Simulation and Identification of the Habitat of Antarctic Krill Based on Vessel Position Data and Integrated Species Distribution Model: A Case Study of Pumping-Suction Beam Trawl Fishing Vessels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental and Fishery Data
2.2. Vessel Position Data
2.3. Habitat Simulation Based on the Integrated Species Distribution Model
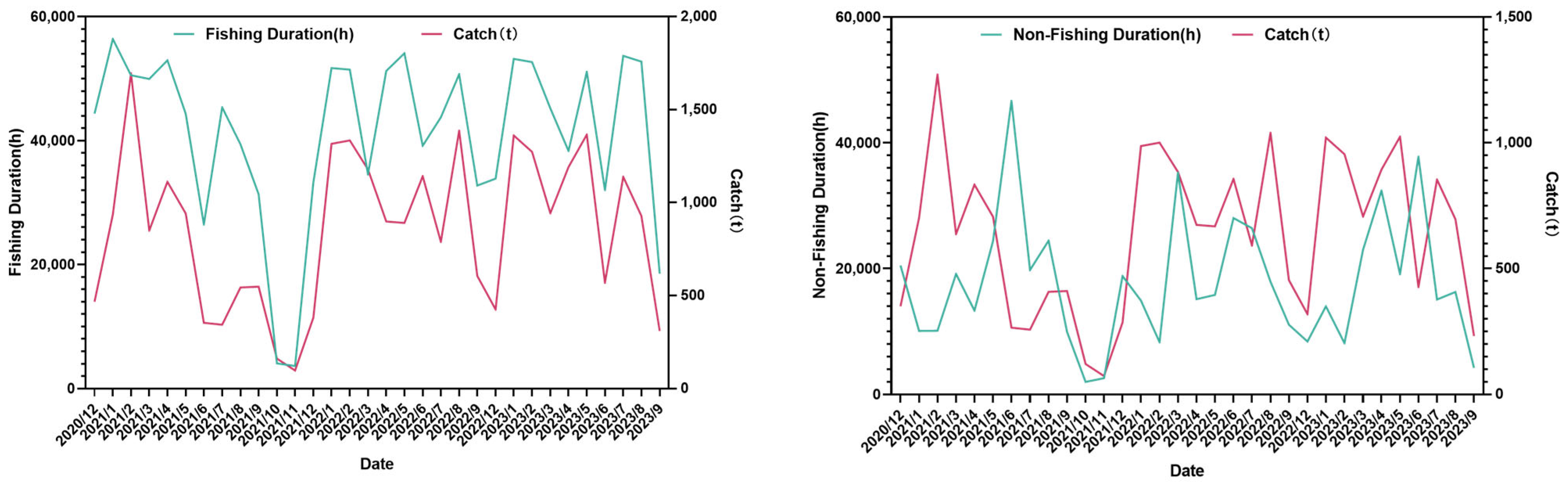
2.4. The Relationship Among the Change in Antarctic Krill’s Habitat Area, Fishing Duration, and Catch
3. Results
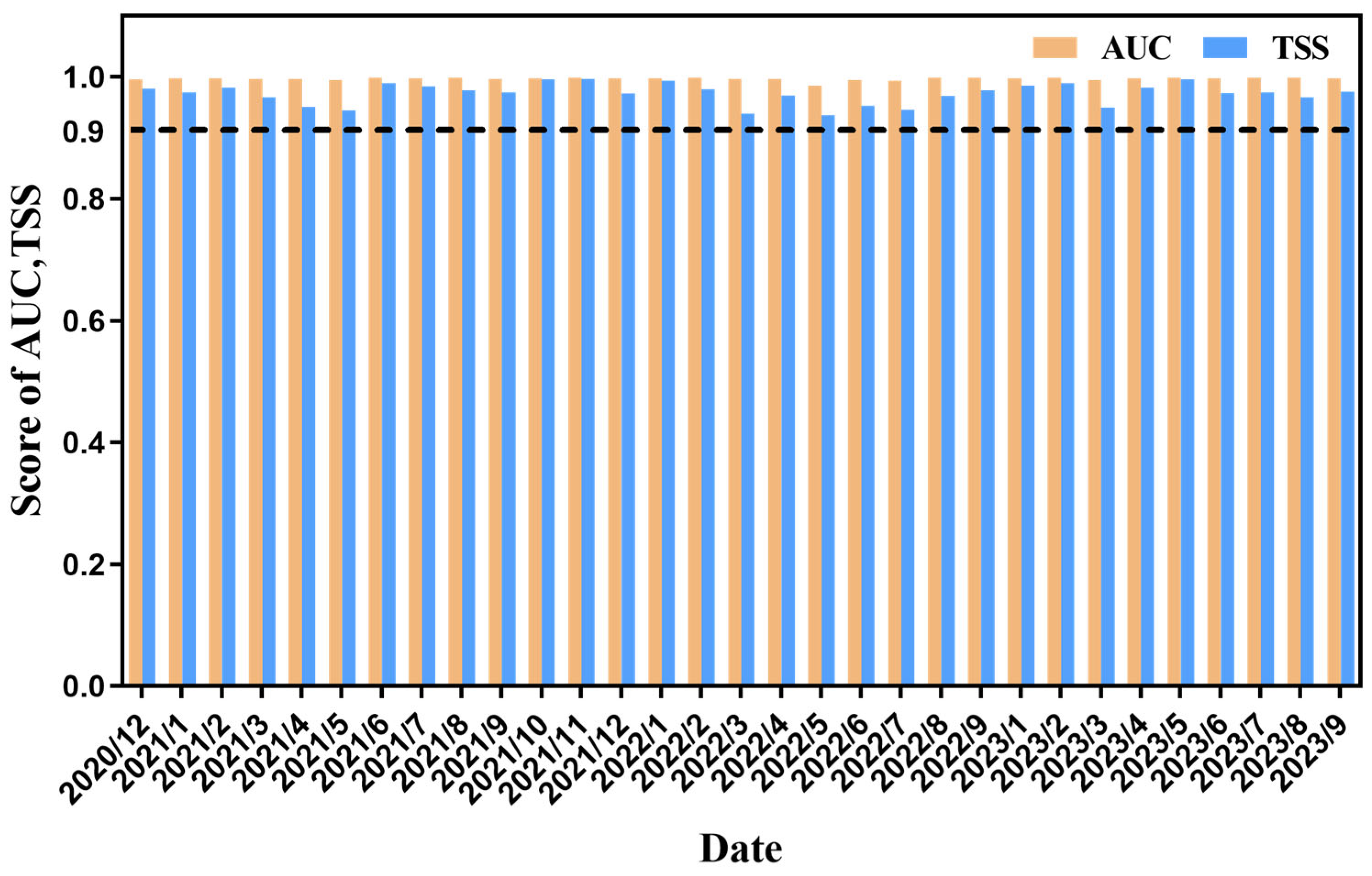
3.1. Performance Evaluation of the Integrated Species Distribution Model
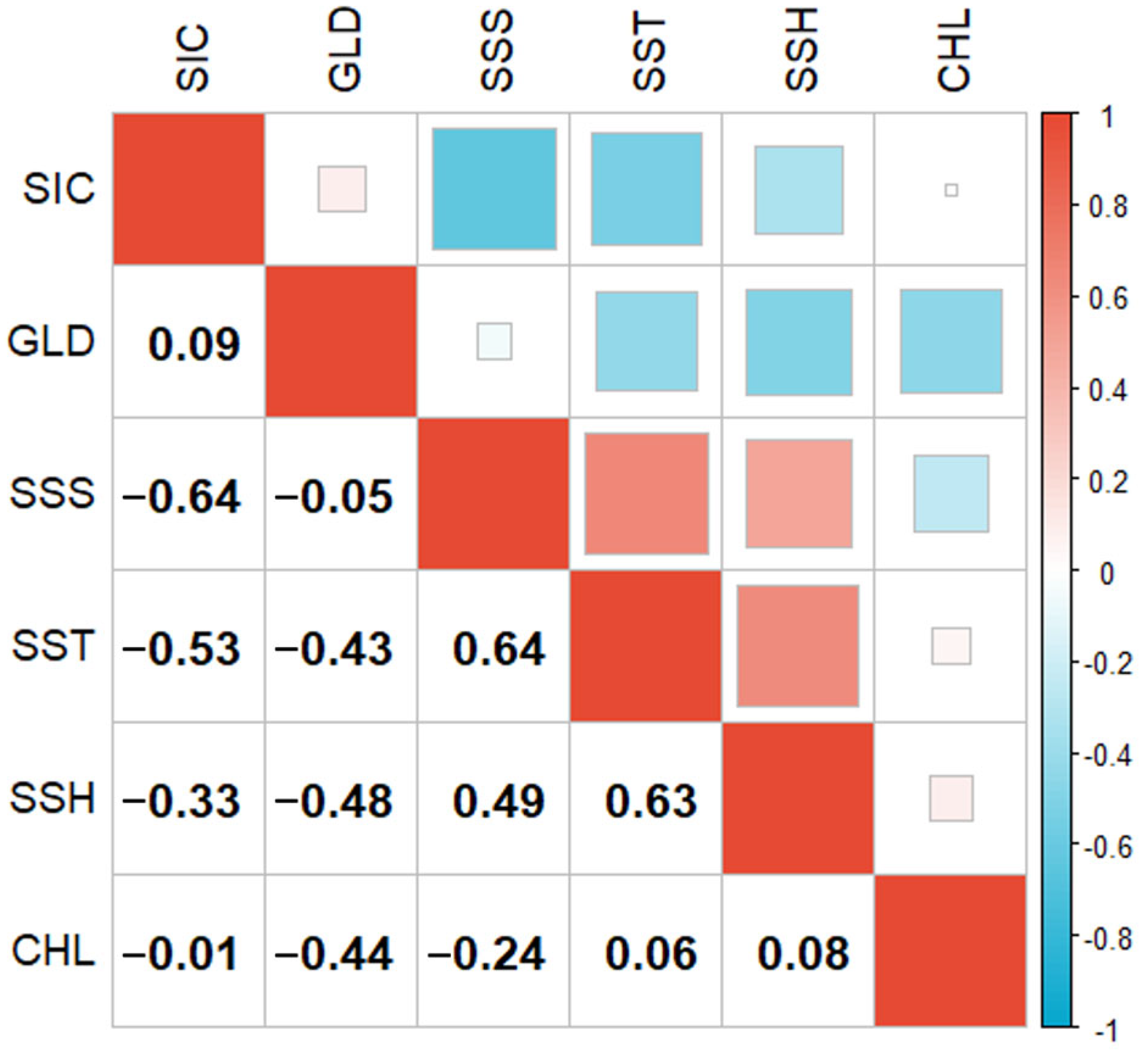
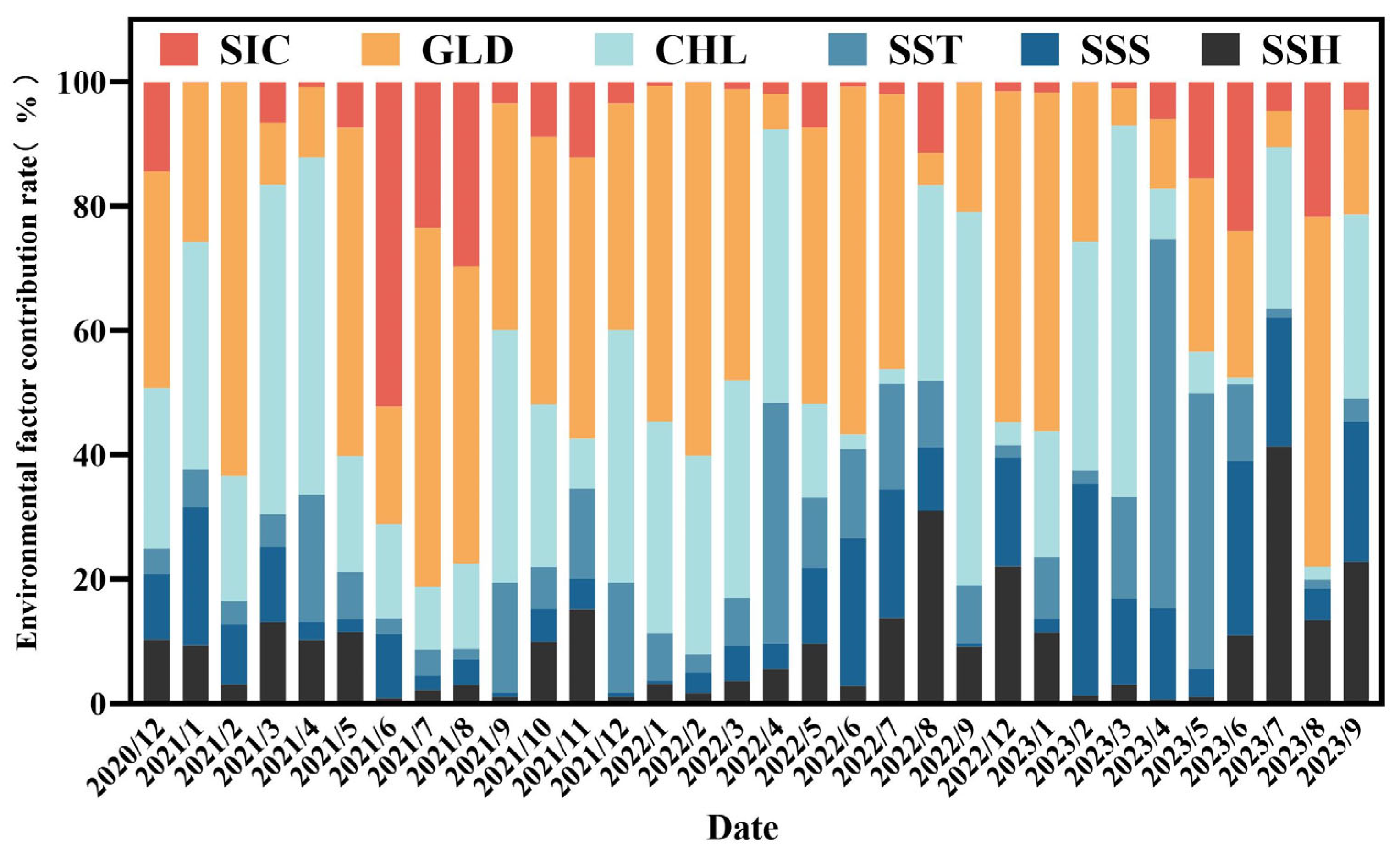
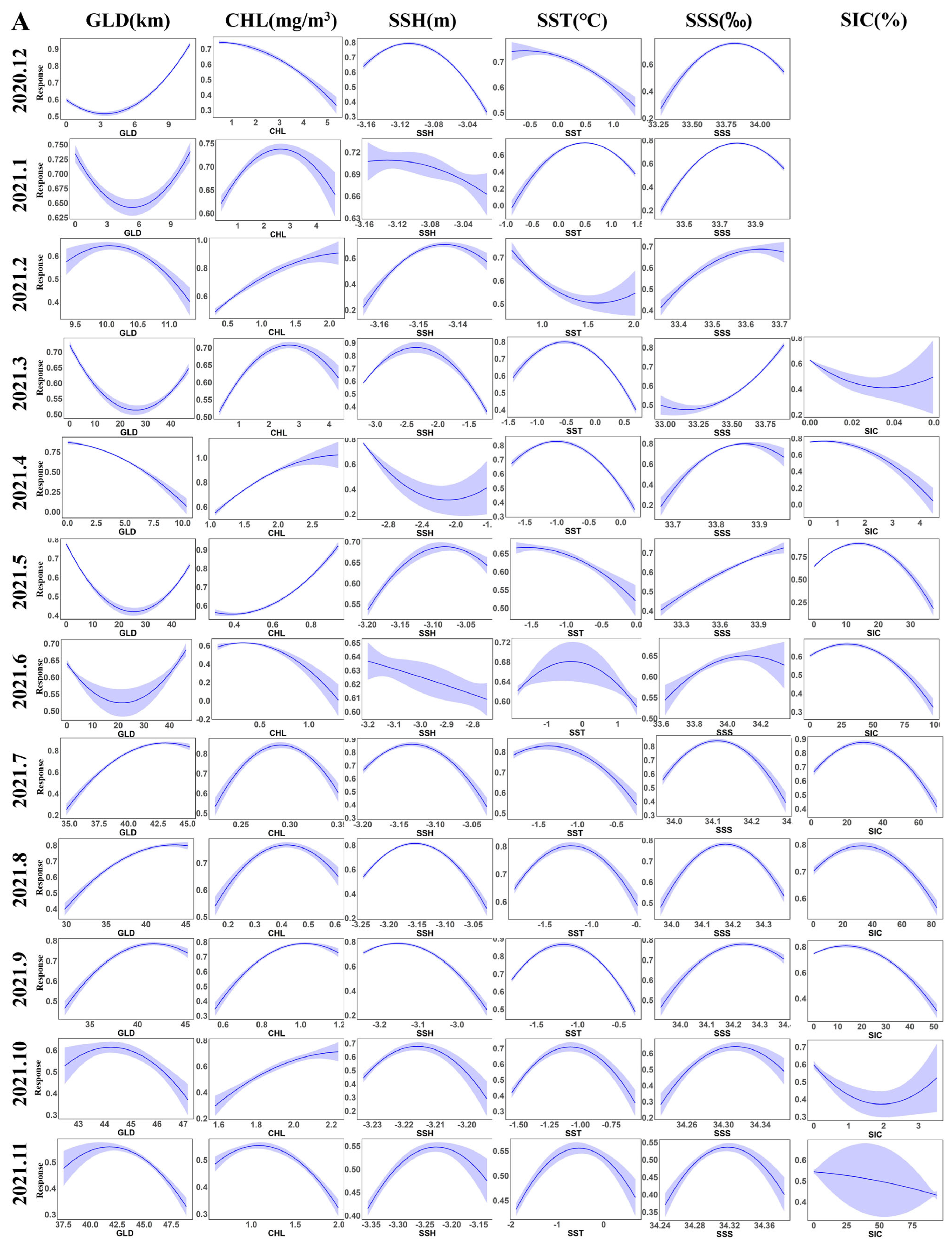
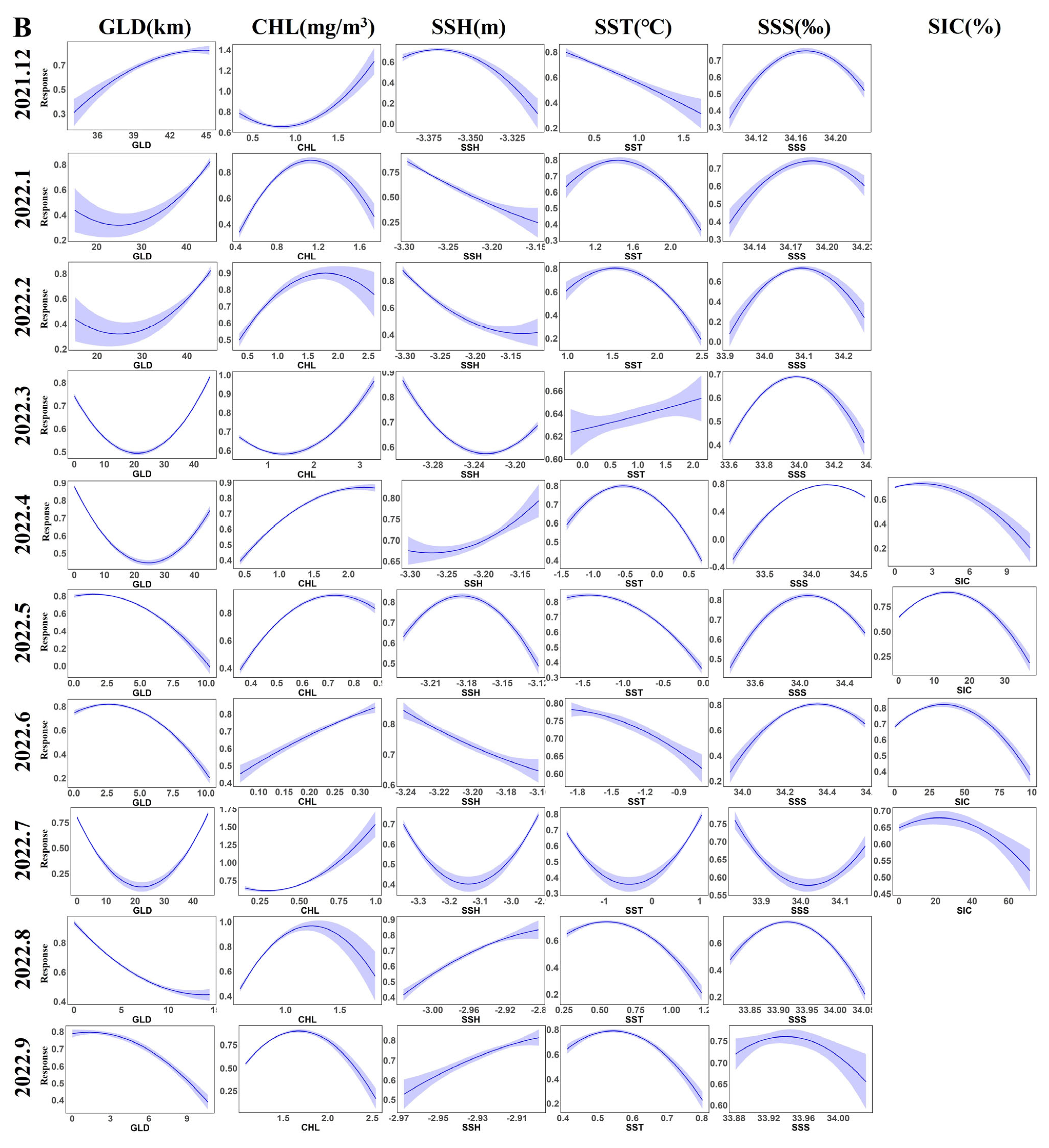
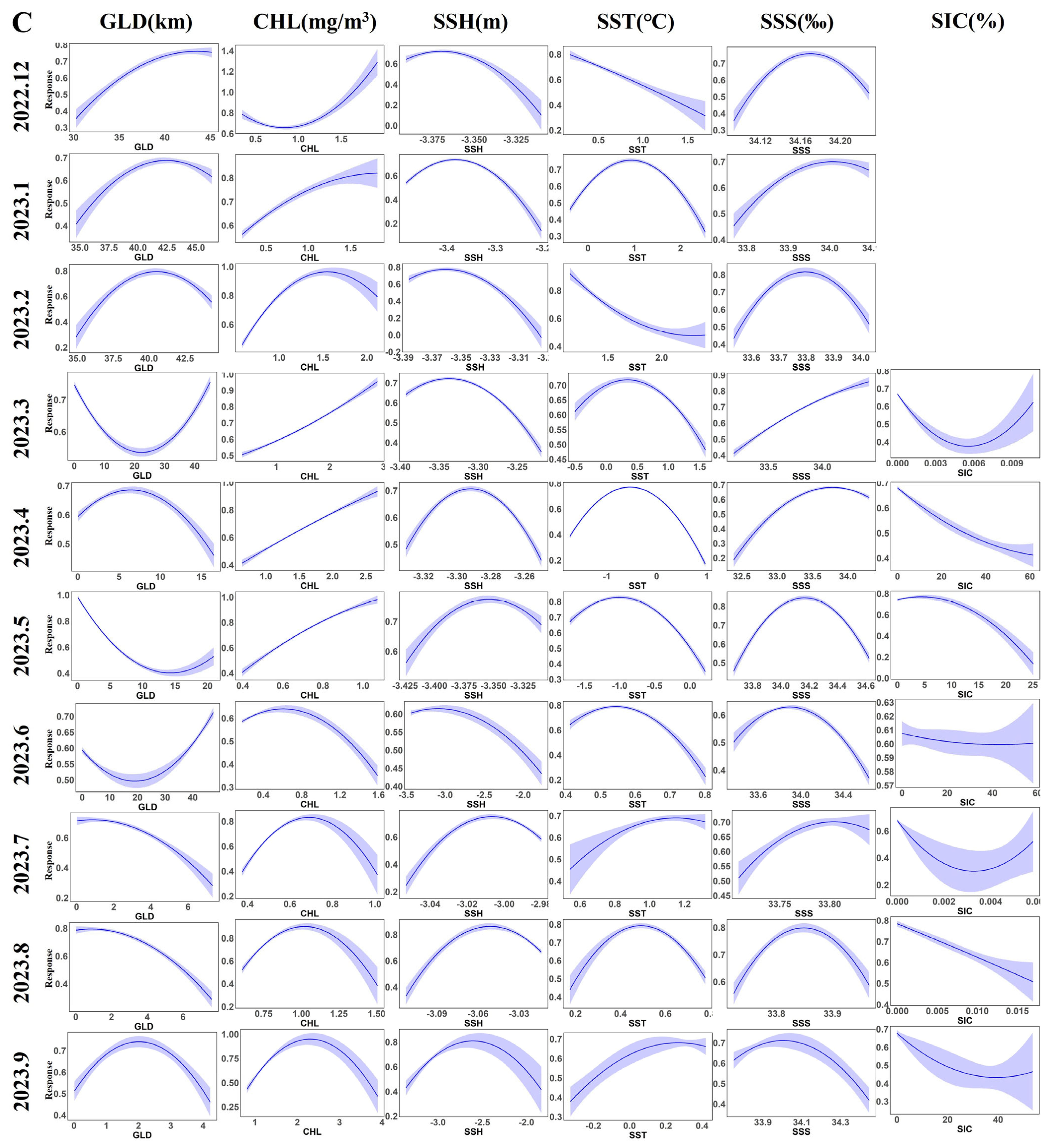
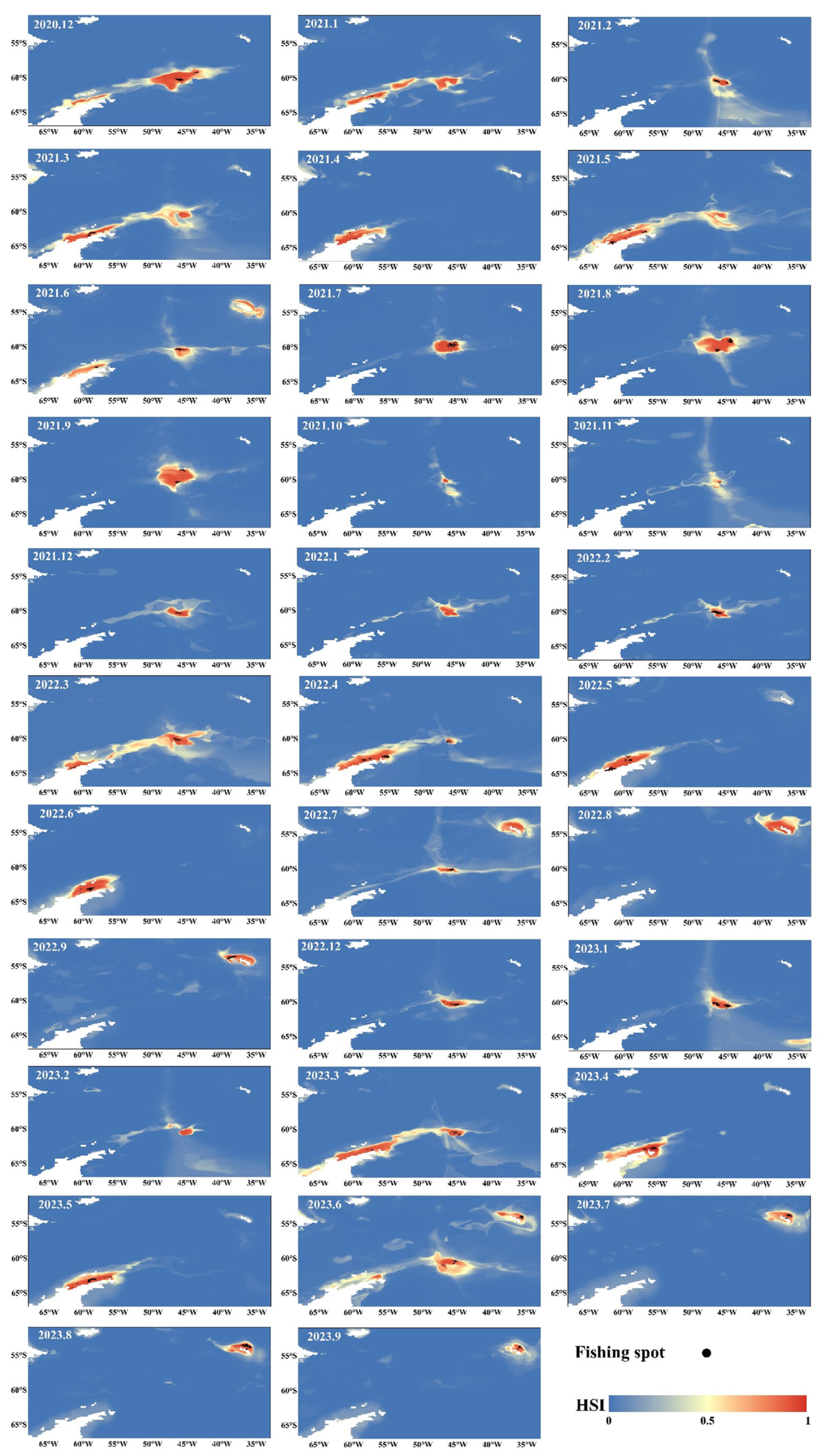
3.2. Contribution Rates of Environmental Factors and Distribution of Krill Habitats
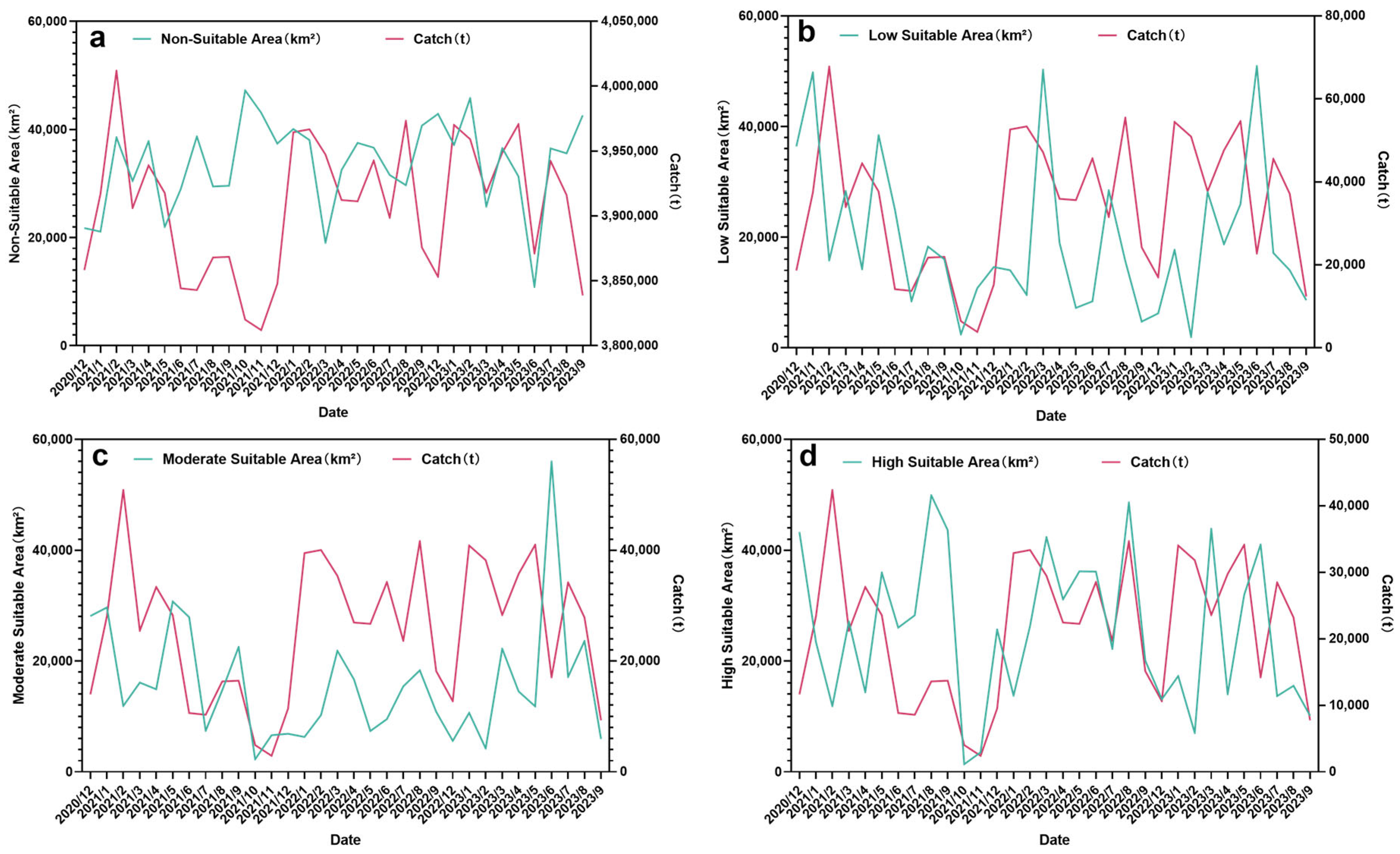
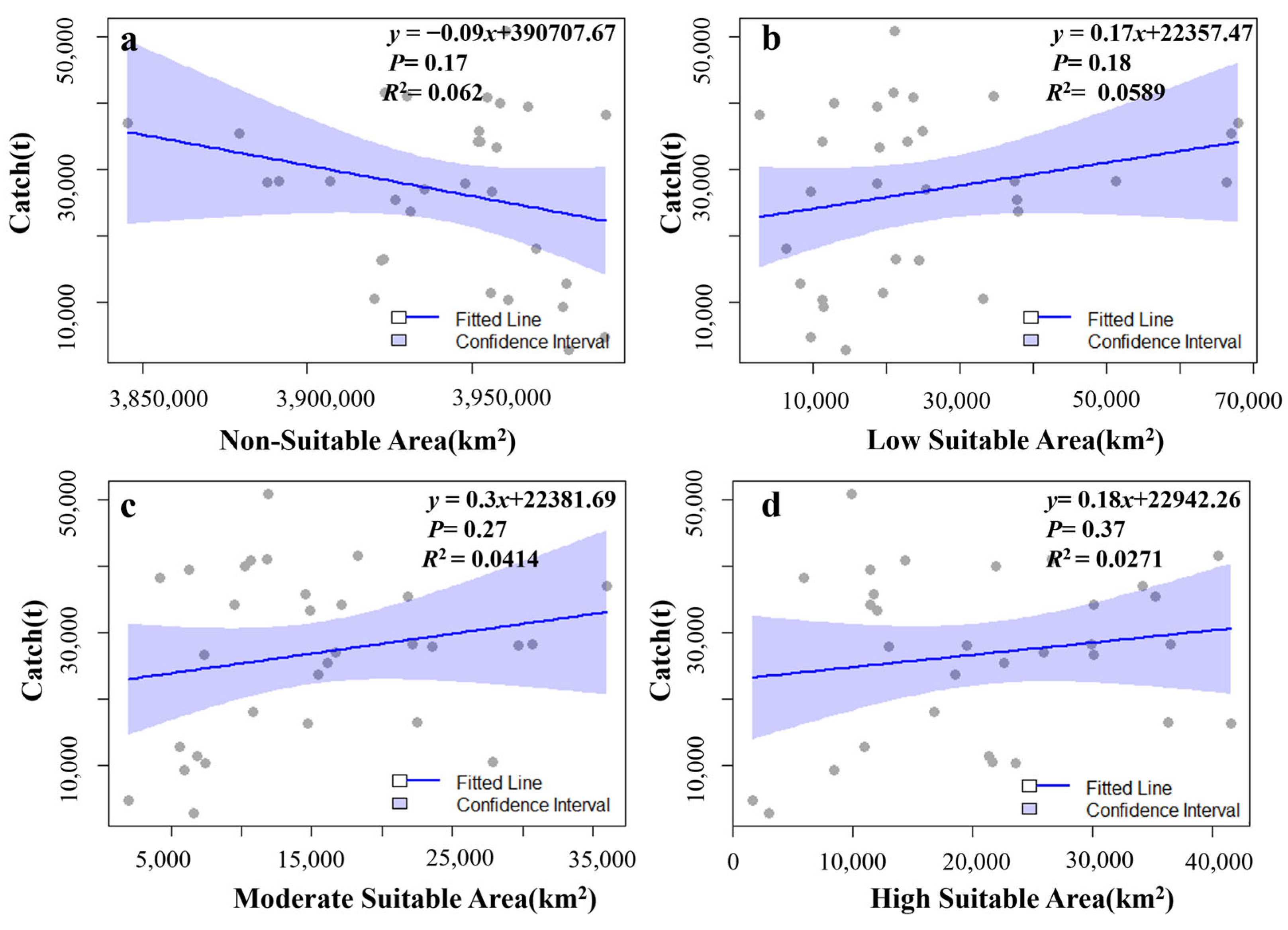
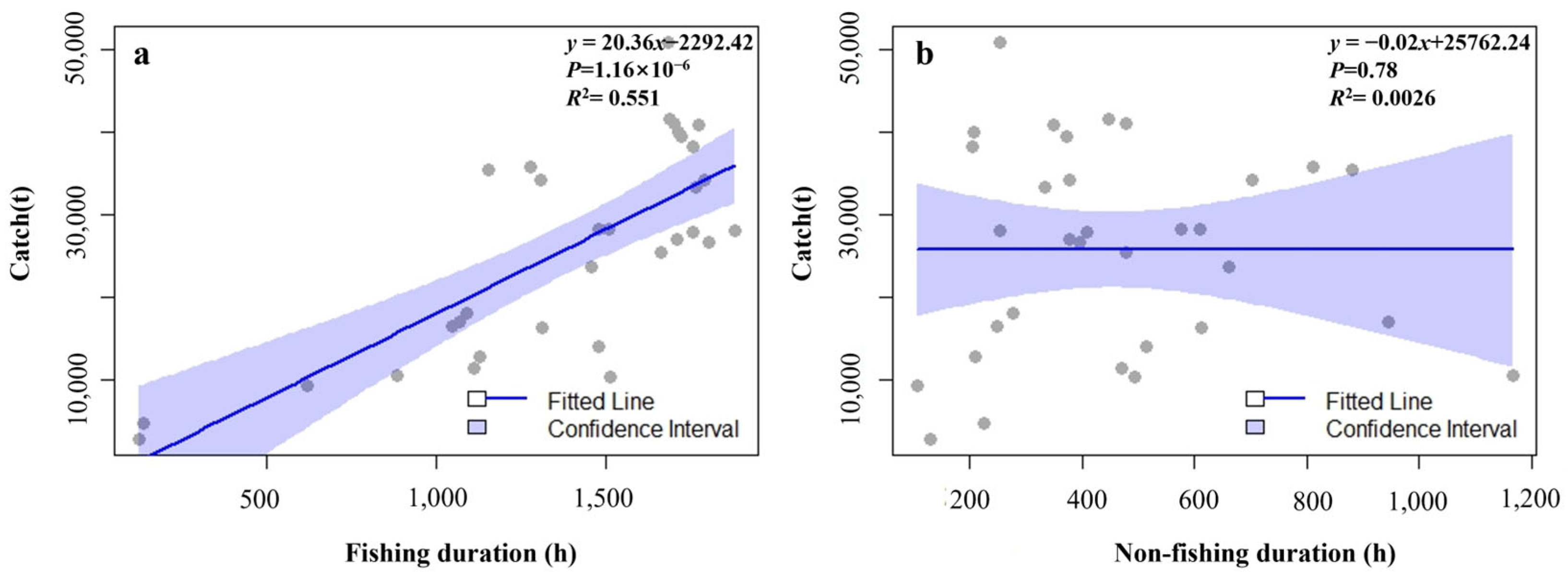
3.3. The Relationship Among the Habitat Area of Krill, the Fishing Duration, and the Catch
4. Discussion
4.1. Accuracy of the Integrated Species Distribution Model Based on Vessel Position Data
4.2. Key Environmental Factors of the Habitat of Krill and Their Impacts
4.3. The Relationship Between the Habitat Area of Antarctic Krill, the Fishing Duration, and the Catch
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Dorland, R.D. Aggregation and vertical migration behavior of Euphausia superba. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 2119–2137. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Zhang, H.; Cheng, T.; Yang, S. Interannual and monthly variations of catch per unit effort and the relation with sea surface temperature and chlorophyll concentration in fishing grounds (fishing area 48) of Antarctic krill. Chin. J. Ecol. 2020, 39, 1685–1694. [Google Scholar] [CrossRef]
- Constable, A.J.; Nicol, S.; Strutton, P.G. Southern Ocean productivity in relation to spatial and temporal variation in the physical environment. J. Geophys. Res. Ocean. 2003, 108, 8079. [Google Scholar] [CrossRef]
