Effects of Hematological Parameters and Plasma Components of Starry Flounder, Platichthys stellatus, by Waterborne Copper Exposure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Experimental Environment
2.2. Lethal Concentration (LC50)
2.3. Hematological Characteristics
2.4. Plasma Components
2.5. Statistical Analysis Method
2.6. Ethics Approval and Consent to Participate
3. Results
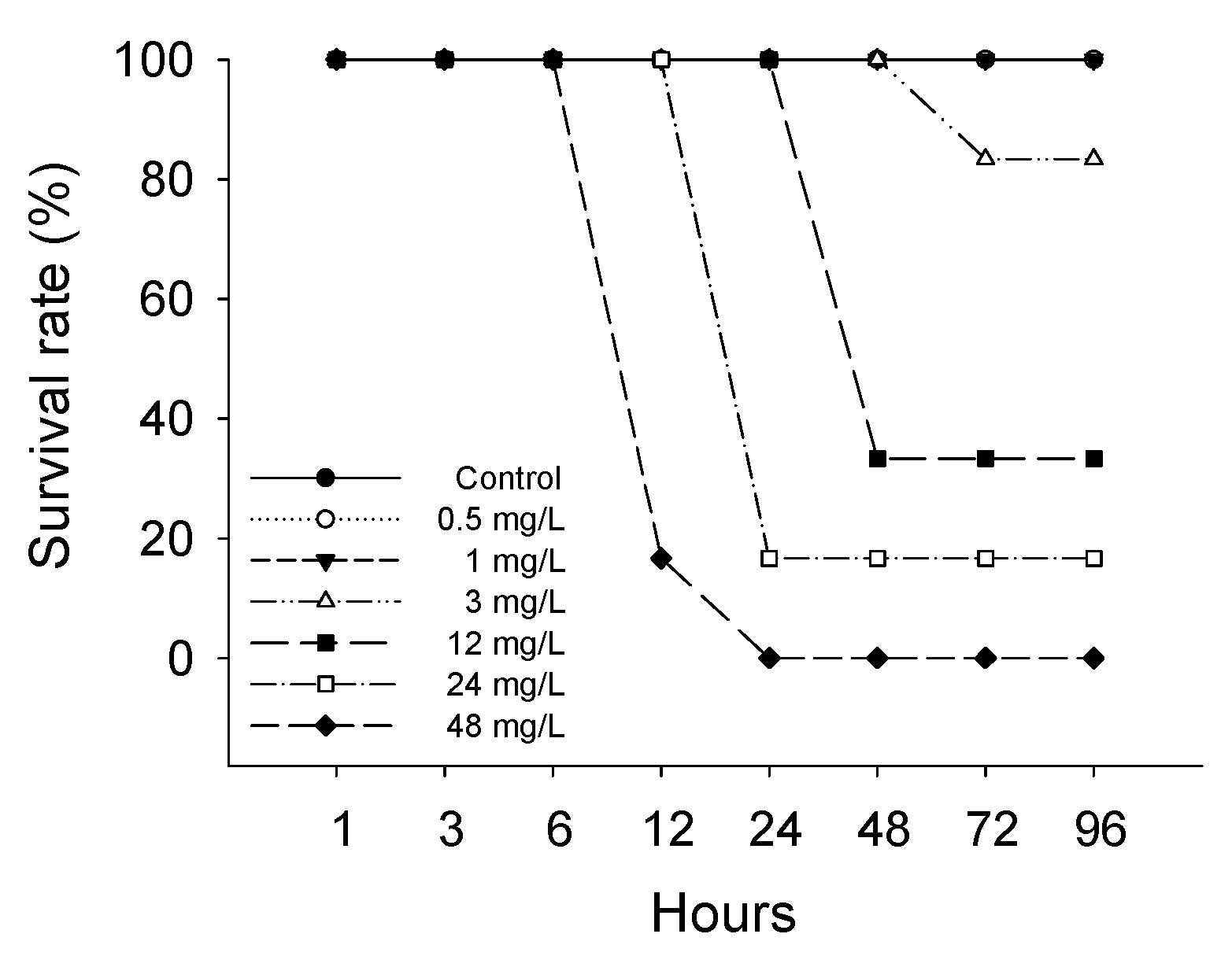
3.1. Survival Rate and Lethal Concentration (LC50)
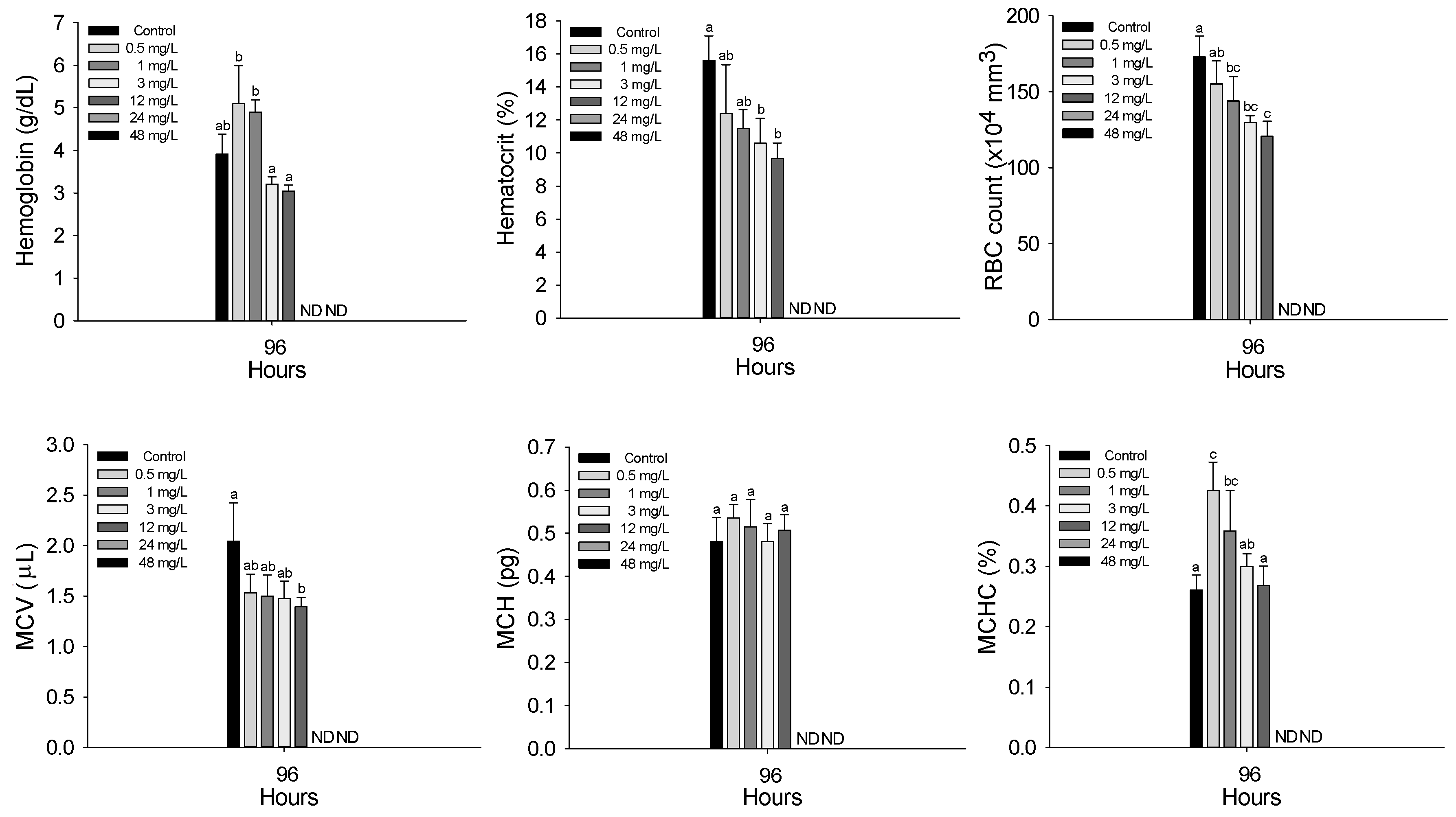
3.2. Hematological Parameters
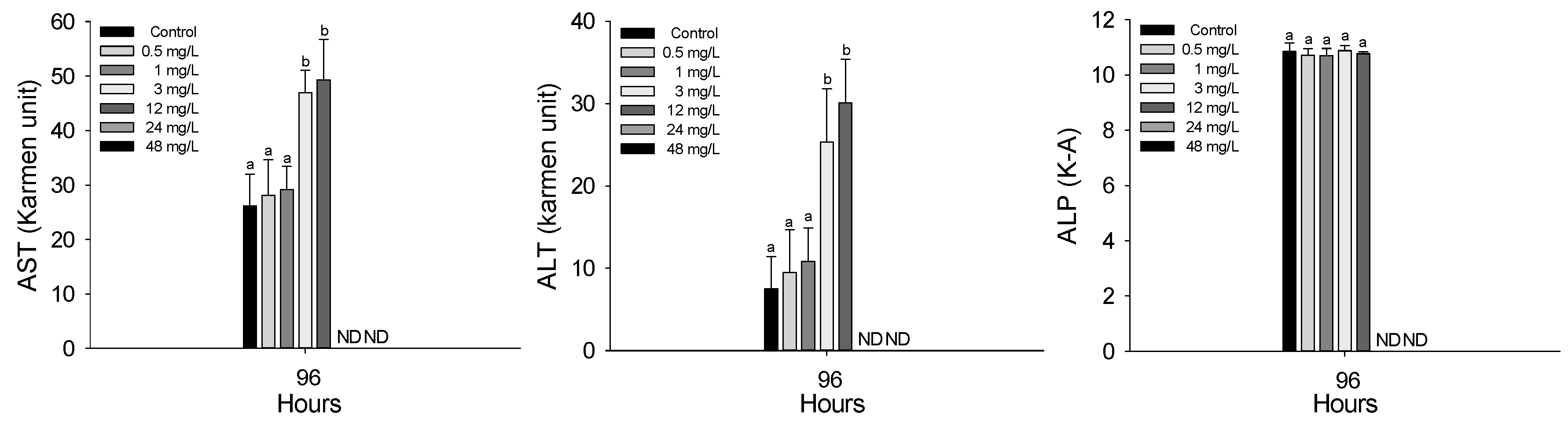
3.3. Plasma Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, S.W.; Cachot, J.; Gourves, P.Y.; Clérandeau, C.; Morin, B.; Gonzalez, P. Sub-lethal effects of waterborne copper in early developmental stages of rainbow trout (Oncorhynchus mykiss). Ecotoxicol. Environ. Saf. 2019, 170, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Fernandes, A.; Ferreira-Cardoso, J.V.; Garcia-Santos, S.; Monteiro, S.M.; Carrola, J.; Matos, P.; Fontaínhas-Fernandes, A. Histopathological changes in liver and gill epithelium of Nile tilapia (Oreochromis niloticus), exposed to waterborne copper. Pesqui. Vet. Bras. 2007, 27, 103–109. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Isani, G.; Andreani, G.; Carpenè, E.; Di Molfetta, S.; Eletto, D.; Spisni, E. Effects of waterborne Cu exposure in gilthead sea bream (Sparus aurata): A proteomic approach. Fish Shellfish Immunol. 2011, 31, 1051–1058. [Google Scholar] [CrossRef]
- Doria, H.B.; Ferreira, M.B.; Rodrigues, S.D.; Lo, S.M.; Domingues, C.E.; Nakao, L.S.; de Campos, S.X.; de Oliveira Ribeiro, C.A.; Randi, M.A.F. Time does matter! Acute copper exposure abolishes rhythmicity of clock gene in Danio rerio. Ecotoxicol. Environ. Saf. 2018, 155, 26–36. [Google Scholar] [CrossRef]
- Zhang, L.H.; Luo, Z.; Song, Y.F.; Shi, X.; Pan, Y.X.; Fan, Y.F.; Xu, Y.H. Effects and mechanisms of waterborne copper exposure influencing ovary development and related hormone secretion in yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 2016, 178, 88–98. [Google Scholar] [CrossRef]
