Neoplastic and Non-Neoplastic Proliferative Mammary Gland Lesions in Female and Male Guinea Pigs: Histological and Immunohistochemical Characterization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Signalment
2.2. Histological Processing
2.3. Histological Examination
2.3.1. Evaluation of Hematoxylin and Eosin-Stained Tissue Sections
2.3.2. Immunohistochemistry
2.4. Classification of Mammary Gland Proliferative Lesions
2.5. Statistical Evaluation
3. Results
3.1. Epidemiology
3.2. Mammary Gland Proliferative Lesions
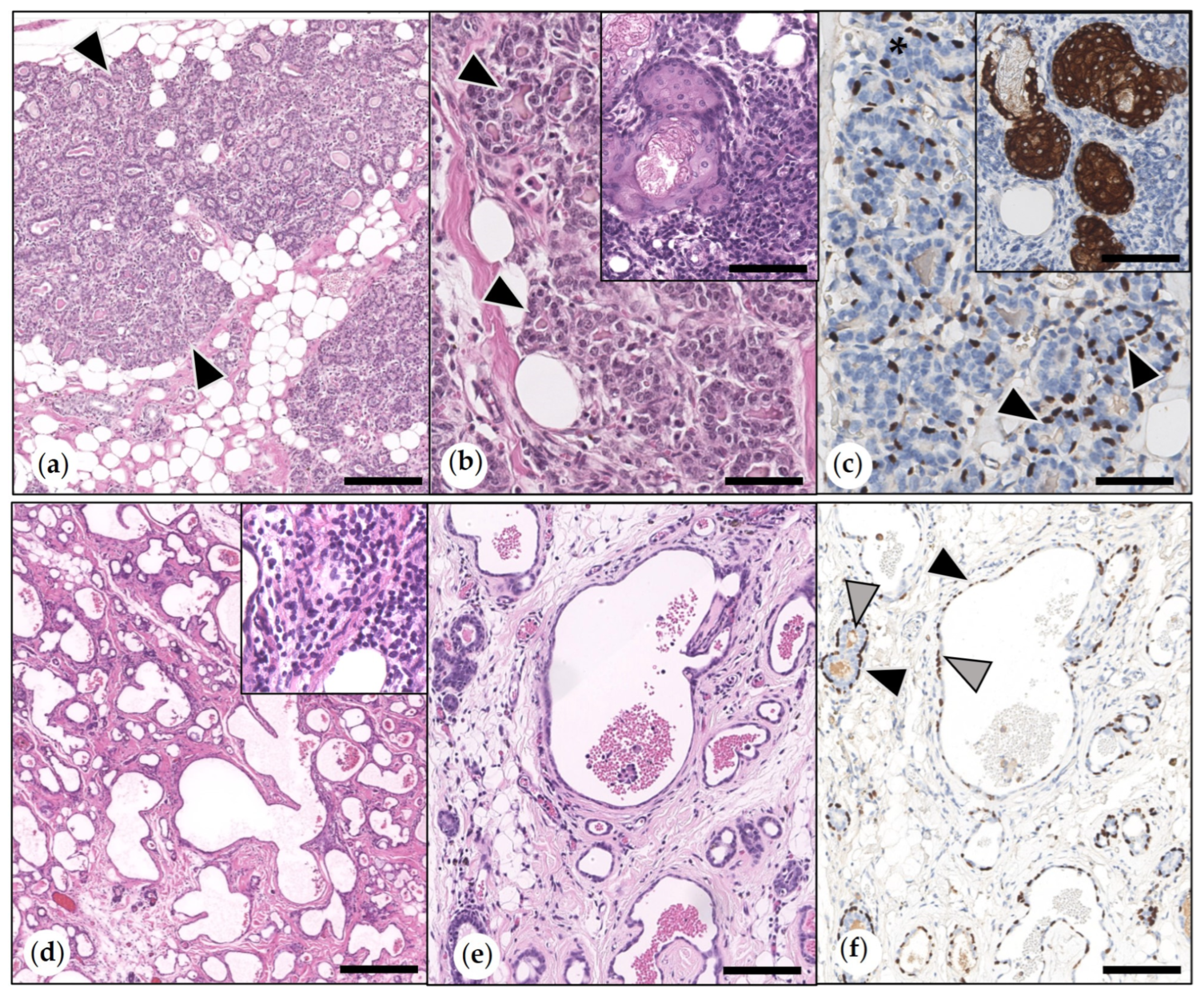
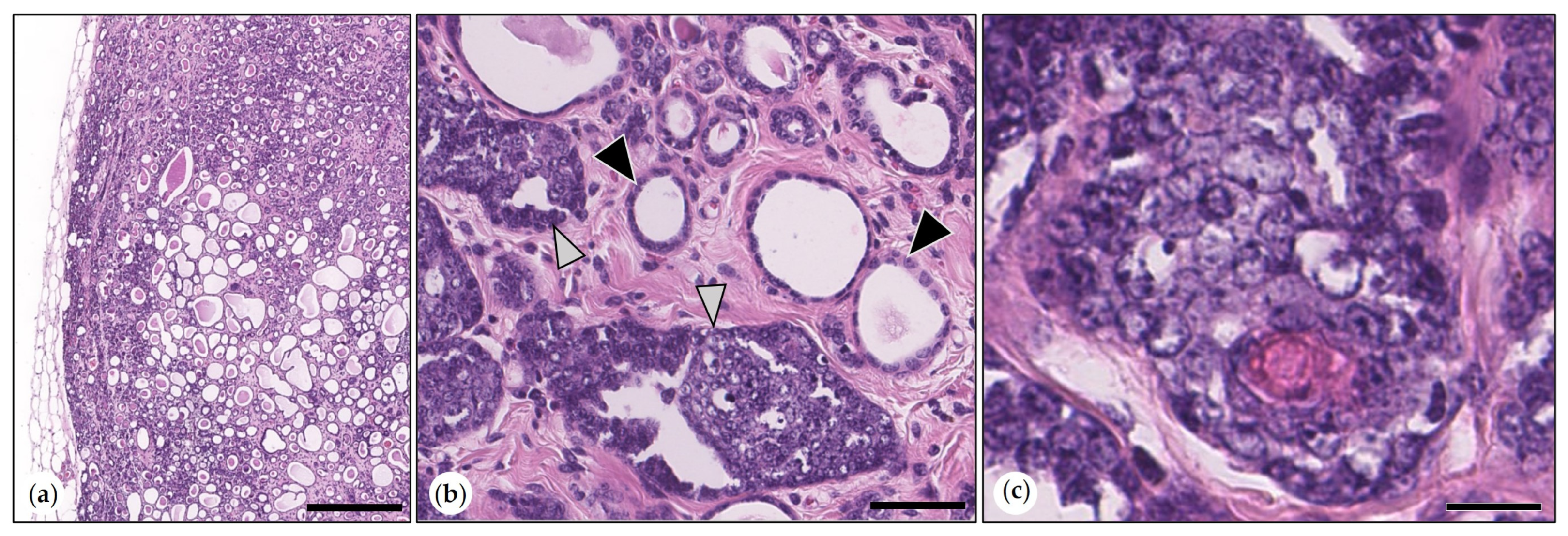
3.2.1. Non-Neoplastic Proliferative Mammary Lesions
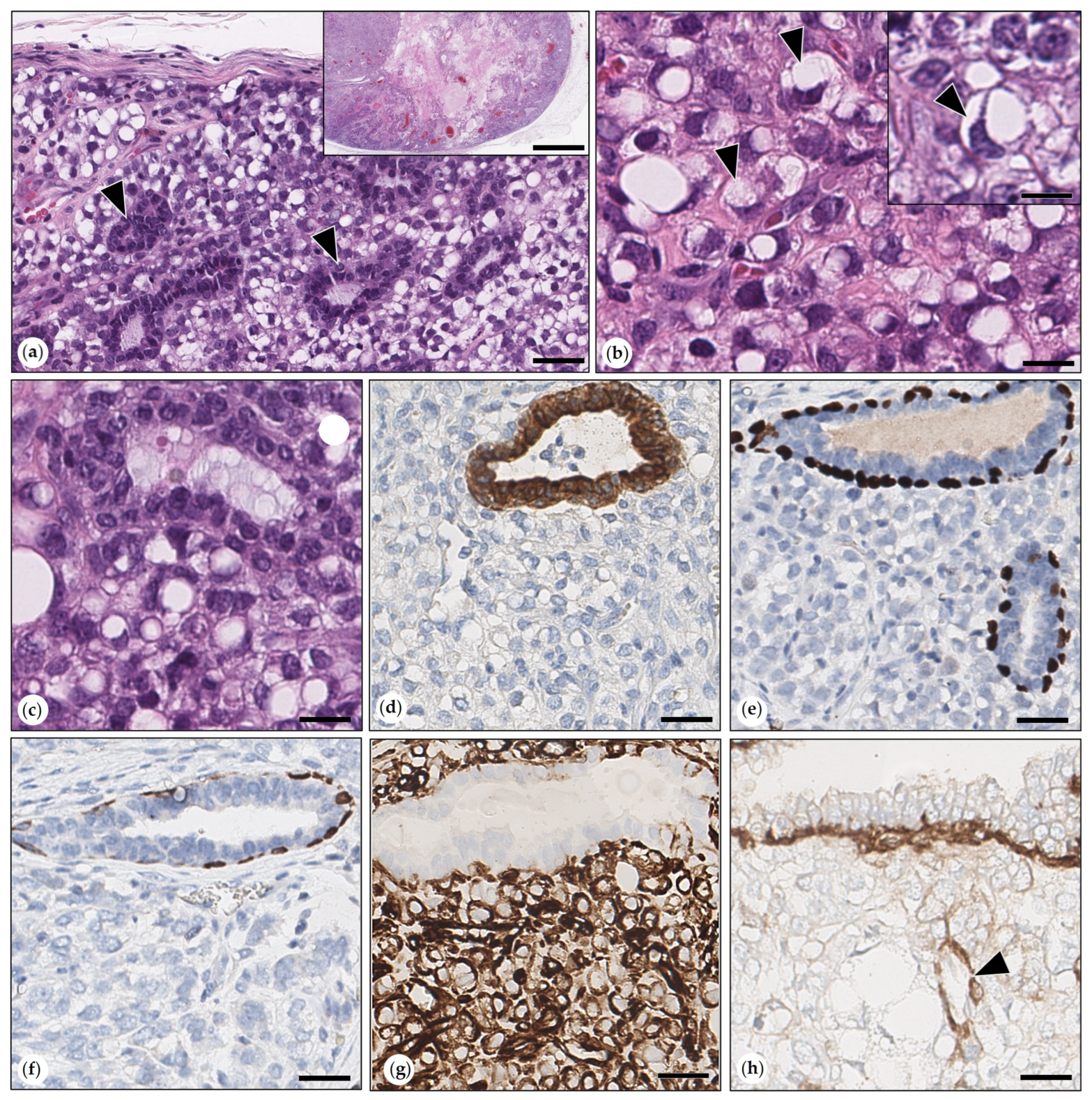
3.2.2. Benign Mammary Tumors
- Simple Adenomas
- Benign Mixed Tumors (Adenolipomas)
- Intraductal Papillary Adenoma (synonym: Duct Papilloma)
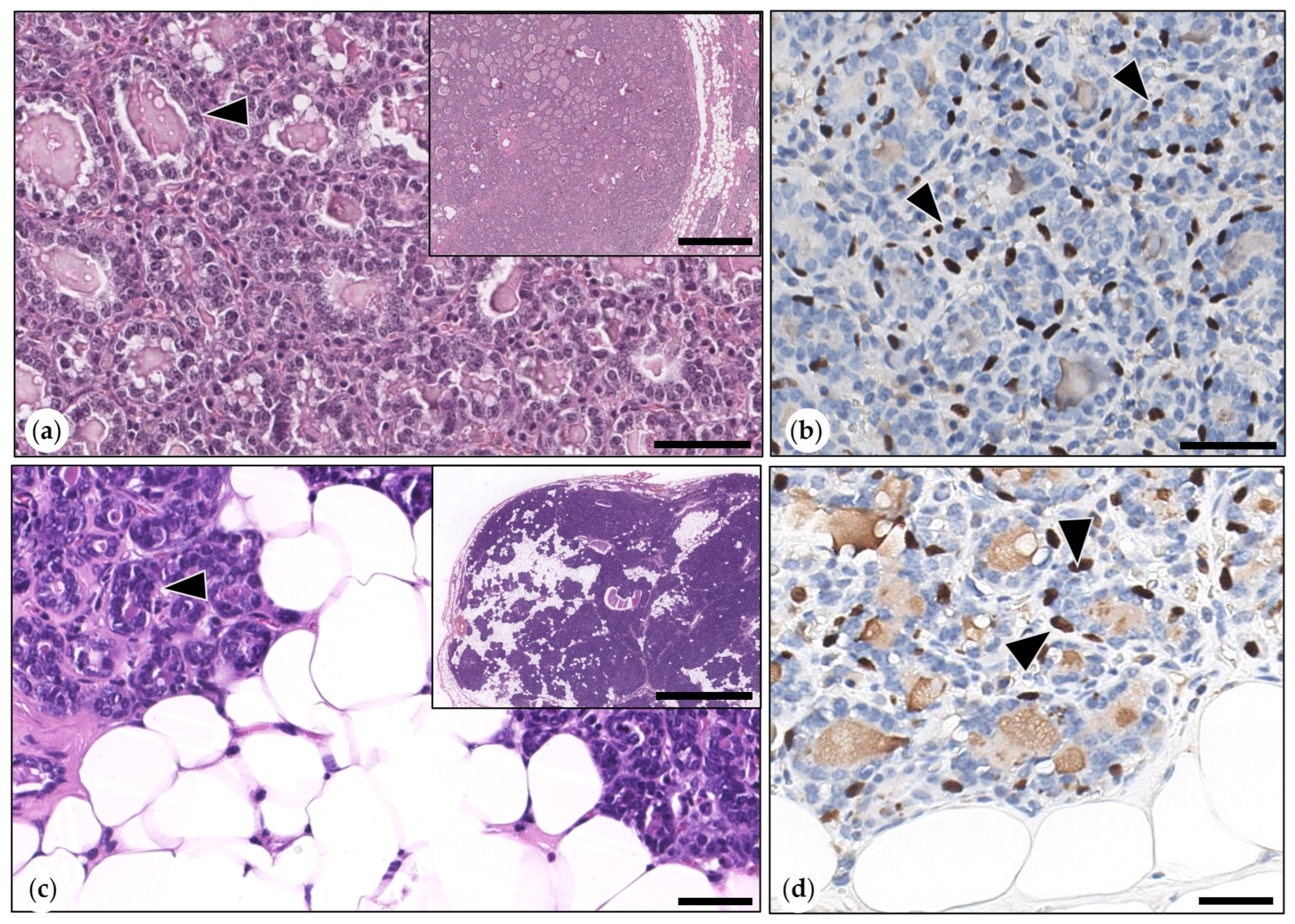