- Atkinson, A.; Shreeve, R.S.; Hirst, A.G.; Rothery, P.; Tarling, G.A.; Pond, D.W.; Korb, R.E.; Murphy, E.J.; Watkins, J.L. Natural growth rates in Antarctic krill (Euphausia superba): II. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnol. Oceanogr. 2006, 51, 973–987. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G. Habitat of Antarctic krill (Euphausia superba) in the Bransfield Straitbased on ensembled species distribution model. J. Fish. China 2022, 46, 390–401. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Zhu, G. Habitat suitability of Antarctic krill (Euphausia superba) in the Antarctic Peninsula. J. Fish. China 2024, 48, 48–57. [Google Scholar] [CrossRef]
- Cresswell, K.A.; Tarling, G.A.; Thorpe, S.E.; Burrows, M.T.; Wiedenmann, J.; Mangel, M. Diel vertical migration of Antarctic krill (Euphausia superba) is flexible during advection across the Scotia Sea. J. Plankton Res. 2009, 31, 1265–1281. [Google Scholar] [CrossRef]
- Zhao, G.; Qiu, S.; Huang, H.; Li, L.; Qiu, T. Effects of abrupt and gradual changes in salinity on survival status and molting of Euphausia superba. Mar. Fish. 2018, 40, 360–367. [Google Scholar] [CrossRef]
- Taconet, M.; Kroodsma, D.; Fernandes, J.A. Global Atlas of AIS-Based Fishing Activity—Challenges and Opportunities; ResearchGate; FAO: Rome, Italy, 2019; Available online: https://openknowledge.fao.org/handle/20.500.14283/ca7012en (accessed on 22 May 2025).
- Wang, W.; Liu, Y.; Ning, X. Development Status and Trend of Antarctic Krill Factory Trawler and Equipment. Ship Eng. 2020, 42, 33–39+93. [Google Scholar] [CrossRef]
- Last, P.; Bahlke, C.; Hering-Bertram, M.; Linsen, L. Comprehensive analysis of automatic identification system (AIS) data in regard to vessel movement prediction. J. Navig. 2014, 67, 791–809. [Google Scholar] [CrossRef]
- Deng, R.; Dichmont, C.; Milton, D.; Haywood, M.; Vance, D.; Hall, N.; Die, D. Can vessel monitoring system data also be used to study trawling intensity and population depletion? The example of Australia’s northern prawn fishery. Can. J. Fish. Aquat. Sci. 2005, 62, 611–622. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, F.; Jin, S.; Wu, Y.; Xu, L.; Dai, Y. Trawler state and net times extraction based on data from Beidou vessel monitoring system. Fish. Inf. Strategy 2015, 30, 205–211. [Google Scholar] [CrossRef]
- Cui, X.; Tang, F.; Zhang, H.; Wu, Y.; Fan, W. The Establishment of Northwest Pacific Ommastrephes bartramii Fishing Ground Forecasting Model Based on Naive Bayes Method. Period. Ocean Univ. China 2015, 45, 37–43. [Google Scholar] [CrossRef]
