, C.E.; Smoglica, C.; Profeta, F.; Farooq, M.; Di Giannatale, E.; Toscani, T.; Marsilio, F. Research Note: Detection of Antibiotic-Resistance Genes in Commercial Poultry and Turkey Flocks from Italy. Poult. Sci. 2021, 100, 101084. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-Spectrum β-Lactamases in the 21st Century: Characterization, Epidemiology, and Detection of This Important Resistance Threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Rastuti, M.R.; Budayanti, N.N.S.; Dwija, I.B.N.P. Various Types of Extended Spectrum β-Lactamases: A Literature Review. J. Clin. Microbiol. Infect. Dis. 2023, 3, 29–34. [Google Scholar] [CrossRef]
- Weldhagen, G.F. GES: An Emerging Family of Extended Spectrum Beta-Lactamases. Clin. Microbiol. Newsl. 2006, 28, 145–149. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Guibert, M.; Chaibi, E.B.; Labia, R.; Nordmann, P. Molecular and Biochemical Characterization of VEB-1, a Novel Class A Extended-Spectrum β-Lactamase Encoded by an Escherichia coli Integron Gene. Antimicrob. Agents Chemother. 1999, 43, 573–581. [Google Scholar] [CrossRef]
- Kuhnke, D.; Werner, N.; Kreienbrock, L. Occurrence of ESBL-Producing Escherichia coli in Healthy, Living Food-Producing Animals in Europe: A Systematic Review. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Danel, F.; Hall, L.M.C.; Gur, D.; Akalin, H.E.; Livermore, D.M. Transferable Production of PER-1 β-Lactamase in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1995, 35, 281–294. [Google Scholar] [CrossRef]
- Romero-Oraá, R.; Herrero-Tudela, M.; López, M.I.; Hornero, R.; García, M. Attention-Based Deep Learning Framework for Automatic Fundus Image Processing to Aid in Diabetic Retinopathy Grading. Comput. Methods Programs Biomed. 2024, 249, 108160. [Google Scholar] [CrossRef]
- Osman, A.-H.; Darkwah, S.; Kotey, F.C.N.; Odoom, A.; Hotor, P.; Dayie, N.T.K.D.; Donkor, E.S. Reservoirs of Nosocomial Pathogens in Intensive Care Units: A Systematic Review. Environ. Health Insights 2024, 18, 11786302241243239. [Google Scholar] [CrossRef]
- Endimiani, A.; Luzzaro, F.; Pini, B.; Amicosante, G.; Maria Rossolini, G.; Toniolo, A.Q. Pseudomonas Aeruginosa Bloodstream Infections: Risk Factors and Treatment Outcome Related to Expression of the PER-1 Extended-Spectrum Beta-Lactamase. BMC Infect. Dis. 2006, 6, 52. [Google Scholar] [CrossRef]
- Ayinla, A.O.; Mateus, A.L.P. Extended-Spectrum Beta-Lactamases in Poultry in Africa: A Systematic Review. Front. Antibiot. 2023, 2, 1140750. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-Spectrum β-Lactamase-Producing and AmpC-Producing Escherichia coli from Livestock and Companion Animals, and Their Putative Impact on Public Health: A Global Perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Gundran, R.S.; Cardenio, P.A.; Villanueva, M.A.; Sison, F.B.; Benigno, C.C.; Kreausukon, K.; Pichpol, D.; Punyapornwithaya, V. Prevalence and Distribution of BlaCTX-M, BlaSHV, BlaTEM Genes in Extended-Spectrum β-Lactamase-Producing E. Coli Isolates from Broiler Farms in the Philippines. BMC Vet. Res. 2019, 15, 227. [Google Scholar] [CrossRef]
- Menck-Costa, M.F.; Baptista, A.A.S.; Gazal, L.E.D.S.; Justino, L.; Sanches, M.S.; de Souza, M.; Nishio, E.K.; Queiroz dos Santos, B.; Cruz, V.D.; Berbert, J.V.M.; et al. High-Frequency Detection of FosA3 and BlaCTX–M–55 Genes in Escherichia coli From Longitudinal Monitoring in Broiler Chicken Farms. Front. Microbiol. 2022, 13, 846116. [Google Scholar] [CrossRef]
- Platell, J.L.; Johnson, J.R.; Cobbold, R.N.; Trott, D.J. Multidrug-Resistant Extraintestinal Pathogenic Escherichia coli of Sequence Type ST131 in Animals and Foods. Vet. Microbiol. 2011, 153, 99–108. [Google Scholar] [CrossRef]
- Casella, T.; Nogueira, M.C.L.; Saras, E.; Haenni, M.; Madec, J.Y. High Prevalence of ESBLs in Retail Chicken Meat despite Reduced Use of Antimicrobials in Chicken Production, France. Int. J. Food Microbiol. 2017, 257, 271–275. [Google Scholar] [CrossRef]
- Bergšpica, I.; Kaprou, G.; Alexa, E.A.; Prieto, M.; Alvarez-Ordóñez, A. Extended Spectrum β-Lactamase (ESBL) Producing Escherichia coli in Pigs and Pork Meat in the European Union. Antibiotics 2020, 9, 678. [Google Scholar] [CrossRef]
- Haeili, M.; Salehzeinali, H.; Mirzaei, S.; Pishnian, Z.; Ahmadi, A. Molecular Characterization of Quinolone Resistance and Antimicrobial Resistance Profiles of Klebsiella Pneumoniae and Escherichia coli Isolated from Human and Broiler Chickens. Int. J. Environ. Health Res. 2022, 32, 1382–1392. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Nordmann, P. Diversity, Epidemiology, and Genetics of Class D β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 24–38. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging Broad-Spectrum Resistance in Pseudomonas Aeruginosa and Acinetobacter Baumannii: Mechanisms and Epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [CrossRef]
- Wang, J.; Stephan, R.; Karczmarczyk, M.; Yan, Q.; Hächler, H.; Fanning, S. Molecular Characterization of Bla ESBL–Harboring Conjugative Plasmids Identified in Multi-Drug Resistant Escherichia coli Isolated from Food-Producing Animals and Healthy Humans. Front. Microbiol. 2013, 4, 188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Stephan, R.; Power, K.; Yan, Q.; Hächler, H.; Fanning, S. Nucleotide Sequences of 16 Transmissible Plasmids Identified in Nine Multidrug-Resistant Escherichia coli Isolates Expressing an ESBL Phenotype Isolated from Food-Producing Animals and Healthy Humans. J. Antimicrob. Chemother. 2014, 69, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Aldea, I.; Gibello, A.; Hernández, M.; Leekitcharoenphon, P.; Bortolaia, V.; Moreno, M.A. Clonal and Plasmid-Mediated Flow of ESBL/AmpC Genes in Escherichia coli in a Commercial Laying Hen Farm. Vet. Microbiol. 2022, 270, 109453. [Google Scholar] [CrossRef] [PubMed]