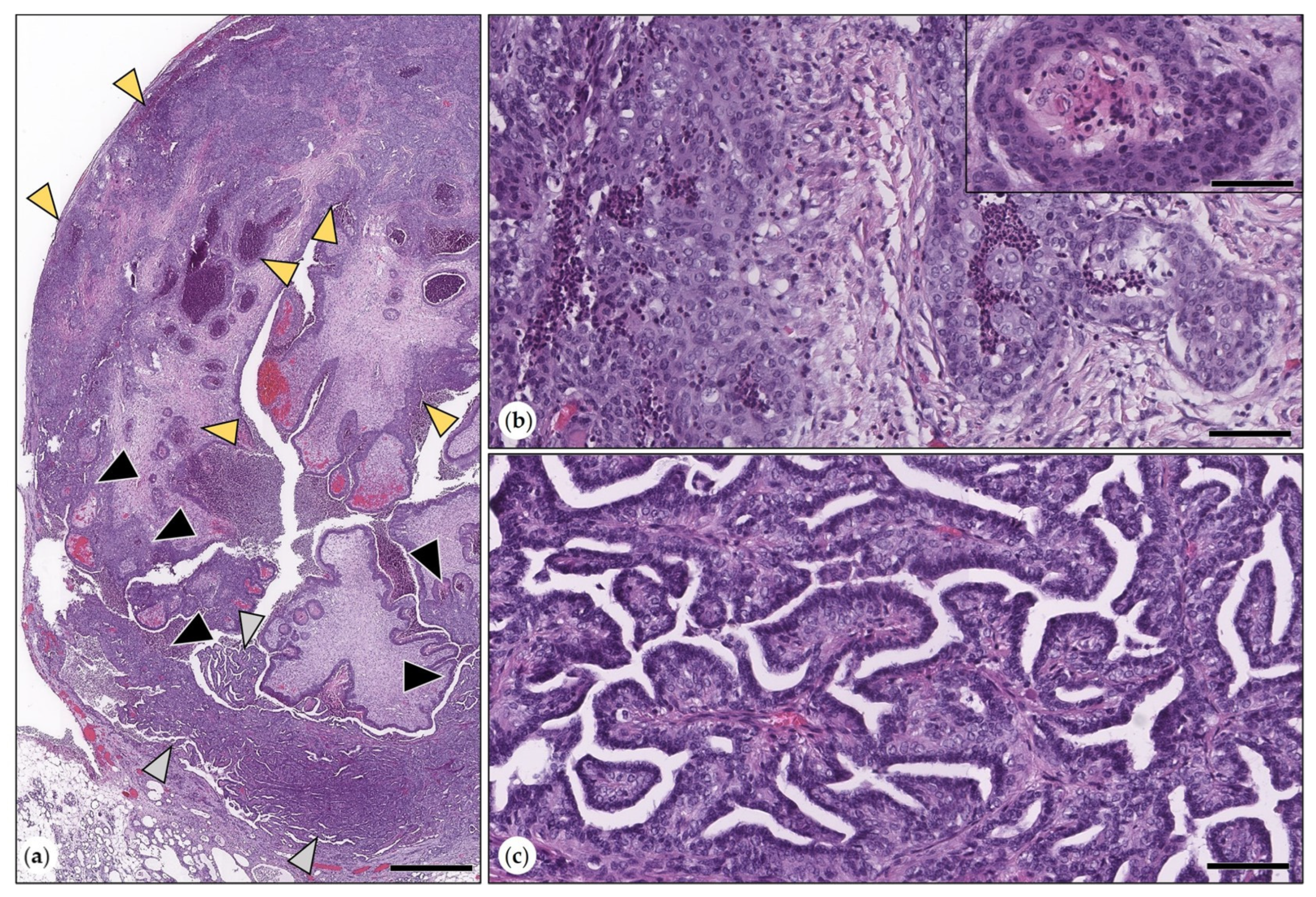
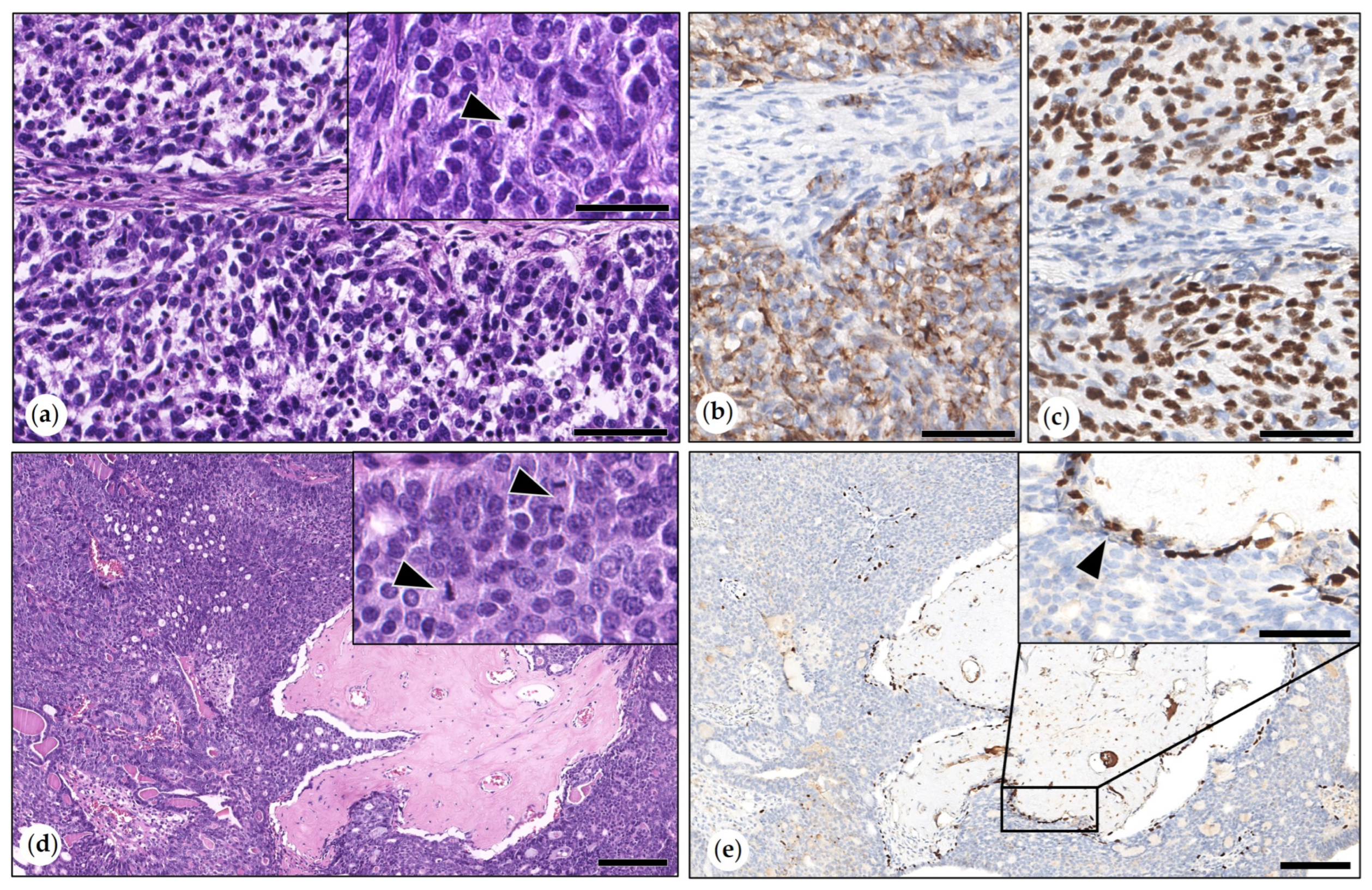
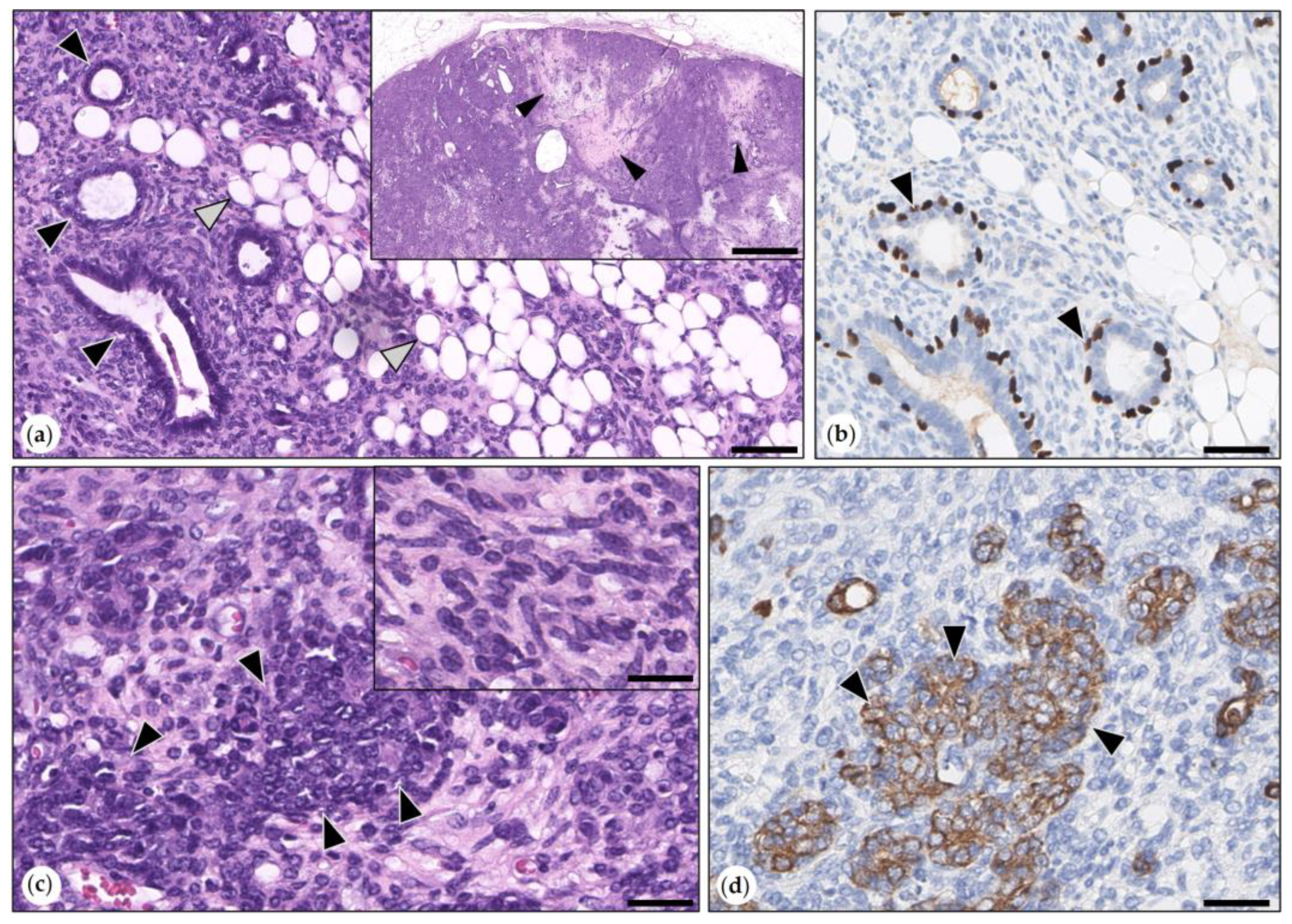
3.2.3. Malignant Mammary Tumors
- Simple Carcinoma
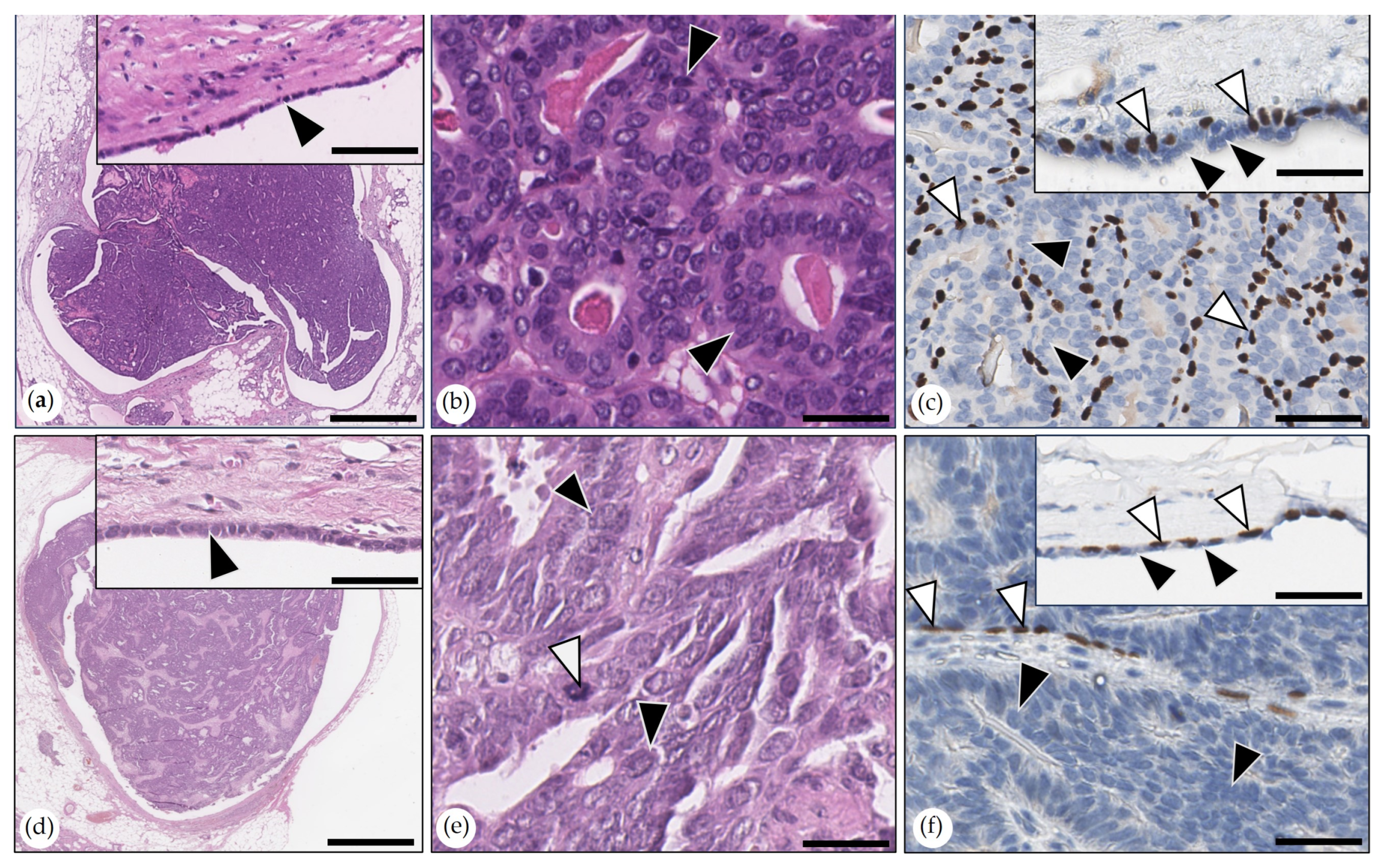
- Intraductal Papillary Carcinomas
- Adenosquamous Carcinoma
- Malignant Myoepithelioma
- Carcinoma Arising in (Simple) Adenoma
- Adenoliposarcoma
- Carcinosarcomas in Benign Mixed Tumors (Adenolipoma)
- Metaplastic Carcinomas with Mesenchymal Differentiation
3.3. Results of Statistical Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harkness, J.E.; Murray, K.A.; Wagner, J.E. Biology and disease of guinea pigs. In Laboratory Animal Medicine, 2nd ed.; Fox, J.G., Anderson, L.C., Loew, F.M., Quimby, F.W., Eds.; Academic Press: London, UK; Oxford, UK; Boston, MA, USA; New York, NY, USA; San Diego, CA, USA, 2007; Volume 1, pp. 203–246. [Google Scholar] [CrossRef]