- Chuaysi, B.; Kiattisin, S. Fishing vessels behavior identification for combating IUU fishing: Enable traceability at sea. Wirel. Pers. Commun. 2020, 115, 2971–2993. [Google Scholar] [CrossRef]
- Su, B.; He, R.; Zhao, G.; Jiang, P.; Li, Y.; Shang, C.; Han, H.; Shen, L.; Zhang, H. Extraction of operational status features of Antarctic krill midwatertrawlers based on vessel position data. Mar. Fish. 2024, 1–26. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Brunner, A.; Márquez, J.R.G.; Domisch, S. Downscaling future land cover scenarios for freshwater fish distribution models under climate change. Limnologica 2024, 104, 126139. [Google Scholar] [CrossRef]
- Xiang, D.; Sun, Y.; Zhu, H.; Wang, J.; Huang, S.; Han, H.; Zhang, S.; Shang, C.; Zhang, H. Prediction of the Relative Resource Abundance of the Argentine Shortfin Squid Illex argentinus in the High Sea in the Southwest Atlantic Based on a Deep Learning Model. Animals 2024, 14, 3106. [Google Scholar] [CrossRef]
- Xie, E.; Wu, Q.; Zhou, Y.; Zhang, S.; Feng, F. Extraction and verification of operational state characteristics of light shield net vessels based on Beidou vessel position data. J. Shanghai Ocean Univ. 2020, 29, 392–400. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Paradinas, I.; Illian, J.B.; Alonso-Fernändez, A.; Pennino, M.G.; Smout, S. Combining fishery data through integrated species distribution models. ICES J. Mar. Sci. 2023, 80, 2579–2590. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Jiang, K.; Xiang, D.; Shi, Y.; Huang, S.; Li, Y.; Han, H. Simulating the changes of the habitats suitability of Chub mackerel (Scomber japonicus) in the high seas of the North Pacific Ocean using ensemble models under medium to long-term future climate scenarios. Mar. Pollut. Bull. 2024, 207, 116873. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shan, X.; Gorfine, H.; Dai, F.; Wu, Q.; Yang, T.; Shi, Y.; Jin, X. Ensemble projections of fish distribution in response to climate changes in the Yellow and Bohai Seas, China. Ecol. Indic. 2023, 146, 109759. [Google Scholar] [CrossRef]