- Anes, J.; Nguyen, S.V.; Eshwar, A.K.; McCabe, E.; Macori, G.; Hurley, D.; Lehner, A.; Fanning, S. Molecular Characterisation of Multi-Drug Resistant Escherichia coli of Bovine Origin. Vet. Microbiol. 2020, 242, 108566. [Google Scholar] [CrossRef]
- Saidani, M.; Messadi, L.; Mefteh, J.; Chaouechi, A.; Soudani, A.; Selmi, R.; Dâaloul-Jedidi, M.; Chehida, F.B.; Mamlouk, A.; Jemli, M.H.; et al. Various Inc-Type Plasmids and Lineages of Escherichia coli and Klebsiella Pneumoniae Spreading BlaCTX-M-15, BlaCTX-M-1 and Mcr-1 Genes in Camels in Tunisia. J. Glob. Antimicrob. Resist. 2019, 19, 280–283. [Google Scholar] [CrossRef]
- Shafiq, M.; Rahman, S.U.; Bilal, H.; Ullah, A.; Noman, S.M.; Zeng, M.; Yuan, Y.; Xie, Q.; Li, X.; Jiao, X. Incidence and Molecular Characterization of ESBL-Producing and Colistin-Resistant Escherichia coli Isolates Recovered from Healthy Food-Producing Animals in Pakistan. J. Appl. Microbiol. 2022, 133, 1169–1182. [Google Scholar] [CrossRef]
- Valentin, L.; Sharp, H.; Hille, K.; Seibt, U.; Fischer, J.; Pfeifer, Y.; Michael, G.B.R.; Nickel, S.; Schmiedel, J.; Falgenhauer, L.; et al. Subgrouping of ESBL-Producing Escherichia coli from Animal and Human Sources: An Approach to Quantify the Distribution of ESBL Types between Different Reservoirs. Int. J. Med. Microbiol. 2014, 304, 805–816. [Google Scholar] [CrossRef]
- Brazelton de Cardenas, J.N.; Garner, C.D.; Su, Y.; Tang, L.; Hayden, R.T. Comparative Evaluation of Assays for Broad Detection of Molecular Resistance Mechanisms in Enterobacterales Isolates. J. Clin. Microbiol. 2021, 59, 10.1128/jcm.01033-21. [Google Scholar] [CrossRef]
- Foti, M.; Grasso, R.; Fisichella, V.; Mascetti, A.; Colnaghi, M.; Grasso, M.; Spena, M.T. Antimicrobial Resistance in Physiological and Potentially Pathogenic Bacteria Isolated in Southern Italian Bats. Animals 2023, 13, 966. [Google Scholar] [CrossRef]
- Chiaverini, A.; Cornacchia, A.; Centorotola, G.; Tieri, E.E.; Sulli, N.; Del Matto, I.; Iannitto, G.; Petrone, D.; Petrini, A.; Pomilio, F. Phenotypic and Genetic Characterization of Klebsiella Pneumoniae Isolates from Wild Animals in Central Italy. Animals 2022, 12, 1347. [Google Scholar] [CrossRef]
- Gambino, D.; Vicari, D.; Vitale, M.; Schirò, G.; Mira, F.; La Giglia, M.; Riccardi, A.; Gentile, A.; Giardina, S.; Carrozzo, A.; et al. Study on Bacteria Isolates and Antimicrobial Resistance in Wildlife in Sicily, Southern Italy. Microorganisms 2021, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Giacopello, C.; Foti, M.; Mascetti, A.; Grosso, F.; Ricciard, D.; Fisichella, V.; Piccolo, F.L. Antimicrobial Resistance Patterns of Enterobacteriaceae in European Wild Bird Species Admitted in a Wildlife Rescue Centre. Vet. Ital. 2016, 52, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Laconi, A.; Tolosi, R.; Apostolakos, I.; Piccirillo, A. Biofilm Formation Ability of ESBL/PAmpC-Producing Escherichia coli Isolated from the Broiler Production Pyramid. Antibiotics 2023, 12, 155. [Google Scholar] [CrossRef]
- Zogg, A.L.; Zurfluh, K.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of ESBL-Producing Enterobacteriaceae and Methicillinresistant Staphylococcus Aureus (MRSA) Isolated from Swiss and Imported Raw Poultry Meat Collected at Retail Level. Schweiz. Arch. Tierheilkd. 2016, 158, 451–456. [Google Scholar] [CrossRef]
- Alessiani, A.; Goffredo, E.; Mancini, M.; Occhiochiuso, G.; Faleo, S.; Didonna, A.; Fischetto, R.; Suglia, F.; De Vito, D.; Stallone, A. Evaluation of Antimicrobial Resistance in Salmonella Strains Isolated from Food, Animal and Human Samples between 2017 and 2021 in Southern Italy. Microorganisms 2022, 10, 812. [Google Scholar] [CrossRef]
- Lettini, A.; Cibin, V.; Pasquale, F.; Mancin, M.; Patuzzi, I. Escherichia coli Producers of ESBL (Extended Spectrum Beta-Lactamase): Prevalence and Genetic Diversity in Isolates from Poultry Farms and Poultry Food in Italy. In Proceedings of the XVI Congresso Nazionale S.I.Di.L.V., Montesilvano, Italy, 30 September–2 October 2015; pp. 63–64. [Google Scholar]
- Castronovo, C.; Agozzino, V.; Schirò, G.; Mira, F.; Di Bella, S.; Lastra, A.; Antoci, F.; Pennisi, M.; Giudice, E.; Guercio, A. Evaluation of the Antimicrobial Resistance of Different Serotypes of Salmonella Enterica from Livestock Farms in Southern Italy. Appl. Sci. 2022, 13, 442. [Google Scholar] [CrossRef]
- Nobili, G.; La Bella, G.; Basanisi, M.G.; Damato, A.M.; Coppola, R.; Migliorelli, R.; Rondinone, V.; Leekitcharoenphon, P.; Bortolaia, V.; La Salandra, G. Occurrence and Characterisation of Colistin-Resistant Escherichia coli in Raw Meat in Southern Italy in 2018–2020. Microorganisms 2022, 10, 1805. [Google Scholar] [CrossRef]
- Stefani, S.; Giovanelli, I.; Anacarso, I.; Condò, C.; Messi, P.; De Niederhausern, S.; Bondi, M.; Iseppi, R.; Sabia, C. Prevalence and Characterization of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Food-Producing Animals in Northern Italy. New Microbiol. 2014, 37, 551–555. [Google Scholar]
- Massaccesi, L.; Albini, E.; Massacci, F.R.; Orsini, S.; Tofani, S.; Blasi, F.; Marchi, L.; Pezzotti, G.; Magistrali, C.F. Longitudinal Study on Antibiotic Susceptibility in Commensal E. Coli from Geese Raised in Free-Range Production Systems. Poult. Sci. 2021, 100, 101230. [Google Scholar] [CrossRef]
- Gambi, L.; Rossini, R.; Menandro, M.L.; Franzo, G.; Valentini, F.; Tosi, G.; D’Incau, M.; Fiorentini, L. Virulence Factors and Antimicrobial Resistance Profile of Escherichia coli Isolated from Laying Hens in Italy. Animals 2022, 12, 1812. [Google Scholar] [CrossRef]