- Afaghi, A.; Zare, S. Effects of exposure to sub-lethal concentrations of copper on hematological and histopathological alterations in common carp, Cyprinus carpio. Arch. Adv. Biosci. 2020, 11, 26–33. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Gao, C.; Wang, C.; Yan, Y.; Zhou, F. Dietary copper for fish: Homeostasis, nutritional functions, toxicity, and affecting factors. Aquaculture 2024, 587, 740875. [Google Scholar] [CrossRef]
- Bakhshalizadeh, S.; Mora-Medina, R.; Fazio, F.; Parrino, V.; Ayala-Soldado, N. Determination of the heavy metal bioaccumulation patterns in muscles of two species of mullets from the Southern Caspian Sea. Animals 2022, 12, 2819. [Google Scholar] [CrossRef]
- Tesser, M.E.; de Paula, A.A.; Risso, W.E.; Monteiro, R.A.; Santo Pereira, A.D.E.; Fraceto, L.F.; dos Reis Martinez, C.B. Sublethal effects of waterborne copper and copper nanoparticles on the freshwater Neotropical teleost Prochilodus lineatus: A comparative approach. Sci. Total Environ. 2020, 704, 135332. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, B.; Liu, Y.; Bai, Y.; Yang, X.; Xu, S. Metabolic responses of golden trout (Oncorhynchus mykiss aguabonita) after acute exposure to waterborne copper. Aquat. Toxicol. 2022, 249, 106236. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Parvez, S.; Ansari, R.A.; Ali, M.; Kaur, M.; Hayat, F.; Ahmad, F.; Raisuddin, S. Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chem. Biol. Interact. 2008, 174, 183–192. [Google Scholar] [CrossRef]
- Molayemraftar, T.; Peyghan, R.; Jalali, M.R.; Shahriari, A. Single and combined effects of ammonia and nitrite on common carp, Cyprinus carpio: Toxicity, hematological parameters, antioxidant defenses, acetylcholinesterase, and acid phosphatase activities. Aquaculture 2022, 548, 737676. [Google Scholar] [CrossRef]
- Rufli, H. Introduction of moribund category to OECD fish acute test and its effect on suffering and LC50 values. Environ. Toxicol. Chem. 2012, 31, 1107–1112. [Google Scholar] [CrossRef]
- Sanchez, W.; Palluel, O.; Meunier, L.; Coquery, M.; Porcher, J.M.; Ait-Aissa, S. Copper-induced oxidative stress in three-spined stickleback: Relationship with hepatic metal levels. Environ. Toxicol. Pharmacol. 2005, 19, 177–183. [Google Scholar] [CrossRef]