- KMN, M.N.; Sreenath, K.; Sreeram, M.P. Change in habitat suitability of the invasive Snowflake coral (Carijoa riisei) during climate change: An ensemble modelling approach. Ecol. Inform. 2023, 76, 102145. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Han, Z. Predicting the effects of climate change on the suitable habitat of Japanese Spanish mackerel (Scomberomorus niphonius) based on the species distribution model. Front. Mar. Sci. 2022, 9, 927790. [Google Scholar] [CrossRef]
- Xu, C.; Xue, Z.; Jiang, M.; Lyu, X.; Zou, Y.; Gao, Y.; Sun, X.; Wang, D.; Li, R. Simulating potential impacts of climate change on the habitats and carbon benefits of mangroves in China. Glob. Ecol. Conserv. 2024, 54, e03048. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, W.; Tang, F. The construction of habitat suitability index forecast model of Ommastrephes bartramii fishing ground based on constrained linear regression. Prog. Fish. Sci. 2018, 39, 64–72. [Google Scholar] [CrossRef]
- Box, J.F. Guinness, Gosset, Fisher, and Small Samples. Stat. Sci. 1987, 2, 45–52. [Google Scholar] [CrossRef]
- Gao, J.; Su, Q. Verification and Discussion on Fractal Model and the General Pattern on Species Abundance in Community. Adv. Earth Sci. 2021, 36, 625–631. [Google Scholar] [CrossRef]
- Atkinson, A.; Siegel, V.; Pakhomov, E.; Rothery, P.; Loeb, V.; Ross, R.; Quetin, L.; Schmidt, K.; Fretwell, P.; Murphy, E. Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 2008, 362, 1–23. [Google Scholar] [CrossRef]
- Perry, F.A.; Atkinson, A.; Sailley, S.F.; Tarling, G.A.; Hill, S.L.; Lucas, C.H.; Mayor, D.J. Habitat partitioning in Antarctic krill: Spawning hotspots and nursery areas. PLoS ONE 2019, 14, e0219325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Luo, J.; Tang, F.; Fan, W.; Song, X.; Yang, C.; Zhang, H. Temporal and Spatial Distribution of Antarctic Krill in 48 Fishing Areas Based on Fishery Data. Prog. Fish. Sci. 2022, 43, 81–92. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H.; Fan, W.; Shi, H.; Fei, Y.; Yuan, S. Behaviour Impact Analysis of Tuna Purse Seiners in the Western and Central Pacific Based on the BRT and GAM Models. Front. Mar. Sci. 2022, 9, 881036. [Google Scholar] [CrossRef]
- Liu, Y.X.; Li, M.L.; Fang, H.; Le, L.; Shu, W. Resources Status and Ecosystem Function in Antarctic krill. Chin. J. Fish. 2019, 32, 55–60. [Google Scholar]