- Kolesnik-Goldmann, N.; Seth-Smith, H.M.B.; Haldimann, K.; Imkamp, F.; Roloff, T.; Zbinden, R.; Hobbie, S.N.; Egli, A.; Mancini, S. Comparison of Disk Diffusion, E-Test, and Broth Microdilution Methods for Testing In Vitro Activity of Cefiderocol in Acinetobacter baumannii. Antibiotics 2023, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Gunjan; Vidic, J.; Manzano, M.; Raj, V.S.; Pandey, R.P.; Chang, C.-M. Comparative Meta-Analysis of Antimicrobial Resistance from Different Food Sources along with One Health Approach in Italy and Thailand. One Health 2023, 16, 100477. [Google Scholar] [CrossRef] [PubMed]
- Campos-Madueno, E.I.; Moser, A.I.; Keller, P.M.; Perreten, V.; Poirel, L.; Nordmann, P.; Endimiani, A. Evaluation of Phenotypic Tests to Detect Extended-Spectrum β-Lactamase (ESBL)-Producing Klebsiella Oxytoca Complex Strains. J. Clin. Microbiol. 2023, 61, e01706-22. [Google Scholar] [CrossRef]
- Zogg, A.L.; Simmen, S.; Zurfluh, K.; Stephan, R.; Schmitt, S.N.; Nüesch-Inderbinen, M. High Prevalence of Extended-Spectrum β-Lactamase Producing Enterobacteriaceae among Clinical Isolates from Cats and Dogs Admitted to a Veterinary Hospital in Switzerland. Front. Vet. Sci. 2018, 5, 361240. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Pasqualin, D.; Tarakdjian, J.; Santini, A.; Cunial, G.; Tonellato, F.; Schiavon, E.; Di Martino, G. Short-Term and Long-Term Effects of Antimicrobial Use on Antimicrobial Resistance in Broiler and Turkey Farms. Avian Pathol. 2022, 51, 120–128. [Google Scholar] [CrossRef]
- Widodo, A.; Effendi, M.H.; Khairullah, A.R. Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli from Livestock. Sys Rev. Pharm. 2020, 11, 382–392. [Google Scholar]
- Condorelli, C.; Nicitra, E.; Musso, N.; Bongiorno, D.; Stefani, S.; Gambuzza, L.V.; Carchiolo, V.; Frasca, M. Prediction of Antimicrobial Resistance of Klebsiella Pneumoniae from Genomic Data through Machine Learning. PLoS ONE 2024, 19, e0309333. [Google Scholar] [CrossRef]
- Guerra, P.R.; Herrero-Fresno, A.; Pors, S.E.; Ahmed, S.; Wang, D.; Thøfner, I.; Antenucci, F.; Olsen, J.E. The Membrane Transporter PotE Is Required for Virulence in Avian Pathogenic Escherichia coli (APEC). Vet. Microbiol. 2018, 216, 38–44. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Wang, Y.; Fanning, S.; Cui, S.; Chen, Q.; Liu, G.; Chen, Q.; Zhou, G.; Yang, B.; et al. Serovar Diversity and Antimicrobial Resistance of Non-Typhoidal Salmonella Enterica Recovered from Retail Chicken Carcasses for Sale in Different Regions of China. Food Control 2017, 81, 46–54. [Google Scholar] [CrossRef]
- Garcias, B.; Aguirre, L.; Seminati, C.; Reyes, N.; Allepuz, A.; Obón, E.; Molina-Lopez, R.A.; Darwich, L. Extended-Spectrum β-Lactam Resistant Klebsiella Pneumoniae and Escherichia coli in Wild European Hedgehogs (Erinaceus Europeus) Living in Populated Areas. Animals 2021, 11, 2837. [Google Scholar] [CrossRef]
- Dunn, S.J.; Connor, C.; McNally, A. The Evolution and Transmission of Multi-Drug Resistant Escherichia coli and Klebsiella Pneumoniae: The Complexity of Clones and Plasmids. Curr. Opin. Microbiol. 2019, 51, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Du Fraysseix, L.; François, P.; Drapeau, A.; Bralet, T.; Madec, J.-Y.; Boulinier, T.; Duriez, O. Occurrence of ESBL- and AmpC-Producing E. Coli in French Griffon Vultures Feeding on Extensive Livestock Carcasses. Antibiotics 2023, 12, 1160. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Silva, V.; de Lurdes Enes Dapkevicius, M.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Yahya, K.; Alhetar, A.; Sekawi, Z.; Mariana, S.; Neela, V. Molecular Characterization of Extended-Spectrum Beta-Lactimase (ESBL) Producing Extra-Intestinal Pathogenic Escherichia coli. Afr. J. Microbiol. Res. 2011, 5, 5662–5668. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ahmad, S.; Khan, I. Incidence of ESBL-Producing-Escherichia coli in Poultry Farm Environment and Retail Poultry Meat. Pak. Vet. J. 2018, 39, 116–120. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the Use of Antibiotics in Food-Producing Animals and Its Associations with Antibiotic Resistance in Food-Producing Animals and Human Beings: A Systematic Review and Meta-Analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Blaak, H.; Hamidjaja, R.A.; Van Hoek, A.H.A.M.; De Heer, L.; De Roda Husman, A.M.; Schets, F.M. Detection of Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli on Flies at Poultry Farms. Appl. Environ. Microbiol. 2014, 80, 239–246. [Google Scholar] [CrossRef]
- Olsen, R.H.; Bisgaard, M.; Löhren, U.; Robineau, B.; Christensen, H. Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolated from Poultry: A Review of Current Problems, Illustrated with Some Laboratory Findings. Avian Pathol. 2014, 43, 199–208. [Google Scholar] [CrossRef]
- Dierikx, C.; van Essen-Zandbergen, A.; Veldman, K.; Smith, H.; Mevius, D. Increased Detection of Extended Spectrum Beta-Lactamase Producing Salmonella Enterica and Escherichia coli Isolates from Poultry. Vet. Microbiol. 2010, 145, 273–278. [Google Scholar] [CrossRef]
- Ewers, C.; de Jong, A.; Prenger-Berninghoff, E.; El Garch, F.; Leidner, U.; Tiwari, S.K.; Semmler, T. Genomic Diversity and Virulence Potential of ESBL- and AmpC-β-Lactamase-Producing Escherichia coli Strains from Healthy Food Animals Across Europe. Front. Microbiol. 2021, 12, 626774. [Google Scholar] [CrossRef]