- Gu, P.; Li, Q.; Zhang, W.; Gao, Y.; Sun, K.; Zhou, L.; Zheng, Z. Biological toxicity of fresh and rotten algae on freshwater fish: LC50, organ damage, and antioxidant response. J. Hazard. Mater. 2021, 407, 124620. [Google Scholar] [CrossRef]
- Park, S.W.; An, S.M.; Jo, A.H.; Kim, J.H. Effects of lethal concentration, hematological parameters and plasma components of common carp, Cyprinus carpio by waterborne acute nitrite exposure. J. Fish Pathol. 2023, 36, 349–360. [Google Scholar] [CrossRef]
- Sampaio, F.G.; de Lima Boijink, C.; Oba, E.T.; dos Santos, L.R.B.; Kalinin, A.L.; Rantin, F.T. Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 43–51. [Google Scholar] [CrossRef]
- Thangam, Y.; Jayaprakash, S.; Perumayee, M. Effect of copper toxicity on hematological parameters to freshwater fish Cyprinus carpio (common carp). J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 50–60. [Google Scholar]
- Fazio, F.; Saoca, C.; Costa, G.; Zumbo, A.; Piccione, G.; Parrino, V. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture 2019, 513, 734398. [Google Scholar] [CrossRef]
- Hwang, I.K.; Kim, K.W.; Kim, J.H.; Kang, J.C. Toxic effects and depuration after the dietary lead (II) exposure on the bioaccumulation and hematological parameters in starry flounder (Platichthys stellatus). Environ. Toxicol. Pharmacol. 2016, 45, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Lee, J.H.; Kim, W.J.; Kim, H.C. Morphological specificity in cultured starry flounder Platichthys stellatus reared in artificial facility. Fish Aquat. Sci. 2012, 15, 117–123. [Google Scholar] [CrossRef]
- Jeong, J.; Tongjoo, S.U.H. Regional Specialization and Economic Impacts of Aquaculture in Korea: An Analysis of Key Species. Asian J. Bus. Environ. 2024, 14, 27–39. [Google Scholar] [CrossRef]
- de Sousa Miranda, D.H.; Maltez, L.C.; Santo Campello, M.E.; Córdova, J.F.L.; Rodrigues, R.V.; Sampaio, L.A.; Okamoto, M.H. Acute toxicity and sublethal effects of nitrite on oxidative stress in early juvenile Brazilian flounder, Paralichthys orbignyanus. Aquac. Res. 2022, 53, 1939–1946. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Fernandes, L.F.L.; Bianchini, A.; Chippari-Gomes, A.R.; Silva, B.F.; Brandão, G.P.; Gomes, L.C. Acute copper toxicity in juvenile fat snook Centropomus parallelus (Teleostei: Centropomidae) in sea water. Neotrop. Ichthyol. 2014, 12, 845–852. [Google Scholar] [CrossRef]