- Huang, H.; Chen, X.; Liu, J.; Li, L.; Wu, Y.; Yang, J.; Zhou, B.; Qi, G.; Chen, S.; Xu, G. Analysis of the status and trend of the antarctic krill fishery. Chin. J. Polar Res. 2015, 27, 25–30. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Chang, Y.; Lee, M.-A.; Wu, R.-F.; Hsiao, S.-C. Predicting skipjack tuna fishing grounds in the Western and Central Pacific Ocean based on high-spatial-temporal-resolution satellite data. Remote Sens. 2021, 13, 861. [Google Scholar] [CrossRef]
- Yang, W.J.; Xu, L.X. A review:research progress on environmental factors affecting resource distribution of Antarctic krill. J. Dalian Ocean Univ. 2014, 29, 316–322. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, G. Spatial-temporal variation on Euphausia superba fishing ground in the northern Antarctic Peninsula based on point pattern model. J. Fish. China 2018, 42, 356–365. [Google Scholar] [CrossRef]
- Klevjer, T.A.; Tarling, G.A.; Fielding, S. Swarm characteristics of Antarctic krill Euphausia superba relative to the proximity of land during summer in the Scotia Sea. Mar. Ecol. Prog. Ser. 2010, 409, 157–170. [Google Scholar] [CrossRef]
- Suqing, X.; Liqi, C. Using satellite data to analyze the interannual distribution characteristics of chlorophyll, temperature, and salinity in the surface waters of the Southern Ocean during summer. Prog. Nat. Sci. 2009, 19, 760–767. [Google Scholar]
- Yang, G.; Li, C.; Zhang, Y.; Liu, Q. Vertical distribution of zooplankton community in South Shetland islands, antarctica, during austral summer. Chin. J. Polar Res. 2015, 27, 17–24. [Google Scholar] [CrossRef]
- Zhou, G. Effects of temporal and environmental factors on the fishing ground of Antarctic krill (Euphausia superba) in the northern Antarctic Peninsula based on generalized additive model. J. Fish. China 2012, 36, 1863–1871. [Google Scholar] [CrossRef]
- Chen, F.; Chen, X.J.; Liu, B.L.; Zhu, G.; Xu, L. Effect of sea ice on the abundance index of Antarctic krill euphausua superba. Oceanol. Limnol. Sin. 2011, 42, 495–499. [Google Scholar]
- Brierley, A.S.; Fernandes, P.G.; Brandon, M.A.; Armstrong, F.; Millard, N.W.; McPhail, S.D.; Stevenson, P.; Pebody, M.; Perrett, J.; Squires, M. Antarctic krill under sea ice: Elevated abundance in a narrow band just south of ice edge. Science 2002, 295, 1890–1892. [Google Scholar] [CrossRef]