- Carattoli, A. Animal Reservoirs for Extended Spectrum β-Lactamase Producers. Clin. Microbiol. Infect. 2008, 14, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Briñas, L.; Moreno, M.A.; Zarazaga, M.; Porrero, C.; Sáenz, Y.; García, M.; Dominguez, L.; Torres, C. Detection of CMY-2, CTX-M-14, and SHV-12 β-Lactamases in Escherichia coli Fecal-Sample Isolates from Healthy Chickens. Antimicrob. Agents Chemother. 2003, 47, 2056–2058. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, L.; Pal, A.; Biswas, R.; Batabyal, K.; Dey, S.; Joardar, S.N.; Dutta, T.K.; Bandyopadhyay, S.; Pal, S.; Samanta, I. In-Silico Insights of ESBL Variants and Tracking the Probable Sources of ESBL-Producing Escherichia coli in a Small-Scale Poultry Farm. Microb. Pathog. 2024, 192, 106710. [Google Scholar] [CrossRef] [PubMed]
- Sakthikarthikeyan, S.; Sivakumar, M.; Manikandan, R.; Kumar, P.S.; Kumar, V.S.; Malmarugan, S.; Prabhu, M.; Ramakrishnan, V. Prevalence and Molecular Characterization of Multidrug-Resistant ESBL-Producing E. Coli in Commercial Poultry. Indian. J. Anim. Res. 2024, 1, 6. [Google Scholar] [CrossRef]
- Oikarainen, P.E.; Pohjola, L.K.; Pietola, E.S.; Heikinheimo, A. Direct Vertical Transmission of ESBL/PAmpC-Producing Escherichia coli Limited in Poultry Production Pyramid. Vet. Microbiol. 2019, 231, 100–106. [Google Scholar] [CrossRef]
- Suresh, Y.; Kiranmayi, C.B.; Rao, T.S.; Srivani, M.; Subhashini, N.; Chaitanya, G.; Vimala, B.S.; Suresh, B. Multidrug Resistance and ESBL Profile of Salmonella Serovars Isolated from Poultry Birds and Foods of Animal Origin. Pharma Innov. J. 2019, 8, 277–282. [Google Scholar]
- Klimienė, I.; Virgailis, M.; Kerzienė, S.; Šiugždinienė, R.; Mockeliūnas, R.; Ružauskas, M. Evaluation of Genotypical Antimicrobial Resistance in ESBL Producing Escherichia coli Phylogenetic Groups Isolated from Retail Poultry Meat. J. Food Saf. 2018, 38, e12370. [Google Scholar] [CrossRef]
- Samanta, I.; Joardar, S.N.; Das, P.K.; Sar, T.K. Comparative Possession of Shiga Toxin, Intimin, Enterohaemolysin and Major Extended Spectrum Beta Lactamase (ESBL) Genes in Escherichia coli Isolated from Backyard and Farmed Poultry. Iran. J. Vet. Res. 2015, 16, 90–93. [Google Scholar]
- Laube, H.; Friese, A.; von Salviati, C.; Guerra, B.; Rösler, U. Transmission of ESBL/AmpC-Producing Escherichia coli from Broiler Chicken Farms to Surrounding Areas. Vet. Microbiol. 2014, 172, 519–527. [Google Scholar] [CrossRef]
- Lamba, M.; Ahammad, S.Z. Sewage Treatment Effluents in Delhi: A Key Contributor of β-Lactam Resistant Bacteria and Genes to the Environment. Chemosphere 2017, 188, 249–256. [Google Scholar] [CrossRef]
- Coque, T.M.; Baquero, F.; Cantón, R. Increasing Prevalence of ESBL-Producing Enterobacteriaceae in Europe. Eurosurveillance 2008, 13, 19044. [Google Scholar] [CrossRef] [PubMed]
- Durairajan, R.; Murugan, M.; Karthik, K.; Porteen, K. Farmer’s Stance on Antibiotic Resistance to E. Coli and Extended Spectrum-β-Lactamase Producing (ESBL) E. coli Isolated from Poultry Droppings. Asian J. Dairy Food Res. 2021, 40, 88–93. [Google Scholar] [CrossRef]
- Blanc, V.; Mesa, R.; Saco, M.; Lavilla, S.; Prats, G.; Miró, E.; Navarro, F.; Cortés, P.; Llagostera, M. ESBL- and Plasmidic Class C β-Lactamase-Producing E. Coli Strains Isolated from Poultry, Pig and Rabbit Farms. Vet. Microbiol. 2006, 118, 299–304. [Google Scholar] [CrossRef]
- Blaak, H.; Van Hoek, A.H.A.M.; Hamidjaja, R.A.; Van Der Plaats, R.Q.J.; Kerkhof-De Heer, L.; De Roda Husman, A.M.; Schets, F.M. Distribution, Numbers, and Diversity of ESBL-Producing E. Coli in the Poultry Farm Environment. PLoS ONE 2015, 10, e0135402. [Google Scholar] [CrossRef]
- Liebhart, D.; Bilic, I.; Grafl, B.; Hess, C.; Hess, M. Diagnosing Infectious Diseases in Poultry Requires a Holistic Approach: A Review. Poultry 2023, 2, 252–280. [Google Scholar] [CrossRef]
- Chowdhury, M.; Bardhan, R.; Pal, S.; Banerjee, A.; Batabyal, K.; Joardar, S.N.; Mandal, G.P.; Bandyopadhyay, S.; Dutta, T.K.; Sar, T.K.; et al. Comparative Occurrence of ESBL/AmpC Beta-lactamase-producing Escherichia coli and Salmonella in Contract Farm and Backyard Broilers. Lett. Appl. Microbiol. 2022, 74, 53–62. [Google Scholar] [CrossRef]
- Abreu, R.; Castro, B.; Espigares, E.; Rodríguez-Álvarez, C.; Lecuona, M.; Moreno, E.; Espigares, M.; Arias, Á. Prevalence of CTX-M-Type Extended-Spectrum β-Lactamases in Escherichia coli Strains Isolated in Poultry Farms. Foodborne Pathog. Dis. 2014, 11, 868–873. [Google Scholar] [CrossRef]
- Gregova, G.; Kmetova, M.; Kmet, V.; Venglovsky, J.; Feher, A. Antibiotic Resistance of Escherichia coli Isolated from a Poultry Slaughterhouse. Ann. Agric. Environ. Med. 2012, 19, 75–77. [Google Scholar]
- Stuart, J.C.; van den Munckhof, T.; Voets, G.; Scharringa, J.; Fluit, A.; Leverstein-Van Hall, M. Comparison of ESBL Contamination in Organic and Conventional Retail Chicken Meat. Int. J. Food Microbiol. 2012, 154, 212–214. [Google Scholar] [CrossRef]
- Ilyas, S.; Rasool, M.H.; Arshed, M.J.; Qamar, M.U.; Aslam, B.; Almatroudi, A.; Khurshid, M. The Escherichia coli Sequence Type 131 Harboring Extended-Spectrum Beta-Lactamases and Carbapenemases Genes from Poultry Birds. Infect. Drug Resist. 2021, 14, 805–813. [Google Scholar] [CrossRef]
- van Hoek, A.H.A.M.; Veenman, C.; Florijn, A.; Huijbers, P.M.C.; Graat, E.A.M.; de Greeff, S.; Dierikx, C.M.; van Duijkeren, E. Longitudinal Study of ESBL Escherichia coli Carriage on an Organic Broiler Farm. J. Antimicrob. Chemother. 2018, 73, 3298–3304. [Google Scholar] [CrossRef] [PubMed]
- Mezhoud, H.; Chantziaras, I.; Iguer-Ouada, M.; Moula, N.; Garmyn, A.; Martel, A.; Touati, A.; Smet, A.; Haesebrouck, F.; Boyen, F. Presence of Antimicrobial Resistance in Coliform Bacteria from Hatching Broiler Eggs with Emphasis on ESBL/AmpC-Producing Bacteria. Avian Pathol. 2016, 45, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Chika, E.; Ifeanyichukwu, I.; Clement, O.A.; Malachy, U.; Peter, E.; Chidinma, I.; Lilian, O.; Chinedu, O. Multiple Antibiotic Resistance, Antibiogram and Phenotypic Detection of Metallo-Beta-Lactamase (MBL) from Escherichia coli of Poultry Origin. J. Appl. Microbiol. Biochem. 2017, 1, 15–20. [Google Scholar] [CrossRef]
