A Novel Postbiotic Reduces Canine Halitosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Treatment
2.4. Breath Sampling
2.5. Breath Scoring
2.6. Statistical Analysis
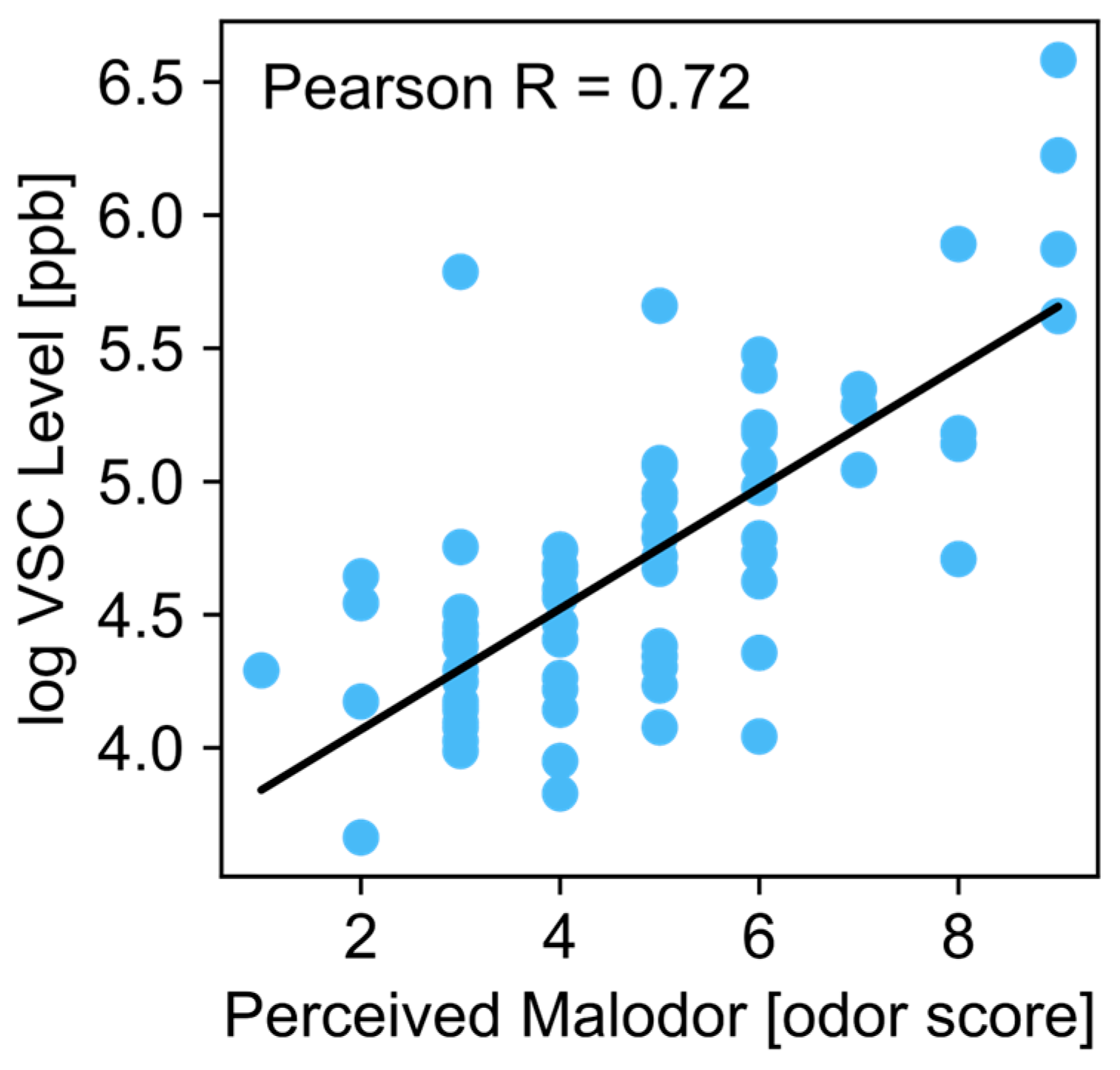
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Li, J.; Fu, R.; Liu, J.; Wen, X.; Zhang, L. Halitosis: Etiology, Prevention, and the Role of Microbiota. Clin. Oral Investig. 2023, 27, 6383–6393. [Google Scholar] [CrossRef] [PubMed]
- Croft, J.M.; Patel, K.V.; Inui, T.; Ruparell, A.; Staunton, R.; Holcombe, L.J. Effectiveness of Oral Care Interventions on Malodour in Dogs. BMC Vet. Res. 2022, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Shin, S.-I.; Hong, J.-Y. Investigation of Volatile Sulfur Compound Level and Halitosis in Patients with Gingivitis and Periodontitis. Sci. Rep. 2023, 13, 13175. [Google Scholar] [CrossRef]
- Krespi, Y.P.; Shrime, M.G.; Kacker, A. The Relationship between Oral Malodor and Volatile Sulfur Compound-Producing Bacteria. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2006, 135, 671–676. [Google Scholar] [CrossRef]
- Azodo, C.C.; Ogbebor, O.G. Social Distance towards Halitosis Sufferers. SWISS Dent. J. SSO Sci. Clin. Top. 2019, 129, 1026–1030. [Google Scholar] [CrossRef]
- de Jongh, A.; van Wijk, A.J.; Horstman, M.; de Baat, C. Self-Perceived Halitosis Influences Social Interactions. BMC Oral Health 2016, 16, 31. [Google Scholar] [CrossRef]
- Fernandes, N.A.; Borges, A.P.B.; Reis, E.C.C.; Sepúlveda, R.V.; de Pontes, K.C.S. Prevalence of Periodontal Disease in Dogs and Owners’ Level of Awareness—A Prospective Clinical Trial. Rev. Ceres 2012, 59, 446–451. [Google Scholar] [CrossRef]
- Enlund, K.B.; Brunius, C.; Hanson, J.; Hagman, R.; Höglund, O.V.; Gustås, P.; Pettersson, A. Dog Owners’ Perspectives on Canine Dental Health—A Questionnaire Study in Sweden. Front. Vet. Sci. 2020, 7, 298. [Google Scholar] [CrossRef]
- Izidoro, C.; Botelho, J.; Machado, V.; Reis, A.M.; Proença, L.; Alves, R.; Mendes, J.J. Periodontitis, Halitosis and Oral-Health-Related Quality of Life—A Cross-Sectional Study. J. Clin. Med. 2021, 10, 4415. [Google Scholar] [CrossRef]
- Kim, S.-E.; Shim, K.-M.; Yoo, K.-H.; Ryu, J.-W.; Koh, H.-B.; Moon, C.-J.; Kim, J.-C.; Kim, S.-H.; Kang, S.-S.; Bae, C.-S. Association Between Halitosis and Periodontal Disease Related Parameters in Dogs. J. Vet. Clin. 2007, 24, 192–196. [Google Scholar]
- Simone, A.J.; Logan, E.I.; Livgren, R.; Suelzer, M. Oral Malodor in Beagles: Association with Indicators of Periodontal Disease. J. Clin. Dent. 1997, 8, 163–168. [Google Scholar] [PubMed]