- Siegel, V.; Reiss, C.S.; Dietrich, K.S.; Haraldsson, M.; Rohardt, G. Distribution and abundance of Antarctic krill (Euphausia superba) along the Antarctic Peninsula. Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 77, 63–74. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X. Influences of photosynthetically active radiation on abundance and distribution of jumbo flying squid Dosidicus gigas in the Southeast Pacific Ocean off Peru. Acta Oceanol. Sin. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Lian, X.; Tan, Y.; Liu, Y.; Huang, L.; Chen, Q.; Zhou, L. Comparison of capture efficiency for zooplankton in the northern South China Sea, using two plankton mesh sizes. J. Trop. Oceanogr. 2013, 32, 33–39. [Google Scholar] [CrossRef]
- Melbourne-Thomas, J.; Corney, S.; Trebilco, R.; Meiners, K.; Stevens, R.; Kawaguchi, S.; Sumner, M.; Constable, A. Under ice habitats for Antarctic krill larvae: Could less mean more under climate warming? Geophys. Res. 2016, 43, 310,322–310,327. [Google Scholar] [CrossRef]
- Li, S.; Zhao, G.; Yang, J.; Li, L.; Rao, X.; Huang, H. Aggregation characteristics of fishable Antarctic krill population and their influences on fishery CPUE. Mar. Fish. 2023, 45, 691–698. [Google Scholar] [CrossRef]
- Lee, J.; South, A.B.; Jennings, S. Developing reliable, repeatable, and accessible methods to provide high-resolution estimates of fishing-effort distributions from vessel monitoring system (VMS) data. ICES J. Mar. Sci. 2010, 67, 1260–1271. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, S.; Dai, Y.; Fan, W.; Huang, H. Algorithm of fishing effort extraction in trawling based on Beidou vessel monitoring system data. J. Fish. China 2014, 38, 1190–1199. [Google Scholar] [CrossRef]
| Month | GLD (km) | CHL (mg/m3) | SSH (m) | SST (℃) | SSS (‰) | SIC(%) |
|---|---|---|---|---|---|---|
| 2020.12 | 0.84~44.93 (43.24) | 0.59~3.83 (0.83) | −3.15~−3.05 (−3.13) | −0.59~1.12 (0.39) | 33.51~34.16 (33.88) | 0~0.01 (0) |
| 2021.01 | 0.57~44.85 (2.48) | 0.57~3.77 (2.91) | −3.14~−3.03 (−3.04) | 0.04~1.05 (0.48) | 33.54~33.99 (33.84) | 0~0 (0) |
| 2021.02 | 2.29~2.54 (2.4) | 0.45~2.13 (1.87) | −3.16~−3.14 (−3.15) | 0.67~1.32 (0.83) | 33.44~33.65 (33.53) | 0~0 (0) |
| 2021.03 | 0.21~44.17 (1.34) | 0.57~3.73 (3.06) | −3.18~−3.04 (−3.06) | −0.51~0.87 (−0.16) | 33.48~33.89 (33.81) | 0~0 (0) |
| 2021.04 | 0.04~4.86 (0.76) | 1.21~2.38 (2.05) | −3.1~−3.06 (−3.07) | −1.28~0.37 (−1.08) | 33.76~33.88 (33.83) | 0.01~1.4 (0.38) |
| 2021.05 | 0.21~44.37 (1.18) | 0.36~0.94 (0.85) | −3.19~−3.03 (−3.04) | −1.64~−0.54 (−1.53) | 33.64~34.06 (33.83) | 0.16~15.89 (4.64) |