- Quesenberry, K.E. Guinea pigs. Vet. Clin. N. Am. Small Anim. Pract. 1994, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.W.; Gomez, E.T. The normal development of the mammary gland of the male and female guinea pig. Res. Bull. 1933, 194, 5–31. [Google Scholar]
- Amorim, I.; Faria, F.; Rema, A.; Gärtner, F. Immunohistochemical characterization of a mammary carcinoma in a male guinea pig (Cavia porcellus). J. Comp. Pathol. 2009, 141, 279. [Google Scholar] [CrossRef]
- Oliveira, R.E.M.; Oliveira, R.K.M.; Dalmagro, M.J.; Silva, D.N.; Gurgel, J.V.O.; Sousa, A.C.F.C.; Attademo, F.L.N.; Soares, M.K.F.; Arcoverde, K.N.; Olinda, R.G.; et al. Mammary tubular carcinoma in a guinea pig (Cavia porcellus): Case report. Braz. J. Case Rep. 2023, 3, 18–23. [Google Scholar] [CrossRef]
- Felínek, F. Spontaneous Tumours in guinea pigs. Acta Vet. Brno 2003, 72, 221–228. [Google Scholar] [CrossRef]
- Suárez-Bonnet, A.; Martín de Las Mulas, J.; Millán, M.Y.; Herráez, P.; Rodríguez, F.; Espinosa de los Monteros, A. Morphological and immunohistochemical characterization of spontaneous mammary gland tumors in the guinea pig (Cavia porcellus). Vet. Pathol. 2010, 47, 298–305. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Wu, J.-L.; Lin, W.-H. Common spontaneous tumors and tumor-like lesions in 70 pet rodents and negative MMTV detection in mammary tumors. Animals 2024, 14, 1469. [Google Scholar] [CrossRef]
- Minarikova, A.; Hauptman, K.; Jeklova, E.; Knotek, Z.; Jekl, V. Diseases in pet guinea pigs: A retrospective study in 1000 animals. Vet. Rec. 2015, 177, 200. [Google Scholar] [CrossRef]
- Dobromylskyj, M.J.; Hederer, R.; Smith, K.C. Lumpy, bumpy guinea pigs: A retrospective study of 619 biopsy samples of externally palpable masses submitted from pet guinea pigs for histopathology. J. Comp. Pathol. 2023, 203, 13–18. [Google Scholar] [CrossRef]