- Wallis, C.; Holcombe, L.J. A Review of the Frequency and Impact of Periodontal Disease in Dogs. J. Small Anim. Pract. 2020, 61, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Yaegaki, K. Oral Malodorous Compounds Are Periodontally Pathogenic and Carcinogenic. Jpn. Dent. Sci. Rev. 2008, 44, 100–108. [Google Scholar] [CrossRef]
- Murata, T.; Yaegaki, K.; Qian, W.; Herai, M.; Calenic, B.; Imai, T.; Sato, T.; Tanaka, T.; Kamoda, T.; Ii, H. Hydrogen Sulfide Induces Apoptosis in Epithelial Cells Derived from Human Gingiva. J. Breath Res. 2008, 2, 017007. [Google Scholar] [CrossRef] [PubMed]
- Calenic, B.; Yaegaki, K.; Murata, T.; Imai, T.; Aoyama, I.; Sato, T.; Ii, H. Oral Malodorous Compound Triggers Mitochondrial-Dependent Apoptosis and Causes Genomic DNA Damage in Human Gingival Epithelial Cells. J. Periodontal Res. 2010, 45, 31–37. [Google Scholar] [CrossRef]
- Basic, A.; Alizadehgharib, S.; Dahlén, G.; Dahlgren, U. Hydrogen Sulfide Exposure Induces NLRP3 Inflammasome-Dependent IL-1β and IL-18 Secretion in Human Mononuclear Leukocytes in Vitro. Clin. Exp. Dent. Res. 2017, 3, 115–120. [Google Scholar] [CrossRef]
- Basic, A.; Serino, G.; Leonhardt, Å.; Dahlén, G. H2S Mediates Increased Interleukin (IL)-1β and IL-18 Production in Leukocytes from Patients with Periodontitis. J. Oral Microbiol. 2019, 11, 1617015. [Google Scholar] [CrossRef]
- Ni, K.; Hua, Y. Hydrogen Sulfide Exacerbated Periodontal Inflammation and Induced Autophagy in Experimental Periodontitis. Int. Immunopharmacol. 2021, 93, 107399. [Google Scholar] [CrossRef]
- Ratkay, L.G.; Waterfield, J.D.; Tonzetich, J. Stimulation of Enzyme and Cytokine Production by Methyl Mercaptan in Human Gingival Fibroblast and Monocyte Cell Cultures. Arch. Oral Biol. 1995, 40, 337–344. [Google Scholar] [CrossRef]
- Johnson, P.; Yaegaki, K.; Tonzetich, J. Effect of Methyl Mercaptan on Synthesis and Degradation of Collagen. J. Periodontal Res. 1996, 31, 323–329. [Google Scholar] [CrossRef]
- Ng, W.; Tonzetich, J. Effect of Hydrogen Sulfide and Methyl Mercaptan on the Permeability of Oral Mucosa. J. Dent. Res. 1984, 63, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial Pathogenesis of Periodontitis: Keystones, Pathobionts, and Host Response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Glickman, L.T.; Glickman, N.W.; Moore, G.E.; Goldstein, G.S.; Lewis, H.B. Evaluation of the Risk of Endocarditis and Other Cardiovascular Events on the Basis of the Severity of Periodontal Disease in Dogs. J. Am. Vet. Med. Assoc. 2009, 234, 486–494. [Google Scholar] [CrossRef]