| 2021.06 | 1.26~45.48 (44.25) | 0.24~0.42 (0.29) | −3.18~−2.76 (−3.18) | −1.81~1.36 (−1.63) | 33.79~34.19 (33.85) | 0~67.69 (12.13) |
| 2021.07 | 38.04~44.93 (43.96) | 0.23~0.33 (0.3) | −3.19~−3.06 (−3.11) | −1.81~−0.34 (−0.73) | 33.98~34.22 (34.1) | 3.28~58.06 (7.44) |
| 2021.08 | 33.85~44.93 (44.12) | 0.19~0.6 (0.42) | −3.23~−3.07 (−3.19) | −1.79~−0.57 (−1.61) | 34.02~34.29 (34.21) | 1.53~75.92 (27.73) |
| 2021.09 | 35.24~44.85 (44.46) | 0.7~1.13 (1.06) | −3.24~−3.08 (−3.21) | −1.77~−0.49 (−1.58) | 34.05~34.39 (34.26) | 0~36.37 (5.04) |
| 2021.10 | 43.75~44.85 (44.66) | 1.9~2.15 (2.13) | −3.22~−3.22 (−3.22) | −1.27~−1.07 (−1.21) | 34.29~34.31 (34.3) | 0~0 (0) |
| 2021.11 | 42.35~44.3 (43.32) | 0.81~1.18 (1.04) | −3.24~−3.23 (−3.24) | −0.37~−0.33 (−0.35) | 34.29~34.31 (34.29) | 0~0 (0) |
| 2021.12 | 37.97~44.93 (43.68) | 0.55~1.16 (1) | −3.27~−3.25 (−3.26) | 0.48~0.98 (0.59) | 34.23~34.33 (34.3) | 0~0 (0) |
| 2022.01 | 39.84~45.16 (44.65) | 0.63~1.74 (1.18) | −3.29~−3.26 (−3.27) | 1.15~1.97 (1.23) | 34.15~34.22 (34.19) | 0~0 (0) |
| 2022.02 | 39.84~45.16 (44.65) | 0.53~2.68 (1.42) | −3.3~−3.26 (−3.28) | 1.3~1.98 (1.31) | 34.02~34.11 (34.09) | 0~0 (0) |
| 2022.03 | 0.04~45.26 (44.06) | 0.51~3.31 (1.69) | −3.3~−3.19 (−3.29) | −0.22~1.9 (1) | 33.94~34.06 (34.04) | 0~0.01 (0) |
| 2022.04 | 0.04~44.93 (1.4) | 0.59~2.38 (1.69) | −3.29~−3.15 (−3.21) | −1.23~0.38 (−0.77) | 33.8~34.49 (34.3) | 0.01~4.66 (0.35) |
| 2022.05 | 0.04~4.94 (0.43) | 0.44~0.88 (0.76) | −3.23~−3.14 (−3.16) | −1.68~−0.26 (−1.23) | 33.95~34.51 (34.08) | 0.36~30.27 (9.46) |
| 2022.06 | 0.26~5.88 (1.63) | 0.17~0.33 (0.27) | −3.24~−3.17 (−3.21) | −1.78~−0.73 (−1.75) | 34.17~34.56 (34.3) | 0.14~34.83 (24.17) |
| 2022.07 | 0.05~44.85 (0.22) | 0.21~0.82 (0.65) | −3.34~−2.92 (−2.92) | −1.72~0.97 (0.71) | 33.85~34.14 (33.88) | 0~52.68 (0) |
| 2022.08 | 0.01~6.71 (1.77) | 0.66~1.53 (0.8) | −2.96~−2.92 (−2.93) | 0.37~0.76 (0.59) | 33.83~33.96 (33.88) | 0~0 (0) |
| 2022.09 | 0.05~6.34 (2.35) | 1.19~1.82 (1.26) | −2.95~−2.9 (−2.91) | 0.43~0.62 (0.58) | 33.89~33.99 (33.9) | 0~0 (0) |
| 2022.12 | 36.81~44.99 (43.11) | 0.36~1.92 (1.16) | −3.39~−3.36 (−3.37) | 0.21~0.87 (0.39) | 34.13~34.23 (34.18) | 0~0 (0) |
| 2023.01 | 39.04~45.16 (43.28) | 0.34~1.82 (1.82) | −3.38~−3.36 (−3.37) | 0.94~1.64 (0.99) | 33.84~34.09 (34) | 0~0 (0) |
| 2023.02 | 37.83~43.92 (43.05) | 0.7~2.12 (1.35) | −3.38~−3.35 (−3.36) | 1.18~1.45 (1.4) | 33.68~33.98 (33.69) | 0~0 (0) |