- Alkobaby, A.I.; Abd El-Wahed, R.K. The acute toxicity of copper to Nile tilapia (Oreochromis niloticus) fingerlings and its effects on gill and liver histology. J. Aquac. Res. Dev. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Oliva, M.; Garrido, M.C.; Márquez, D.S.; de Canales, M.G. Sublethal and lethal toxicity in juvenile Senegal sole (Solea senegalensis) exposed to copper: A preliminary toxicity range-finding test. Exp. Toxicol. Pathol. 2009, 61, 113–121. [Google Scholar] [CrossRef]
- De Boeck, G.; Meeus, W.; De Coen, W.; Blust, R. Tissue-specific Cu bioaccumulation patterns and differences in sensitivity to waterborne Cu in three freshwater fish: Rainbow trout (Oncorhynchus mykiss), common carp (Cyprinus carpio), and gibel carp (Carassius auratus gibelio). Aquat. Toxicol. 2004, 70, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mazandarani, M.; Hoseini, S.M. Anaemia and plasma lipid profile in common carp (Cyprinus carpio) exposed to ambient copper sulfate and nanoscale copper oxide. Aquac. Res. 2017, 48, 844–852. [Google Scholar] [CrossRef]
- Van Vuren, J.H.J.; Van der Merwe, M.; Du Preez, H.H. The effect of copper on the blood chemistry of Clarias gariepinus (Clariidae). Ecotoxicol. Environ. Saf. 1994, 29, 187–199. [Google Scholar] [CrossRef]
- Simonato, J.D.; Mela, M.; Doria, H.B.; Guiloski, I.C.; Randi, M.A.; Carvalho, P.S.; Martinez, C.B. Biomarkers of waterborne copper exposure in the Neotropical fish Prochilodus lineatus. Aquat. Toxicol. 2016, 170, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Abdullah, S.; Afzal, M.; Hussain, M. Assessment of acute metals toxicity in Catla catla through hematological and biochemical blood markers. Pak. J. Agric. Sci. 2018, 55, 449–454. [Google Scholar] [CrossRef]
- Al-Tamimi, A.H.; Al-Azzawi, A.J. The acute and chronic toxicity of copper on the behavioral responses and hematological parameters of freshwater fish, common carp (Cyprinus carpio L.). Iraqi J. Sci. 2015, 56, 2835–2845. [Google Scholar]