- Allan, R.M.; Adams, V.J.; Johnston, N.W. Prospective Randomised Blinded Clinical Trial Assessing Effectiveness of Three Dental Plaque Control Methods in Dogs. J. Small Anim. Pract. 2019, 60, 212–217. [Google Scholar] [CrossRef]
- Harvey, C.; Serfilippi, L.; Barnvos, D. Effect of Frequency of Brushing Teeth on Plaque and Calculus Accumulation, and Gingivitis in Dogs. J. Vet. Dent. 2015, 32, 16–21. [Google Scholar] [CrossRef]
- Ray, J.D.; Eubanks, D.L. Dental Homecare: Teaching Your Clients to Care for Their Pet’s Teeth. J. Vet. Dent. 2009, 26, 57–60. [Google Scholar] [CrossRef]
- Gaynor, J.; Dunlop, C.; Wagner, A.; Wertz, E.; Golden, A.; Demme, W. Complications and Mortality Associated with Anesthesia in Dogs and Cats. J. Am. Anim. Hosp. Assoc. 1999, 35, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Gruenheid, M.; Aarnes, T.K.; McLoughlin, M.A.; Simpson, E.M.; Mathys, D.A.; Mollenkopf, D.F.; Wittum, T.E. Risk of Anesthesia-Related Complications in Brachycephalic Dogs. J. Am. Vet. Med Assoc. 2018, 253, 301–306. [Google Scholar] [CrossRef]
- Lindinger, M.I. Reduced Dental Plaque Formation in Dogs Drinking a Solution Containing Natural Antimicrobial Herbal Enzymes and Organic Matcha Green Tea. Scientifica 2016, 2016, 2183623. [Google Scholar] [CrossRef]
- Low, S.B.; Peak, R.M.; Smithson, C.W.; Perrone, J.; Gaddis, B.; Kontogiorgos, E. Evaluation of a Topical Gel Containing a Novel Combination of Essential Oils and Antioxidants for Reducing Oral Malodor in Dogs. Am. J. Vet. Res. 2014, 75, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Torkan, S. Comparison of the Effects of an Herbal Mouthwash with Chlorhexidine on Surface Bacteria Counts of Dental Plaque in Dogs. Biosci. Biotechnol. Res. Asia 2015, 12, 955–959. [Google Scholar] [CrossRef]
- Van Leeuwen, M.P.C.; Slot, D.E.; Van der Weijden, G.A. Essential Oils Compared to Chlorhexidine with Respect to Plaque and Parameters of Gingival Inflammation: A Systematic Review. J. Periodontol. 2011, 82, 174–194. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Arumugham, I.M.; Doraikannan, S.; Rathinavelu, P.K.; Prabakar, J.; Balasubramaniam, A. Comparing the Effectiveness of Herbal and Conventional Dentifrices in Reducing Dental Plaque and Gingivitis: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2021, 11, 601. [Google Scholar] [CrossRef]
- Gorrel, C.; Warrick, J.; Bierer, T.L. Effect of a New Dental Hygiene Chew on Periodontal Health in Dogs. J. Vet. Dent. 1999, 16, 77–81. [Google Scholar] [CrossRef]