- Ziech, R.E.; Lampugnani, C.; Perin, A.P.; Sereno, M.J.; Sfaciotte, R.A.P.; Viana, C.; Soares, V.M.; Pinto, J.P.d.A.N.; Bersot, L.d.S. Multidrug Resistance and ESBL-Producing Salmonella spp. Isolated from Broiler Processing Plants. Braz. J. Microbiol. 2016, 47, 191–195. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.A.; Roshdy, H.; Samir, A.H.; Hamed, E.A. Antibiotic Resistance and Extended-Spectrum β-Lactamase in Escherichia coli Isolates from Imported 1-Day-Old Chicks, Ducklings, and Turkey Poults. Vet. World 2020, 13, 1037–1044. [Google Scholar] [CrossRef]
- Sivaprakasam, A.; Kannan, P.; Ganapathy, S.; Mangalanathan, V.; Muthukrishnan, S. Screening of E. coli Isolates of Poultry Origin for Extended Spectrum Betalactamases (ESBL) Resistance. Int. J. Livest. Res. 2018, 1, 299–306. [Google Scholar] [CrossRef]
- Souza, A.I.S.; Saraiva, M.M.S.; Casas, M.R.T.; Oliveira, G.M.; Cardozo, M.V.; Benevides, V.P.; Barbosa, F.O.; Neto, O.C.F.; Almeida, A.M.; Berchieri, A., Jr. High Occurrence of β-Lactamase-Producing Salmonella Heidelberg from Poultry Origin. PLoS ONE 2020, 15, e0230676. [Google Scholar] [CrossRef]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for Detection of Plasmid-Mediated Quinolone Resistance Qnr Genes in ESBL-Producing Enterobacterial Isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, Y.J. Molecular Characteristics of ESBL-Producing Escherichia coli Isolated from Chickens with Colibacillosis. J. Vet. Sci. 2022, 23, e37. [Google Scholar] [CrossRef]
- Kadry, M.; Nader, S.M.; Elshafiee, E.A.; Ahmed, Z.S. Molecular Characterization of ESBL and Carbapenenemase Producing Salmonella spp. Isolated from Chicken and Its Public Health Importance. Pak. J. Zool. 2021, 53, 2289. [Google Scholar] [CrossRef]
- Wieland, N.; Boss, J.; Lettmann, S.; Fritz, B.; Schwaiger, K.; Bauer, J.; Hölzel, C.S. Susceptibility to Disinfectants in Antimicrobial-Resistant and -Susceptible Isolates of Escherichia coli, Enterococcus Faecalis and Enterococcus Faecium from Poultry-ESBL/AmpC-Phenotype of E. Coli Is Not Associated with Resistance to a Quaternary Ammonium. J. Appl. Microbiol. 2017, 122, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, L.; Alonso, C.A.; Simón, C.; González-Esteban, C.; Orós, J.; Rezusta, A.; Ortega, C.; Torres, C. Wild Birds, Frequent Carriers of Extended-Spectrum β-Lactamase (ESBL) Producing Escherichia coli of CTX-M and SHV-12 Types. Microb. Ecol. 2016, 72, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Koga, V.L.; Scandorieiro, S.; Vespero, E.C.; Oba, A.; De Brito, B.G.; De Brito, K.C.T.; Nakazato, G.; Kobayashi, R.K.T. Comparison of Antibiotic Resistance and Virulence Factors among Escherichia coli Isolated from Conventional and Free-Range Poultry. BioMed Res. Int. 2015, 2015, 618752. [Google Scholar] [CrossRef]
- Martínez-Álvarez, S.; Sanz, S.; Olarte, C.; Hidalgo-Sanz, R.; Carvalho, I.; Fernández-Fernández, R.; Campaña-Burguet, A.; Latorre-Fernández, J.; Zarazaga, M.; Torres, C. Antimicrobial Resistance in Escherichia coli from the Broiler Farm Environment, with Detection of SHV-12-Producing Isolates. Antibiotics 2022, 11, 444. [Google Scholar] [CrossRef]
- Projahn, M.; von Tippelskirch, P.; Semmler, T.; Guenther, S.; Alter, T.; Roesler, U. Contamination of Chicken Meat with Extended-Spectrum Beta-Lactamase Producing- Klebsiella pneumoniae and Escherichia coli during Scalding and Defeathering of Broiler Carcasses. Food Microbiol. 2019, 77, 185–191. [Google Scholar] [CrossRef]
- Egea, P.; López-Cerero, L.; Navarro, M.D.; Rodríguez-Baño, J.; Pascual, A. Assessment of the Presence of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Eggshells and Ready-to-Eat Products. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1045–1047. [Google Scholar] [CrossRef]
- Langkabel, N.; Burgard, J.; Freter, S.; Fries, R.; Meemken, D.; Ellerbroek, L. Detection of Extended-Spectrum β-Lactamase (ESBL) E. Coli at Different Processing Stages in Three Broiler Abattoirs. Microorganisms 2023, 11, 2541. [Google Scholar] [CrossRef]
- Bhave, S.; Kolhe, R.; Mahadevaswamy, R.; Bhong, C.; Jadhav, S.; Nalband, S.; Gandhale, D. Phylogrouping and Antimicrobial Resistance Analysis of Extraintestinal Pathogenic Escherichia coli Isolated from Poultry Species. Turk. J. Vet. Anim. Sci. 2019, 43, 117–126. [Google Scholar] [CrossRef]
- Ebrahem, A.F.; El-Demerdash, A.S.; Orady, R.M.; Nabil, N.M. Modulatory Effect of Competitive Exclusion on the Transmission of ESBL E. Coli in Chickens. Probiotics Antimicrob. Proteins 2024, 16, 1087–1098. [Google Scholar] [CrossRef]
- Yasir, M.; Qureshi, A.K.; Kensarah, E.A.; Bibi, F.; Al-Zahrani, I.A.; Abd El Ghany, M.; Azhar, E.I. Draft Genome Sequence of Colistin-Resistant and Extended-Spectrum β-Lactamase (ESBL)-Producing Multidrug-Resistant Escherichia coli Isolated from Poultry Meat. J. Glob. Antimicrob. Resist. 2021, 27, 112–114. [Google Scholar] [CrossRef]
- Becker, E.; Projahn, M.; Burow, E.; Käsbohrer, A. Are There Effective Intervention Measures in Broiler Production against the ESBL/AmpC Producer Escherichia coli? Pathogens 2021, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Kurittu, P.; Khakipoor, B.; Aarnio, M.; Nykäsenoja, S.; Brouwer, M.; Myllyniemi, A.-L.; Vatunen, E.; Heikinheimo, A. Plasmid-Borne and Chromosomal ESBL/AmpC Genes in Escherichia coli and Klebsiella pneumoniae in Global Food Products. Front. Microbiol. 2021, 12, 592291. [Google Scholar] [CrossRef] [PubMed]