- Sawsan, H.A.; Amira, H.M.; Mostafa, M.B.; Nashaat, A.M.M. Hematological and serum biochemical studies in fresh water fish exposed to acute and chronic copper and mercury toxicity. J. Fish Pathol. 2017, 30, 25–39. [Google Scholar] [CrossRef]
- Naz, S.; Hussain, R.; Guangbin, Z.; Chatha, A.M.M.; Rehman, Z.U.; Jahan, S.; Liaquat, M.; Khan, A. Copper sulfate induces clinico-hematological, oxidative stress, serum biochemical, and histopathological changes in freshwater fish Labeo rohita. Front. Vet. Sci. 2023, 10, 1142042. [Google Scholar] [CrossRef] [PubMed]
- Canli, E.G.; Canli, M. Low water conductivity increases the effects of copper on the serum parameters in fish (Oreochromis niloticus). Environ. Toxicol. Pharmacol. 2015, 39, 606–613. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Ferreira, J.; Affonso, E.; Ono, E.; Martins, M. Toxicity and effects of copper sulfate on parasitic control and hematological response of tambaqui Colossoma macropomum. Bol. Inst. Pesca 2011, 37, 355–365. [Google Scholar]
- Baeck, S.; Min, E.; Kang, J.C. Combined effects of copper and temperature on hematological constituents in the rock fish, Sebastes schlegeli. J. Fish Pathol. 2014, 27, 57–65. [Google Scholar] [CrossRef]
- Eyckmans, M.; Tudorache, C.; Darras, V.M.; Blust, R.; De Boeck, G. Hormonal and ion regulatory response in three freshwater fish species following waterborne copper exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 270–278. [Google Scholar] [CrossRef]
- Latif, A.; Khalid, M.; Ali, M. Evaluation of toxic stress of copper sulphate and lead nitrate on hematological and serum biochemical characteristics of freshwater cyprinid (Labeo rohita). Int. J. Eng. Tech. 2014, 4, 366–372. [Google Scholar]
- Monteiro, S.M.; Mancera, J.M.; Fontaínhas-Fernandes, A.; Sousa, M. Copper induced alterations of biochemical parameters in the gill and plasma of Oreochromis niloticus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 375–383. [Google Scholar] [CrossRef]