- Clarke, D.E.; Kelman, M.; Perkins, N. Effectiveness of a Vegetable Dental Chew on Periodontal Disease Parameters in Toy Breed Dogs. J. Vet. Dent. 2011, 28, 230–235. [Google Scholar] [CrossRef]
- Oba, P.M.; Sieja, K.M.; Schauwecker, A.; Somrak, A.J.; Hristova, T.S.; Keating, S.C.J.; Swanson, K.S. Effects of a Novel Dental Chew on Oral Health Outcomes, Halitosis, and Microbiota of Adult Dogs. J. Anim. Sci. 2024, 102, skae071. [Google Scholar] [CrossRef]
- Oba, P.M.; Carroll, M.Q.; Alexander, C.; Somrak, A.J.; Keating, S.C.J.; Sage, A.M.; Swanson, K.S. Dental Chews Positively Shift the Oral Microbiota of Adult Dogs. J. Anim. Sci. 2021, 99, skab100. [Google Scholar] [CrossRef]
- Vaara, M. Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef]
- Stookey, G.K.; Warrick, J.M.; Miller, L.L. Effect of Sodium Hexametaphosphate on Dental Calculus Formation in Dogs. Am. J. Vet. Res. 1995, 56, 913–918. [Google Scholar] [CrossRef]
- Lanigan, R.S. Final Report on the Safety Assessment of Sodium Metaphosphate, Sodium Trimetaphosphate, and Sodium Hexametaphosphate. Int. J. Toxicol. 2001, 20 (Suppl. 3), 75–89. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J.; Jank, M. Ascophyllum Nodosum as a Nutrient Supporting Oral Health in Dogs and Cats: A Review. Pol. J. Vet. Sci. 2023, 26, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J.; Jank, M.; Jodkowska, K.; Klim, E.; Svensson, U.K. Effects of Edible Treats Containing Ascophyllum Nodosum on the Oral Health of Dogs: A Double-Blind, Randomized, Placebo-Controlled Single-Center Study. Front. Vet. Sci. 2018, 5, 168. [Google Scholar] [CrossRef]
- Gawor, J.; Jodkowska, K.; Klim, E.; Jank, M.; Nicolas, C.S. Comparison of a Vegetable-Based Dental Chew to 2 Other Chews for Oral Health Prevention. J. Vet. Dent. 2021, 38, 131–138. [Google Scholar] [CrossRef]
- Carroll, M.Q.; Oba, P.M.; Sieja, K.M.; Alexander, C.; Lye, L.; de Godoy, M.R.C.; He, F.; Somrak, A.J.; Keating, S.C.J.; Sage, A.M.; et al. Effects of Novel Dental Chews on Oral Health Outcomes and Halitosis in Adult Dogs. J. Anim. Sci. 2020, 98, skaa274. [Google Scholar] [CrossRef] [PubMed]
- Quest, B.W. Oral Health Benefits of a Daily Dental Chew in Dogs. J. Vet. Dent. 2013, 30, 84–87. [Google Scholar] [CrossRef]
- Brown, W.Y.; McGenity, P. Effective Periodontal Disease Control Using Dental Hygiene Chews. J. Vet. Dent. 2005, 22, 16–19. [Google Scholar] [CrossRef]