- Raymond, P.; Coutant, T.; Chauvaux, B.; Muffat-es-Jacques, P.; Phouratsamay, A.; Donnelly, T.M.; Pignon, C. Spontaneous mammary neoplasms in guinea pigs: 85 cases (2006–2022). J. Exotic Pet Med. 2024, 50, 19–25. [Google Scholar] [CrossRef]
- Bertram, C.A.; Donovan, T.A.; Bertram, B.; Sabara, J.; Klopfleisch, R. Neoplasia in pet guinea pigs: A retrospective analysis of 2,474 autopsy examinations. J. Vet. Diagn. Investig. 2025, 37, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Paździor-Czapula, K.; Mikiewicz, M.; Fiedorowicz, J.; Otrocka-Domagała, I. Mammary and reproductive tract tumours and tumour-like lesions of 286 small pet mammals: A retrospective study. J. Comp. Pathol. 2024, 213, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Ediger, R.D.; Kovatch, R.M. Spotaneous tumors in the Dunkin-Hartley guinea pig. J. Nat. Cancer Inst. 1976, 56, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.J. Mammary neoplasia in the guinea pig (Cavia porcellus). Cornell Vet. 1976, 66, 82–96. [Google Scholar]
- Hoch-Ligeti, C.; Liebelt, A.G.; Congdon, C.C.; Stewart, H.L. Mammary gland tumors in irradiated and untreated guinea pigs. Toxicol. Pathol. 1986, 14, 289–298. [Google Scholar] [CrossRef]
- Meuten, D.J.; Moore, F.M.; George, J.W. Mitotic count and the field of view area: Time to standardize. Vet. Pathol. 2016, 53, 7–9. [Google Scholar] [CrossRef]
- Zappuli, V.; Peňa, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Mammary tumors. In Surgical pathology of Tumors of Domestic Animals; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2019; Volume 2, pp. 1–269. [Google Scholar]
- Available online: https://vcgp.org/documents/2022/03/mitotic-count-2.pdf/ (accessed on 22 February 2025).