- Geser, N.; Stephan, R.; Hächler, H. Occurrence and Characteristics of Extended-Spectrum β-Lactamase (ESBL) Producing Enterobacteriaceae in Food Producing Animals, Minced Meat and Raw Milk. BMC Vet. Res. 2012, 8, 21. [Google Scholar] [CrossRef]
- Mandujano-Hernández, A.; Martínez-Vázquez, A.V.; Paz-González, A.D.; Herrera-Mayorga, V.; Sánchez-Sánchez, M.; Lara-Ramírez, E.E.; Vázquez, K.; de Jesús de Luna-Santillana, E.; Bocanegra-García, V.; Rivera, G.; et al. The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview. Animals 2024, 14, 2490. [Google Scholar] [CrossRef]
- Olopade, A.; Bitrus, A.A.; Momoh-Zekeri, A.H.H.; Bamaiyi, P.H. Multi-Drug Resistant Phenotypes of Extended-Spectrum β-Lactamase (ESBL)-Producing E. Coli from Layer Chickens. Iraqi J. Vet. Sci. 2022, 36, 945–951. [Google Scholar] [CrossRef]
- Shoaib, M.; Kamboh, A.A.; Sajid, A.; Mughal, G.A.; Leghari, R.A.; Malhi, K.K.; Bughio, S.U.D.; Ali, A.; Alam, S.; Khan, S. Prevalence of Extended Spectrum Beta-Lactamase Producing Enterobacteriaceae in Commercial Broilers and Backyard Chickens. Adv. Anim. Vet. Sci. 2016, 4, 209–214. [Google Scholar] [CrossRef]
- Díaz-Jiménez, D.; García-Meniño, I.; Fernández, J.; García, V.; Mora, A. Chicken and Turkey Meat: Consumer Exposure to Multidrug-Resistant Enterobacteriaceae Including Mcr-Carriers, Uropathogenic E. Coli and High-Risk Lineages Such as ST131. Int. J. Food Microbiol. 2020, 331, 108750. [Google Scholar] [CrossRef]
- Zdovc, I.; Golob, M.; Pirš, T.; Ambrožič-Avguštin, J. Occurrence of ESBL-and AmpC-Producing Escherichia coli Isolates in Poultry Meat. J. Vet. Res. 2013, 57, 513–517. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Mesa, R.J. Extended-Spectrum -Lactamase-Producing Enterobacteriaceae in Different Environments (Humans, Food, Animal Farms and Sewage). J. Antimicrob. Chemother. 2006, 58, 211–215. [Google Scholar] [CrossRef]
- Pacholewicz, E.; Liakopoulos, A.; Swart, A.; Gortemaker, B.; Dierikx, C.; Havelaar, A.; Schmitt, H. Reduction of Extended-Spectrum-β-Lactamase- and AmpC-β-Lactamase-Producing Escherichia coli through Processing in Two Broiler Chicken Slaughterhouses. Int. J. Food Microbiol. 2015, 215, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.H.; Board, M.M.; Crespo, R.; Guard, J.; Paul, N.C.; Faux, C. The Occurrence of Salmonella, Extended-Spectrum β-Lactamase Producing Escherichia coli and Carbapenem Resistant Non-Fermenting Gram-Negative Bacteria in a Backyard Poultry Flock Environment. Zoonoses Public Health 2020, 67, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.S.; Salwa, E.; Sanaa, O.Y.; Elzubeir, I.E.M. Antibiotic Susceptibility and Production of Extended-Spectrum Beta-Lactamase (ESBL) of E. Coli Strains Isolated from Meat. Afr. J. Microbiol. Res. 2021, 15, 370–376. [Google Scholar] [CrossRef]
- Robé, C.; Blasse, A.; Merle, R.; Friese, A.; Roesler, U.; Guenther, S. Low Dose Colonization of Broiler Chickens with ESBL-/AmpC-Producing Escherichia coli in a Seeder-Bird Model Independent of Antimicrobial Selection Pressure. Front. Microbiol. 2019, 10, 2124. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Valentão, P.; Falco, V.; Poeta, P. Antimicrobial Resistance and Clonal Lineages of Escherichia coli from Food-Producing Animals. Antibiotics 2023, 12, 1061. [Google Scholar] [CrossRef]
- Ojer-Usoz, E.; González, D.; Vitas, A.I. Clonal Diversity of ESBL-Producing Escherichia coli Isolated from Environmental, Human and Food Samples. Int. J. Environ. Res. Public Health 2017, 14, 676. [Google Scholar] [CrossRef]
- Uyanik, T.; Gülel, G.T.; Alişarli, M. Characterization of Extended-spectrum Beta-lactamase-producing Enterobacterales from Organic and Conventional Chicken Meats. Lett. Appl. Microbiol. 2021, 72, 783–790. [Google Scholar] [CrossRef]
- Falodun, O.I.; Ikusika, E.O. Extended Spectrum and Metallo-Beta-Lactamase Pseudomonas Species from Poultry and Piggery Waste. MicroMedicine 2019, 7, 37–45. [Google Scholar] [CrossRef]
- Tacão, M.; Moura, A.; Correia, A.; Henriques, I. Co-Resistance to Different Classes of Antibiotics among ESBL-Producers from Aquatic Systems. Water Res. 2014, 48, 100–107. [Google Scholar] [CrossRef]
- Dame-Korevaar, A.; Fischer, E.A.J.; van der Goot, J.; Velkers, F.; van den Broek, J.; Veldman, K.; Ceccarelli, D.; Mevius, D.; Stegeman, A. Effect of Challenge Dose of Plasmid-Mediated Extended-Spectrum β-Lactamase and AmpC β-Lactamase Producing Escherichia coli on Time-until-Colonization and Level of Excretion in Young Broilers. Vet. Microbiol. 2019, 239, 108446. [Google Scholar] [CrossRef]
- Liu, X.; Wei, X.; Liu, L.; Feng, X.; Shao, Z.; Han, Z.; Li, Y. Prevalence and Characteristics of Extended-Spectrum β-Lactamases-Producing Escherichia coli from Broiler Chickens at Different Day-Age. Poult. Sci. 2020, 99, 3688–3696. [Google Scholar] [CrossRef] [PubMed]
- Vitas, A.I.; Naik, D.; Pérez-Etayo, L.; González, D. Increased Exposure to Extended-Spectrum β-Lactamase-Producing Multidrug-Resistant Enterobacteriaceae through the Consumption of Chicken and Sushi Products. Int. J. Food Microbiol. 2018, 269, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, D.; van Essen-Zandbergen, A.; Smid, B.; Veldman, K.T.; Boender, G.J.; Fischer, E.A.J.; Mevius, D.J.; van der Goot, J.A. Competitive Exclusion Reduces Transmission and Excretion of Extended-Spectrum-β-Lactamase-Producing Escherichia coli in Broilers. Appl. Environ. Microbiol. 2017, 83, e03439-16. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Cui, C.-Y.; Zhang, Y.; Liu, X.; Cui, Z.-H.; Ma, X.-Y.; Feng, Y.; Fang, L.-X.; Lian, X.-L.; et al. Plasmid-Encoded Tet (X) Genes That Confer High-Level Tigecycline Resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef]