- Rawlings, J.M.; Gorrel, C.; Markwell, P.J. Effect on Canine Oral Health of Adding Chlorhexidine to a Dental Hygiene Chew. J. Vet. Dent. 1998, 15, 129–134. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A Comprehensive Review of the Application of Probiotics and Postbiotics in Oral Health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Ghafouri, H.; Mashayekhi, P.; Davoudi, M. Control of Opportunistic Oral Cavity Infections Using Postbiotics Secreted by Aerobic Oral Flora, with Minimal Impact on Host Cells. J. Med. Microbiol. Infect. Dis. 2024, 12, 217–223. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, Y.-T.; Ho, H.-H.; Kuo, Y.-W.; Lin, W.-Y.; Chen, J.-F.; Lin, J.-H.; Liu, C.-R.; Lin, C.-H.; Yeh, Y.-T.; et al. Impact of the Food Grade Heat-Killed Probiotic and Postbiotic Oral Lozenges in Oral Hygiene. Aging 2022, 14, 2221–2238. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the Power of Postbiotics: A Revolutionary Approach to Nutrition for Humans and Animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Yamada, M.; Takahashi, N.; Matsuda, Y.; Sato, K.; Yokoji, M.; Sulijaya, B.; Maekawa, T.; Ushiki, T.; Mikami, Y.; Hayatsu, M.; et al. A Bacterial Metabolite Ameliorates Periodontal Pathogen-Induced Gingival Epithelial Barrier Disruption via GPR40 Signaling. Sci. Rep. 2018, 8, 9008. [Google Scholar] [CrossRef]
- Wuri, G.; Liu, F.; Sun, Z.; Fang, B.; Zhao, W.; Hung, W.-L.; Liu, W.-H.; Zhang, X.; Wang, R.; Wu, F.; et al. Lactobacillus Paracasei ET-22 and Derived Postbiotics Reduce Halitosis and Modulate Oral Microbiome Dysregulation-a Randomized, Double-Blind Placebo-Controlled Clinical Trial. Food Funct. 2023, 14, 7335–7346. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, B.; Wuri, G.; Lan, H.; Wang, R.; Sun, Y.; Zhao, W.; Hung, W.-L.; Zhang, M. From Biofilm to Breath: The Role of Lacticaseibacillus Paracasei ET-22 Postbiotics in Combating Oral Malodor. J. Agric. Food Chem. 2024, 72, 27203–27214. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.M.; Culham, N. Halitosis in Dogs and the Effect of Periodontal Therapy. J. Nutr. 1998, 128, S2715–S2716. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Canello, S.; Guidetti, G.; Palmieri, B. Therapeutic Effectiveness of a Dietary Supplement for Management of Halitosis in Dogs. J. Vis. Exp. 2015, 52717. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Tang, Y.; Cai, S.; Zheng, Z.; Yuan, Y.; Zhang, X.; Tang, H.; Chen, X.; Wu, H. Effect of Dental Chew on Reducing Dental Plaque, Dental Calculus and Halitosis in Beagle Dogs. Res. Vet. Sci. 2024, 174, 105304. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Duffield, J.; Spencer, P.; Rosenberg, M.; Corry, D.; Saad, S.; Lenton, P.; Majerus, G.; Nachnani, S.; El-Maaytah, M. Study on the Organoleptic Intensity Scale for Measuring Oral Malodor. J. Dent. Res. 2004, 83, 81–85. [Google Scholar] [CrossRef]