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Schöniger, S.; Horn, L.C.; Schoon, H.A. Tumors and tumor-like lesions in the mammary gland of 24 pet rabbits: A histomorphological and immunohistochemical characterization. Vet. Pathol. 2014, 51, 569–580. [Google Scholar] [CrossRef]
- Degner, S.; Schoon, H.-A.; Laik-Schandelmaier, C.; Aupperle-Lellbach, H.; Schöniger, S. Estrogen receptor-α and progesterone receptor expression in mammary proliferative lesions of female pet rabbits. Vet. Pathol. 2018, 55, 838–848. [Google Scholar] [CrossRef]
- Shimomoto, T.; Yoshida, M.; Takahashi, M.; Maekawa, A. Sebaceous gland metaplasia in a mammary fibroadenoma developing in a female Donryu rat. J. Toxicol. Pathol. 2002, 15, 73–77. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, J.; Lu, H.; Zhang, J.; Liu, Z.; Wei, J.; Zhao, K.; Gao, F.; He, W. Benign mixed mammary tumor with sebaceous differentiation in a dog. J. Vet. Med. Sci. 2024, 86, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Rudmann, D.; Cardiff, R.; Chouinard, L.; Goodman, D.; Küttler, K.; Marxfeld, H.; Molinolo, A.; Treumann, S.; Yoshizawa, K.; INHAND Mammary, Zymbal’s, Preputial, and Clitoral Gland Organ Working Group. Proliferative and nonproliferative lesions of the rat and mouse mammary, Zymbal’s, preputial, and clitoral glands. Toxicol. Pathol. 2012, 40 (Suppl. S6), 7S–39S. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Breast Tumours [Internet]. Lyon (France): International Agency for Research on Cancer. 2019. (WHO Classification of Tumours Series, 5th ed.; Vol. 2). Available online: https://tumourclassification.iarc.who.int/chapters/32 (accessed on 12 March 2025).
- Goldschmidt, M.H.; Peňa, L.; Zappulli, V. Tumors of the mammary gland. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons: Ames, IA, USA, 2017; pp. 723–765. [Google Scholar]
- Schöniger, S.; Degner, S.; Jasani, B.; Schoon, H.A. A review on mammary tumors in rabbits: Translation of pathology into medical care. Animals 2019, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Vergneau-Grosset, C.; Keel, M.K.; Goldsmith, D.; Kass, P.H.; Paul-Murphy, J.; Hawkins, M.G. Description of the prevalence, histologic characteristics, concomitant abnormalities, and outcomes of mammary gland tumors in companion rats (Rattus norvegicus): 100 cases (1990–2015). J. Am. Vet. Med. Assoc. 2016, 249, 1170–1179. [Google Scholar] [CrossRef]
- Anderson, W.F.; Jatoi, I.; Tse, J.; Rosenberg, P.S. Male breast cancer: A population-based comparison with female breast cancer. J. Clin. Oncol. 2010, 28, 232–239. [Google Scholar] [CrossRef]
- Bearss, J.J.; Schulman, F.Y.; Carter, D. Histologic, immunohistochemical, and clinical features of 27 mammary tumors in 18 male dogs. Vet. Pathol. 2012, 49, 602–607. [Google Scholar] [CrossRef]
- Skorupski, K.A.; Overley, B.; Shofer, F.S.; Goldschmidt, M.H.; Miller, C.A.; Sørenmo, K.U. Clinical characteristics of mammary carcinoma in male cats. J. Vet. Intern. Med. 2005, 19, 52–55. [Google Scholar] [CrossRef]
- Graur, D.; Hide, W.; Li, W.H. Is the guinea-pig a rodent? Nature 1991, 351, 649–652. [Google Scholar] [CrossRef]
- D’Erchia, A.; Gissi, C.; Pesole, G.; Saccone, C.; Arnason, U. The guinea-pig is not a rodent. Nature 1996, 381, 597–600. [Google Scholar] [CrossRef]
- Alonso-Diez, Á.; Ramos, A.; Roccabianca, P.; Barreno, L.; Pérez-Alenza, M.D.; Tecilla, M.; Avallone, G.; Gama, A.; Peña, L. Canine spindle cell mammary tumor: A retrospective study of 67 cases. Vet. Pathol. 2019, 56, 526–535. [Google Scholar] [CrossRef]
- Charlot, M.; Béatrix, O.; Chateau, F.; Dubuisson, J.; Golfier, F.; Valette, P.J.; Réty, F. Pathologies of the male breast. Diagn. Interv. Imaging 2013, 94, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Pilny, A. Ovarian cystic disease in guinea pigs. Vet. Clin. Exot. Anim. Pract. 2014, 17, 69–75. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours [Internet]. 5th Lyon (France): International Agency for Research on Cancer; 2020. (WHO Classification of Tumours Series, 5th ed.; Vol. 4). Available online: https://tumourclassification.iarc.who.int/chapters/34 (accessed on 12 March 2025).