- Heydarnejad, M.S.; Khosravian-hemami, M.; Nematollahi, A.; Rahnama, S. Effects of copper at sublethal concentrations on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Int. Rev. Hydrobiol. 2013, 98, 71–79. [Google Scholar] [CrossRef]
- Atli, G.; Ariyurek, S.Y.; Kanak, E.G.; Canli, M. Alterations in the serum biomarkers belonging to different metabolic systems of fish (Oreochromis niloticus) after Cd and Pb exposures. Environ. Toxicol. Pharmacol. 2015, 40, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Öner, M.; Atli, G.; Canli, M. Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ. Toxicol. Chem. 2008, 27, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Barad, V.S.; Kulkarni, R.S. Hematological changes induced by short-term exposure to copper in the Indian freshwater fish, Notopterus notopterus (Pallas). Bioscan 2010, 5, 313–316. [Google Scholar]
- Lee, J.H.; Kang, J.C.; Kim, J.H. Toxic effects of microplastic (Polyethylene) on fish: Accumulation, hematological parameters and antioxidant responses in Korean Bullhead, Pseudobagrus fulvidraco. Sci. Total Environ. 2023, 877, 162874. [Google Scholar] [CrossRef]
- Vutukuru, S.S.; Suma, C.H.; Madhavi, K.R.; Juveria, J.; Pauleena, J.S.; Rao, J.V.; Anjaneyulu, Y. Studies on the development of potential biomarkers for rapid assessment of copper toxicity to freshwater fish using Esomus danricus as model. Int. J. Environ. Res. Public Health 2005, 2, 63–73. [Google Scholar] [CrossRef]
- Kim, S.G.; Kang, J.C. Effect of dietary copper exposure on accumulation, growth and hematological parameters of the juvenile rockfish, Sebastes schlegeli. Mar. Environ. Res. 2004, 58, 65–82. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Rajabiesterabadi, H.; Kordrostami, S. Chronic exposure of Rutilus rutilus caspicus fingerlings to ambient copper: Effects on food intake, growth performance, biochemistry and stress resistance. Toxicol. Ind. Health 2016, 32, 375–383. [Google Scholar] [CrossRef]
- Kavitha, C.; Malarvizhi, A.; Kumaran, S.S.; Ramesh, M. Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem. Toxicol. 2010, 48, 2848–2854. [Google Scholar] [CrossRef]