- Rosenberg, M.; McCulloch, C.A.G. Measurement of Oral Malodor: Current Methods and Future Prospects. J. Periodontol. 1992, 63, 776–782. [Google Scholar] [CrossRef]
- Greenman, J.; El-Maaytah, M.; Duffield, J.; Spencer, P.; Rosenberg, M.; Corry, D.; Saad, S.; Lenton, P.; Majerus, G.; Nachnani, S. Assessing the Relationship between Concentrations of Malodor Compounds and Odor Scores from Judges. J. Am. Dent. Assoc. 2005, 136, 749–757. [Google Scholar] [CrossRef]
- Hayes, J.E.; Stevenson, R.J.; Stuetz, R.M. The Impact of Malodour on Communities: A Review of Assessment Techniques. Sci. Total Environ. 2014, 500–501, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Hennet, P.R.; Delille, B.; Davot, J.L. Oral Malodor Measurements on a Tooth Surface of Dogs with Gingivitis. Am. J. Vet. Res. 1998, 59, 255–257. [Google Scholar] [CrossRef]
- Iwashita, N.; Sugita, K.; Murata, S.; Ichimura, K.; Shirai, M.; Hisasue, M.; Saito, M.; Aoki, T.; Takagi, Y.; Asai, F. Age-Dependent Aggravation of Oral Malodor and Periodontal Disease in Dogs. Fundam. Toxicol. Sci. 2019, 6, 75–79. [Google Scholar] [CrossRef]
- Silva, M.F.; Cademartori, M.G.; Leite, F.R.M.; López, R.; Demarco, F.F.; Nascimento, G.G. Is Periodontitis Associated with Halitosis? A Systematic Review and Meta-Regression Analysis. J. Clin. Periodontol. 2017, 44, 1003–1009. [Google Scholar] [CrossRef]
- Wang, N.; Li, C.; Zhang, J.; Wang, L.; Yang, J. The Association between Halitosis and Periodontitis: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2024, 28, 341. [Google Scholar] [CrossRef]
- Koziol, S.A.; Oba, P.M.; Soto-Diaz, K.; Steelman, A.J.; Suchodolski, J.S.; Eckhardt, E.R.M.; Swanson, K.S. Effects of a Lactobacillus Fermentation Product on the Fecal Characteristics, Fecal Microbial Populations, Immune Function, and Stress Markers of Adult Dogs. J. Anim. Sci. 2023, 101, skad160. [Google Scholar] [CrossRef]
- Maturana, M.; Castillejos, L.; Martin-Orue, S.M.; Minel, A.; Chetty, O.; Felix, A.P.; Adib Lesaux, A. Potential Benefits of Yeast Saccharomyces and Their Derivatives in Dogs and Cats: A Review. Front. Vet. Sci. 2023, 10, 1279506. [Google Scholar] [CrossRef] [PubMed]