- Zeng, L.; Li, W.; Chen, C.-S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.L.; Cowan, G.; Chatterji, S.; Conti, G.; Speirs, V. Exploring the one health paradigm in male breast cancer. J. Mammary Gland. Biol. Neoplasia 2024, 29, 8. [Google Scholar] [CrossRef]
- Schildhaus, H.U.; Schroeder, L.; Merkelbach-Bruse, S.; Binot, E.; Büttner, R.; Kuhn, W.; Rudlowski, C. Therapeutic strategies in male breast cancer: Clinical implications of chromosome 17 gene alterations and molecular subtypes. Breast 2013, 22, 1066–1071. [Google Scholar] [CrossRef]

| Primary Antibody | Antigen Retrieval | ||||
|---|---|---|---|---|---|
| Antigen | Clone | Source | Dilution | ICT | Buffer/ICT |
| PanCK | AE1/AE3 | Mouse | RTU | 60 min | CC1/48 min |
| p63 | 4A4 | Mouse | 1/50 | 20 min | CC1/64 min |
| CK 14 | SP53 | Rabbit | 1/200 | 16 min | CC1/32 min |
| Vim | V9 | Mouse | RTU | 16 min | CC1/36min |
| SMA | EP188 | Rabbit | 1/100 | 32 min | none |
| Group 1 | Group 2 | Analyzed Parameter(s) | ||||
|---|---|---|---|---|---|---|
| Sex | Lesion | N | Sex | Lesion | N | |
| FI, FS | Hyperplasia 1 | 8 | FI, FS | Benign tumor 2 | 26 | Age |
| FI, FS | Hyperplasia 1 | 8 | FI, FS | Malignant tumor 3 | 35 | Age |
| FI, FS | Benign tumor 2 | 26 | FI, FS | Malignant tumor 3 | 35 | Age |
| FI, FS | (TPC, SC, IPC) 4 | 26 | MI, MN | (TPC, SC, IPC) 4 | 41 | Age, mitotic count, tumor grade |
| FI, FS | (TPC, SC, IPC) 4 | 26 | MI, MN | (TPC, SC, IPC) 4 | 40 | Infiltrative growth |
| MI | (TPC, SC, IPC) 4 | 31 | MN | (TPC, SC, IPC) 4 | 10 | Age, mitotic count, tumor grade |
| MI | (TPC, SC, IPC) 4 | 30 | MN | (TPC, SC, IPC) 4 | 10 | Infiltrative growth |
| FI | (TPC, SC, IPC) 4 | 25 | MN | (TPC, SC, IPC) 4 | 10 | Age, mitotic count, tumor grade, infiltrative growth |
| FI | (TPC, SC, IPC) 4 | 25 | MI | (TPC, SC, IPC) 4 | 31 | Age, mitotic count, tumor grade |
| FI | (TPC, SC, IPC) 4 | 25 | MI | (TPC, SC, IPC) 4 | 30 | Infiltrative growth |
| FI, FS | TPC | 18 | MI, MN | TPC | 24 | Age, mitotic count, tumor grade |
| FI, FS | TPC | 18 | MI, MN | TPC | 23 | Infiltrative growth |
| FI, FS | IPC | 6 | MI, MN | IPC | 7 | Age, mitotic count, tumor grade infiltrative growth |
| Mammary Tissue and Lesions | Guinea Pigs | ||
|---|---|---|---|
| Numbers | FI/FS | MI/MN | |
| Non-Neoplastic Lesions | 50 | 49/1 | 0/0 |
| Lobular hyperplasia with secretory activity | 8 | 7/1 | 0/0 |
| Lobular hyperplasia with secretory activity and a tumor | 18 | 18/0 | 0/0 |
| Lobular hyperplasia with fibrosis and a tumor | 17 | 17/0 | 0/0 |
| Lobular hyperplasia with secretory activity and fibrosis, and a tumor | 7 | 7/0 | 0/0 |
| Benign Tumors | 28 | 26 | 0/2 |
| (Simple) adenoma | 20 | 20/0 | 0/0 |
| Adenolipoma | 3 | 3/0 | 0/0 |
| Intraductal papillary adenoma | 5 | 3/0 | 0/2 |
| Malignant Tumors | 81 | 34/1 | 36/10 |
| (Simple) tubular carcinoma | 3 | 1/0 | 2/0 |
| (Simple) tubulopapillary carcinoma | 41 | 17/1 | 19/4 |
| (Simple) solid carcinoma | 11 | 2/0 | 7/2 |
| (Simple) tubulopapillary and (simple) solid carcinoma | 1 | 0/0 | 1/0 |
| Anaplastic carcinoma | 1 | 0/0 | 1/0 |
| Intraductal papillary carcinoma | 13 | 6/0 | 3/4 |
| Adenosquamous carcinoma | 3 | 3/0 | 0/0 |
| Malignant myoepithelioma | 1 | 0/0 | 1/0 |
| Carcinoma in (simple) adenoma | 1 | 1/0 | 0/0 |
| Adenoliposarcoma | 1 | 1/0 | 0/0 |
| Carcinosarcoma in adenolipoma | 3 | 3/0 | 0/0 |
| Metaplastic carcinoma | 2 | 0/0 | 2/0 |
| Normal Mammary Tissue in Sections with a Proliferative Lesion | 105 | 64/2 | 31/8 |
| Carcinomas in More Than 3 Animals (IPC, TPC, SC) | ||||
|---|---|---|---|---|
| Group 1 | Group 2 | Analysed Parameter(s) | Significance | Result |
| FI, FS (n = 26) | MI, MN (n = 41) | Mitotic count | p = 0.05 | Higher in group 2 |
| MI (n = 30) | MN (n = 10) | Infiltrative growth | p = 0.003 | More frequent in group 2 |
| FI (n = 25) | MN (n = 10) | Mitotic count | p = 0.047 | Higher in group 2 |
| FI (n = 25) | MN (n = 10) | Infiltrative growth | p = 0.010 | More frequent in group 1 |
| Tubulopapillary carcinomas | ||||
| Group 1 | Group 2 | Analysed Parameter(s) | Significance | Result |
| FI, FS (n = 18) | MI, MN (n = 24) | Mitotic count | p = 0.002 | Higher in group 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöniger, S.; Schandelmaier, C.; Aupperle-Lellbach, H.; Koppel, C.; Zhang, Q.; Schildhaus, H.-U. Neoplastic and Non-Neoplastic Proliferative Mammary Gland Lesions in Female and Male Guinea Pigs: Histological and Immunohistochemical Characterization. Animals 2025, 15, 1573. https://doi.org/10.3390/ani15111573
Schöniger S, Schandelmaier C, Aupperle-Lellbach H, Koppel C, Zhang Q, Schildhaus H-U. Neoplastic and Non-Neoplastic Proliferative Mammary Gland Lesions in Female and Male Guinea Pigs: Histological and Immunohistochemical Characterization. Animals. 2025; 15(11):1573. https://doi.org/10.3390/ani15111573
Chicago/Turabian StyleSchöniger, Sandra, Claudia Schandelmaier, Heike Aupperle-Lellbach, Christina Koppel, Qian Zhang, and Hans-Ulrich Schildhaus. 2025. "Neoplastic and Non-Neoplastic Proliferative Mammary Gland Lesions in Female and Male Guinea Pigs: Histological and Immunohistochemical Characterization" Animals 15, no. 11: 1573. https://doi.org/10.3390/ani15111573
APA StyleSchöniger, S., Schandelmaier, C., Aupperle-Lellbach, H., Koppel, C., Zhang, Q., & Schildhaus, H.-U. (2025). Neoplastic and Non-Neoplastic Proliferative Mammary Gland Lesions in Female and Male Guinea Pigs: Histological and Immunohistochemical Characterization. Animals, 15(11), 1573. https://doi.org/10.3390/ani15111573