- Celik, C.; Kalin, G.; Cetinkaya, Z.; Ildiz, N.; Ocsoy, I. Recent Advances in Colorimetric Tests for the Detection of Infectious Diseases and Antimicrobial Resistance. Diagnostics 2023, 13, 2427. [Google Scholar] [CrossRef]
- Veloo, Y.; Thahir, S.S.A.; Rajendiran, S.; Hock, L.K.; Ahmad, N.; Muthu, V.; Shaharudin, R. Multidrug-Resistant Gram-Negative Bacteria and Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae from the Poultry Farm Environment. Microbiol. Spectr. 2022, 10, e02694-21. [Google Scholar] [CrossRef]
- Bobbadi, S.; Kiranmayi Chinnam, B.; Nelapati, S.; Tumati, S.R.; Kandhan, S.; Gottapu, C.; Boddu, S.V. Occurrence and Genetic Diversity of ESBL Producing Klebsiella Species Isolated from Livestock and Livestock Products. J. Food Saf. 2020, 40, e12738. [Google Scholar] [CrossRef]
- Martiny, A.C.; Martiny, J.B.H.; Weihe, C.; Field, A.; Ellis, J.C. Functional Metagenomics Reveals Previously Unrecognized Diversity of Antibiotic Resistance Genes in Gulls. Front. Microbiol. 2011, 2, 238. [Google Scholar] [CrossRef]
- Martinez, J.L. The Role of Natural Environments in the Evolution of Resistance Traits in Pathogenic Bacteria. Proc. R. Soc. B Biol. Sci. 2009, 276, 2521–2530. [Google Scholar] [CrossRef]
- Mazzariol, A.; Cornaglia, G.; Nikaido, H. Contributions of the AmpC β-Lactamase and the AcrAB Multidrug Efflux System in Intrinsic Resistance of Escherichia coli K-12 to β-Lactams. Antimicrob. Agents Chemother. 2000, 44, 1387–1390. [Google Scholar] [CrossRef]
- Rekadwad, B.N.; Pramod, N.; Rao, M.P.N.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F. Identification and Specificity Validation of Unique and Antimicrobial Resistance Genes to Trace Suspected Pathogenic AMR Bacteria and to Monitor the Development of AMR in Non-AMR Strains in the Environment and Clinical Settings. Saudi J. Biol. Sci. 2023, 30, 103869. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Farooq, M.; Ruffini, F.; Marsilio, F.; Di Francesco, C.E. Microbial Community and Abundance of Selected Antimicrobial Resistance Genes in Poultry Litter from Conventional and Antibiotic-Free Farms. Antibiotics 2023, 12, 1461. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Barco, L.; Angelucci, S.; Orsini, M.; Marsilio, F.; Antonucci, A.; Di Francesco, C.E. Whole Genome Sequencing of Escherichia coli and Enterococcus spp. in Wildlife-Livestock Interface: A Pilot Study. J. Glob. Antimicrob. Resist. 2023, 32, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.U.; Aatika; Chughtai, M.I.; Ejaz, H.; Mazhari, B.B.Z.; Maqbool, U.; Alanazi, A.; Alruwaili, Y.; Junaid, K. Antibiotic-Resistant Bacteria, Antimicrobial Resistance Genes, and Antibiotic Residue in Food from Animal Sources: One Health Food Safety Concern. Microorganisms 2023, 11, 161. [Google Scholar] [CrossRef]
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A.R.; Antonucci, A.; Moschetti, L.; Farooq, M.; Marsilio, F.; Di Francesco, C.E. Resistance Patterns, Mcr-4 and OXA-48 Genes, and Virulence Factors of Escherichia coli from Apennine Chamois Living in Sympatry with Domestic Species, Italy. Animals 2022, 12, 129. [Google Scholar] [CrossRef]
- Musa, L.; Stefanetti, V.; Casagrande Proietti, P.; Grilli, G.; Gobbi, M.; Toppi, V.; Brustenga, L.; Magistrali, C.F.; Franciosini, M.P. Antimicrobial Susceptibility of Commensal E. Coli Isolated from Wild Birds in Umbria (Central Italy). Animals 2023, 13, 1776. [Google Scholar] [CrossRef]
- Mukerji, S.; O’Dea, M.; Barton, M.; Kirkwood, R.; Lee, T.; Abraham, S. Development and Transmission of Antimicrobial Resistance among Gram-Negative Bacteria in Animals and Their Public Health Impact. Essays Biochem. 2017, 61, 23–35. [Google Scholar] [CrossRef]


| Sampling Area | Sampling Site | Sampled Species | Sample Source | Sample Size | Health Status | Bacterial Species | Detection Method | Genes | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Nationwide | Slaughterhouses | Chicken | Carcass, Meat Products | 85 | Diseased | Salmonella spp. | Phenotypic, PCR ***, WGS * | blaCTX-M-1 | [49] |
| Farms | Chicken | Faecal, skin, liver, meat products | 87 | Diseased | Salmonella spp. | Phenotypic, PCR ***, RFLP ** | blaCTX-M-1 | [50] | |
| Farms | Chicken, pigs, cattle | Faecal, caecal, intestinal contents | 194 | Diseased | Escherichia coli | Phenotypic, PCR ***, WGS * | blaCTX-M-15 | [51] | |
| Northern Italy | Farms | Chicken | Cloacal swab | 229 | Diseased | Escherichia coli | Phenotypic, PCR ***, RFLP * | blaCTX-M-1 | [52] |
| Farms | Chicken | Faecal | 6 | Healthy | Salmonella spp. | Phenotypic, PCR *** | blaCTX-M-1 | [53] | |
| Slaughterhouses | Chicken | Carcass, faecal, cloacal swabs | 513 | Diseased | Escherichia coli | Phenotypic, PCR *** | blaCTX-M-1, blaSHV | [54] | |
| Farms | Chicken | Faecal | 67 | Healthy | Escherichia coli | Phenotypic, PCR ***, RFLP ** | blaTEM-1, blaSHV | [55] | |
| Farms | Chicken | Faecal, meat products | 1053 | Diseased | Salmonella spp. | Phenotypic, PCR, RFLP ** | blaSHV-12 | [56] | |
| Mixed | Chicken | Carcass, meat | 33 | Diseased | Escherichia coli | Phenotypic, PCR ***, WGS *, RFLP ** | blaTEM | [57] | |
| Farms | Chicken | Carcass, meat | 142 | Diseased | Escherichia coli | Phenotypic, PCR *** | blaTEM | [58] | |
| Farms | Chicken | Rectal swabs | 308 | Healthy | Klebsiella pneumoniae | Phenotypic, WGS * | blaCMX-15, blaSHV-27 | [59] | |
| Farms | Chicken | Cloacal swabs | 100 | Diseased | Escherichia coli | WGS * | blaCMY-2, blaCTX-M-65,55 | [60] | |
| Central Italy | Mixed | Chicken | Faecal | 42 | Healthy and Diseased | Salmonella spp. | Phenotypic, PCR ***, WGS * | blaCTX-M-1 | [61] |
| Farms | Chicken | Skin, cloacal swabs | 406 | Healthy | Escherichia coli | Phenotypic | ND | [62] | |