| 2023.03 | 0.04~44.67 (42.59) | 0.4~2.75 (1.88) | −3.39~−3.29 (−3.39) | −0.14~1.22 (1.12) | 33.45~34.42 (33.74) | 0~0 (0) |
| 2023.04 | 0.45~11.64 (2.45) | 0.93~2.64 (2.16) | −3.32~−3.26 (−3.29) | −0.98~0.02 (−0.54) | 33.61~34.27 (33.82) | 0.02~2.54 (0.11) |
| 2023.05 | 0.04~7.56 (0.35) | 0.55~1.07 (0.85) | −3.41~−3.32 (−3.34) | −1.59~−0.5 (−1.43) | 33.75~34.62 (34.15) | 0.07~7.74 (2.58) |
| 2023.06 | 0.05~45.26 (44.85) | 0.22~1.19 (0.25) | −3.43~−2.98 (−3.41) | −1.74~2.11 (−0.94) | 33.75~34.14 (33.79) | 0~48.99 (8.56) |
| 2023.07 | 0.05~3.81 (2.04) | 0.47~1.01 (0.51) | −3.02~−2.98 (−3) | 0.67~1.29 (1.03) | 33.75~33.82 (33.8) | 0~0 (0) |
| 2023.08 | 0.05~3.71 (1.11) | 0.68~1.51 (0.77) | −3.08~−3.02 (−3.03) | 0.18~0.71 (0.58) | 33.72~33.94 (33.83) | 0~0.01 (0) |
| 2023.09 | 0.05~3.38 (2.06) | 1.05~1.99 (1.36) | −3.08~−3.03 (−3.04) | 0.25~0.37 (0.34) | 33.83~33.95 (33.88) | 0~0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Sun, Y.; Zhu, H.; Xiang, D.; Wang, J.; Zhang, F.; Huang, S.; Li, Y. Simulation and Identification of the Habitat of Antarctic Krill Based on Vessel Position Data and Integrated Species Distribution Model: A Case Study of Pumping-Suction Beam Trawl Fishing Vessels. Animals 2025, 15, 1557. https://doi.org/10.3390/ani15111557
Zhang H, Sun Y, Zhu H, Xiang D, Wang J, Zhang F, Huang S, Li Y. Simulation and Identification of the Habitat of Antarctic Krill Based on Vessel Position Data and Integrated Species Distribution Model: A Case Study of Pumping-Suction Beam Trawl Fishing Vessels. Animals. 2025; 15(11):1557. https://doi.org/10.3390/ani15111557
Chicago/Turabian StyleZhang, Heng, Yuyan Sun, Hanji Zhu, Delong Xiang, Jianhua Wang, Famou Zhang, Sisi Huang, and Yang Li. 2025. "Simulation and Identification of the Habitat of Antarctic Krill Based on Vessel Position Data and Integrated Species Distribution Model: A Case Study of Pumping-Suction Beam Trawl Fishing Vessels" Animals 15, no. 11: 1557. https://doi.org/10.3390/ani15111557
APA StyleZhang, H., Sun, Y., Zhu, H., Xiang, D., Wang, J., Zhang, F., Huang, S., & Li, Y. (2025). Simulation and Identification of the Habitat of Antarctic Krill Based on Vessel Position Data and Integrated Species Distribution Model: A Case Study of Pumping-Suction Beam Trawl Fishing Vessels. Animals, 15(11), 1557. https://doi.org/10.3390/ani15111557