- Vutukuru, S.S.; Arun Prabhath, N.; Raghavender, M.; Yerramilli, A. Effect of arsenic and chromium on the serum amino-transferases activity in Indian major carp, Labeo rohita. Int. J. Environ. Res. Public Health 2007, 4, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Fırat, Ö.; Coğun, H.Y.; Yüzereroğlu, T.A.; Gök, G.; Fırat, Ö.; Kargın, F.; Kötemen, Y. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) on serum biochemistry of Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2011, 37, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Do, J.W.; Saravanan, M.; Nam, S.E.; Lim, H.J.; Rhee, J.S. Waterborne manganese modulates immunity, biochemical, and antioxidant parameters in the blood of red seabream and black rockfish. Fish Shellfish Immunol. 2019, 88, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, J.C. The lead accumulation and hematological findings in juvenile rock fish Sebastes schlegelii exposed to the dietary lead (II) concentrations. Ecotoxicol. Environ. Saf. 2015, 115, 33–39. [Google Scholar] [CrossRef]


| 95% Confidence Limits | |
|---|---|
| Probability | Estimate (mg/L) |
| 0.01 | −3.107 |
| 0.10 | 5.315 |
| 0.20 | 8.861 |
| 0.30 | 11.418 |
| 0.40 | 13.602 |
| 0.50 | 15.644 |
| 0.60 | 17.687 |
| 0.70 | 19.871 |
| 0.80 | 22.428 |
| 0.90 | 25.974 |
| 0.99 | 34.396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.-M.; Choi, C.Y.; Kim, J.-H. Effects of Hematological Parameters and Plasma Components of Starry Flounder, Platichthys stellatus, by Waterborne Copper Exposure. Animals 2025, 15, 1549. https://doi.org/10.3390/ani15111549
An S-M, Choi CY, Kim J-H. Effects of Hematological Parameters and Plasma Components of Starry Flounder, Platichthys stellatus, by Waterborne Copper Exposure. Animals. 2025; 15(11):1549. https://doi.org/10.3390/ani15111549
Chicago/Turabian StyleAn, Su-Min, Cheol Young Choi, and Jun-Hwan Kim. 2025. "Effects of Hematological Parameters and Plasma Components of Starry Flounder, Platichthys stellatus, by Waterborne Copper Exposure" Animals 15, no. 11: 1549. https://doi.org/10.3390/ani15111549
APA StyleAn, S.-M., Choi, C. Y., & Kim, J.-H. (2025). Effects of Hematological Parameters and Plasma Components of Starry Flounder, Platichthys stellatus, by Waterborne Copper Exposure. Animals, 15(11), 1549. https://doi.org/10.3390/ani15111549