| Farms | Chicken, turkey | Faecal, caecal | 1044 | Diseased | Salmonella spp. | Phenotypic, PCR *** | blaCTX-M-1 | [63] | |
| Farms | Chicken | Carcass, meat | 324 | Diseased | Salmonella spp. | Phenotypic, PCR ***, WGS * | blaCTX-M | [64] | |
| Mixed | Chicken | Meat products | 80 | Diseased | Salmonella spp. | Phenotypic, PCR ***, WGS * | blaCTX-M-1, blaTEM | [65] | |
| Farms | Chicken, pigs | Insect contaminated Meat | 105 | Diseased | Salmonella spp. | Phenotypic, PCR *** | blaTEM | [66] | |
| ND | Chicken | Meat | 5 | Diseased | Salmonella spp. | WGS * | blaCTX-M-1, blaCTX-M-15 | [67] | |
| Farms | Chicken | Carcasses, Litter | 180 + 6 * | Healthy | E. coli, Salmonella spp. | Phenotypic, PCR *** | blaTEM-1 | [68] | |
| Farm | Chicken | Meat products | 96 | Healthy | Klebsiella spp. | Phenotypic, WGS * | blaCTX-M-15, blaDHA-1 | [69] | |
| Slaughterhouses | Chicken | Caecal | 809 | Diseased | Escherichia coli | Phenotypic, PCR ***, WGS * | blaCTX-M-1 | [70] | |
| Farms | Chicken | Caecal | 855 | Healthy | Escherichia coli | Phenotypic, PCR *** | blaCTX-M-15, blaTEM, blaSHV | [71] | |
| Southern Italy | Market | Chicken, turkey | Meat | 38 | Healthy | Escherichia coli | Phenotypic | ND | [72] |
| Farms | chicken, layer, turkey | Carcass, faecal | 17 | Healthy | Salmonella spp. | Phenotypic, PCR ***, WGS * | blaSHV-12 | [73] | |
| Farms | Chicken | Meat | 237 | Diseased | Escherichia coli | PCR *** | blaCTX-M-1, blaCTX-M-15 | [74] | |
| ND | Chicken | Meat | 163 | Diseased | Escherichia coli | Phenotypic, PCR *** | blaTEM-1, blaCTX-M-1 | [75] | |
| ND | Chicken | Meat | 145 | Diseased | Salmonella spp. | Phenotypic | ND | [76] | |
| Farms | Chicken, turkey | Meat | 103 | Diseased | Salmonella spp. | Phenotypic, PCR ***, WGS * | blaCTX-M-1 | [77] |
| Location | Sampling Date | Antibiotic Susceptibility Test | Phenotypic Confirmatory Test | Genotypic Detection Test | Accreditation Body | Bacterial Species | Reference |
|---|---|---|---|---|---|---|---|
| Whole Country | 2016–2019 | Disk diffusion method | Broth microdilution method | PCR | EUCAST, CLSI | Salmonella | [49] |
| 2016–2017 | Broth microdilution method | Broth microdilution method | PCR | EUCAST | Salmonella | [50] | |
| 2016–2017 | Broth microdilution method | ND | PCR | EUCAST | E. coli | [51] | |
| Northern Italy | 2008–2012 | Disk diffusion method | ND | PCR | CLSI | E. coli | [52] |
| 2009 | Broth microdilution method | ND | PCR | EUCAST | E. coli | [53] | |
| 2017–2018 | ND | Double disk synergy method | PCR | CLSI | Salmonella | [54] | |
| 2009 | Disk diffusion method | Double disk synergy method | PCR | CLSI | Salmonella | [55] | |
| 2006–2007 | Disk diffusion method | Broth microdilution method | PCR | CLSI | Salmonella | [56] | |
| 2010–2018 | ND | Broth microdilution method | PCR | CLSI, EUCAST | E. coli | [57] | |
| 2019–2021 | ND | Double disk synergy method | PCR | CLSI | E. coli | [58] | |
| 2017–2018 | Broth microdilution method | ND | WGS | EUCAST | Klebsiella | [59] | |
| 2020 | ND | Double disk synergy method | PCR | CLSI | E. coli | [60] | |
| Central Italy | 2011–2014 | Disk diffusion method | Broth microdilution method | PCR | CLSI | Salmonella | [61] |
| 2020 | Disk diffusion method, broth microdilution | ND | ND | CLSI | E. coli | [62] | |
| 2018 | Broth microdilution method | Broth microdilution method | PCR | EUCAST | Salmonella | [63] | |
| 2016–2017 | Broth microdilution method | Broth microdilution method | PCR | EUCAST | Salmonella | [64] | |
| 2020 | Disk diffusion method | ND | WGS | EUCAST | E. coli | [65] | |
| 2019 | Disk diffusion method | ND | PCR | CLSI | Salmonella | [66] | |
| 2016–2017 | ND | ND | WGS | ND | Salmonella | [67] | |
| 2023 | VITEK® 2 system | VITEK® 2 system | PCR | CLSI | E. coli and Salmonella | [68] | |
| 2018–2022 | Kirby–Bauer method, disk diffusion method | Double disk synergy method | WGS | EUCAST | Klebsiella | [69] | |
| 2017–2018 | Disk diffusion method | Double disk synergy method | PCR | ND | E. coli | [70] | |
| 2017–2018 | Disk diffusion method | ND | PCR | EUCAST | E. coli | [71] | |
| Southern Italy | 2015 | Disk diffusion method | ND | ND | CLSI | E. coli | 72] |
| 2014–2019 | Broth microdilution method | ND | WGS | EUCAST | Salmonella | [73] | |
| 2013–2015 | ND | Double Disk Synergy Method | PCR | EUCAST | E. coli | [74] | |
| 2014–2015 | Disk diffusion method | Double Disk Synergy Method | PCR | ND | E. coli | [75] | |
| 2019–2021 | Kirby–Bauer method | ND | ND | CLSI | Salmonella | [76] | |
| 2017–2020 | Broth microdilution method | ND | WGS | EUCAST | Salmonella | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.T.S.; Formenti, N.; Tosi, G.; Guarneri, F.; Scali, F.; Saleemi, M.K.; Monti, E.; Alborali, G.L. Prevalence of ESBL-Resistant Genes in Birds in Italy—A Comprehensive Review. Animals 2025, 15, 1598. https://doi.org/10.3390/ani15111598
Khan MTS, Formenti N, Tosi G, Guarneri F, Scali F, Saleemi MK, Monti E, Alborali GL. Prevalence of ESBL-Resistant Genes in Birds in Italy—A Comprehensive Review. Animals. 2025; 15(11):1598. https://doi.org/10.3390/ani15111598
Chicago/Turabian StyleKhan, Muhammad Tahir Sarfraz, Nicoletta Formenti, Giovanni Tosi, Flavia Guarneri, Federico Scali, Muhammad Kashif Saleemi, Eugenio Monti, and Giovanni Loris Alborali. 2025. "Prevalence of ESBL-Resistant Genes in Birds in Italy—A Comprehensive Review" Animals 15, no. 11: 1598. https://doi.org/10.3390/ani15111598
APA StyleKhan, M. T. S., Formenti, N., Tosi, G., Guarneri, F., Scali, F., Saleemi, M. K., Monti, E., & Alborali, G. L. (2025). Prevalence of ESBL-Resistant Genes in Birds in Italy—A Comprehensive Review. Animals, 15(11), 1598. https://doi.org/10.3390/ani15111598