- Belà, B.; Coman, M.M.; Verdenelli, M.C.; Gramenzi, A.; Pignataro, G.; Fiorini, D.; Silvi, S. In Vitro Assessment of Postbiotic and Probiotic Commercial Dietary Supplements Recommended for Counteracting Intestinal Dysbiosis in Dogs. Vet. Sci. 2024, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Wambacq, W.A.; Apper, E.; Le Bourgot, C.; Barbe, F.; Lyu, Y.; Pelst, M.; Broeckx, B.J.G.; Devriendt, B.; Cox, E.; Hesta, M. A New Combination of a Prebiotic and Postbiotic Mitigates Immunosenescence in Vaccinated Healthy Senior Dogs. Front. Vet. Sci. 2024, 11, 1392985. [Google Scholar] [CrossRef] [PubMed]
- Kayser, E.; He, F.; Nixon, S.; Howard-Varona, A.; Lamelas, A.; Martinez-Blanch, J.; Chenoll, E.; Davenport, G.M.; de Godoy, M.R.C. Effects of Supplementation of Live and Heat-Treated Bifidobacterium Animalis Subspecies Lactis CECT 8145 on Glycemic and Insulinemic Response, Fecal Microbiota, Systemic Biomarkers of Inflammation, and White Blood Cell Gene Expression of Adult Dogs. J. Anim. Sci. 2024, 102, skae291. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Daristotle, L.; Quesnell, R.; Reinhart, G.A.; Frantz, N.Z. Altered Fecal Microbiota, IgA, and Fermentative End-Products in Adult Dogs Fed Prebiotics and a Nonviable Lactobacillus Acidophilus. J. Anim. Sci. 2021, 99, skab347. [Google Scholar] [CrossRef]
- Wilson, S.M.; Oba, P.M.; Applegate, C.C.; Koziol, S.A.; Panasevich, M.R.; Norton, S.A.; Swanson, K.S. Effects of a Saccharomyces Cerevisiae Fermentation Product-Supplemented Diet on Fecal Characteristics, Oxidative Stress, and Blood Gene Expression of Adult Dogs Undergoing Transport Stress. J. Anim. Sci. 2023, 101, skac378. [Google Scholar] [CrossRef]
- Rui, W.; Zhong, S.; Li, X.; Tang, X.; Wang, L.; Yang, J. Evaluating the Role of Postbiotics in the Modulation of Human Oral Microbiota: A Randomized Controlled Clinical Trial. Probiotics Antimicrob. Proteins 2024, 16, 1–11. [Google Scholar] [CrossRef]

| Timepoint | |||
|---|---|---|---|
| Group | Day 0 | Day 7 | Day 14 |
| Placebo | 119.4 ± 61 | 154.3 ± 148 | 207.9 ± 190 |
| COHP | 119.4 ± 48 | 90.0 ± 46 | 126.8 ± 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sordillo, A.; Casella, L.; Turcotte, R.; Sheth, R.U. A Novel Postbiotic Reduces Canine Halitosis. Animals 2025, 15, 1596. https://doi.org/10.3390/ani15111596
Sordillo A, Casella L, Turcotte R, Sheth RU. A Novel Postbiotic Reduces Canine Halitosis. Animals. 2025; 15(11):1596. https://doi.org/10.3390/ani15111596
Chicago/Turabian StyleSordillo, Aylesse, Liza Casella, Raphaël Turcotte, and Ravi U. Sheth. 2025. "A Novel Postbiotic Reduces Canine Halitosis" Animals 15, no. 11: 1596. https://doi.org/10.3390/ani15111596
APA StyleSordillo, A., Casella, L., Turcotte, R., & Sheth, R. U. (2025). A Novel Postbiotic Reduces Canine Halitosis. Animals, 15(11), 1596. https://doi.org/10.3390/ani15111596






