Simple Summary
Antimicrobial resistance is a frequent finding during bacterial urinary tract infection in dogs and cats. This study evaluated bacterial species, antimicrobial resistance patterns and multi-drug resistance rate in clinical isolates of dogs and cats in a European veterinary university hospital; moreover, we evaluated multi-drug resistance trend in a long-term follow-up, applying the International Society for Companion Animal Infectious Disease urinary tract infections guidelines as a guide for antimicrobial stewardship. In our study, almost half of the cases were classified as upper urinary tract infection and recurrent cystitis; also, this study showed that antimicrobial resistance reached 75% of clinical isolates and the multi-drug resistance percentage was 37%, including resistance mostly against first-line antibiotics such as penicillins for sporadic cystitis and fluoroquinolones for pyelonephritis, respectively. Applying urinary tract infection guidelines during a 30-month period of follow-up, led to a significant decrease in multi-drug resistance. Our work highlights how the application of international urinary tract infection guidelines as a tool for antimicrobial stewardship can significantly reduce antimicrobial resistance in daily practice for a long-term follow-up; moreover, it emphasizes the bacteriological assessment as a fundamental examination for urinary tract infection treatment, guiding antibiotics prescription in an evidence-based manner to reduce antimicrobial resistance spreading.
Abstract
Bacterial urinary tract infections (UTIs) are common in small animal practice and their inappropriate treatment contributes to the antimicrobial resistance (AMR) spreading. This study assessed bacterial prevalence, non-susceptibility percentages, antimicrobial prescription and the impact of the application of international guidelines redacted by the International Society for Companion Animals Infectious Disease (ISCAID) in dogs and cats with UTIs evaluated at a European veterinary university hospital, over a 30-month period. A total of 729 bacterial isolates were included. The most frequently isolated bacterial species was Escherichia coli in both dogs (52.8%) and cats (45.7%). Following ISCAID guidelines, almost half of the cases were classified as upper UTIs (24.9%) or recurrent cystitis (24.8%). Multidrug resistance (MDR) percentage was 37.3% (n = 272). Over five semesters, MDR significantly decreased (p = 0.001). Additionally, a significant decrease was recorded for specimens from patients previously treated (p = 0.018) and under treatment at sampling (p < 0.001). Previous treatment with amoxicillin-clavulanate (p = 0.001), marbofloxacin (p < 0.001), enrofloxacin (p < 0.001) and piperacillin-tazobactam (p = 0.016) was linked with higher MDR rates. This study highlighted that companion animals are potential reservoirs for AMR; moreover, international guidelines applied in the daily practice guiding antimicrobial stewardship can lead to a reduction in AMR over time.
1. Introduction
The spread of antimicrobial resistance (AMR) is a major threat for both human and animal health worldwide. The selective pressure exerted by antimicrobial use (including misuse and overuse) is considered one of the most important factors for an AMR rise [1]. In veterinary medicine, companion animals are gaining interest regarding their role in the overall AMR epidemiology. Indeed, although few evidence-based data are available to date, their close proximity with people could facilitate the interspecies transmission of AMR, and poses a significant One Health challenge [2]. Moreover, infections in dogs and cats are mostly treated with the same antibiotics used in human medicine, such as fluoroquinolones, penicillins and cephalosporins [3], increasing the risk of development of the same drug resistances. Specifically, urinary tract infections (UTIs) are commonly encountered in veterinary practice [4,5,6,7], affecting a substantial proportion of companion animals each year. It has been estimated that close to 14% of dogs evaluated by a veterinarian will develop UTI during their lifetime, with the average age being 7.8–8 years [8,9]. Therefore, similarly to human medicine [10,11], UTIs are also a frequent reason for antimicrobial prescription in small animal practices [3,4,12,13].
While antimicrobial therapy has traditionally been the cornerstone for UTI treatment, the emergence of multidrug-resistant (MDR) pathogens has complicated management strategies, raising serious public health implications. UTIs represent a significant source of morbidity among companion animals, leading to clinical signs of different severity, a decreased quality of life and potential life-threatening complications if left untreated. Additionally, the increasing involvement of AMR bacteria is also paramount for humans they cohabit with. Moreover, multi-drug resistance is exacerbated by factors such as inappropriate antimicrobial selection, suboptimal dosing and the difficulties to establish robust antimicrobial stewardship programs in veterinary settings [14,15]. In 2019, the International Society for Companion Animals Infectious Disease (ISCAID) redacted specific guidelines for UTI treatment in companion animals [16]; notably, some of the antibiotics suggested for UTI treatment in dogs and cats are also used in human medicine, and some of them, quinolones and third-generation cephalosporins, specifically, are considered of highest priority and of critically importance (HPCIAs) by the World Health Organization [17]. These guidelines are general recommendations, requiring a proper adjustment considering national legislation on antimicrobial prescriptions, specific geographical AMR rates and epidemiological nuances [16]. In Italy, few recent studies that focused on this specific topic highlighted overall MDR rates up to 44% [18] and 60% in animals previously treated [19], with alarming resistance rates for HPCIAs such as fluoroquinolones (36%) and third-generation cephalosporins (29.7%) in uropathogenic Escherichia coli [20]. Given these premises, there is an urgent need for comprehensive research addressing AMR in UTIs among companion animals, including local data about etiology, AMR rates and their relationship with antibiotics administration [21].
This study had different aims: (1) to describe the prevalence of bacteria isolated from samples collected in dogs and cats with UTI evaluated at a Veterinary University Hospital (VUH); (2) to determine non-susceptibility patterns of such isolates; (3) to describe antimicrobial usage patterns in positive specimens; (4) to evaluate the impact of the application of the ISCAID guidelines on both AMR rates and antimicrobial usage over a 30-month period.
2. Materials and Methods
A prospective observational study, part of a larger surveillance program whose partial results were published in 2023 [22] was conducted at the Veterinary laboratory of bacteriology and at the VUH of the Department of Veterinary Medical Sciences (University of Bologna), from December 2020 to May 2023. This program included a specific part for the application of the ISCAID guidelines [11] for the diagnosis, classification, and management of UTIs in dogs and cats. The implementation of the ISCAID guidelines was preceded and facilitated by specific internal training meetings within the medical staff of the veterinary hospital and subsequently supervised by clinicians of the nephrology and urology unit. Specifically, around 70% of the cases included in this study were followed up directly by the clinicians in this unit, and for an additional 20%, a specific consultation was required for UTI treatment. Additionally, another part of the surveillance program was constituted by meetings between the bacteriology unit and the clinicians, where data collected were systematically shared and discussed.
Samples collected at the VUH from dogs and cats with suspected UTIs were submitted for bacteriological diagnostic purposes and results were recorded and analyzed. Standard microbiological procedures were used and are listed in Table A1. Specimens were classified as urine, bladder stones and urinary bladder biopsies. After an incubation phase of 24–48 h at 37 ± 1 °C, plates with adequate bacterial growth were considered positive according to previous published guidelines [23]. Colonies were morphologically evaluated, and the identification of bacterial species was assessed using the matrix-assisted laser desorption–ionization time-of-flight mass spectrometry method (MALDI-TOF MS) (Biotyper, Bruker Daltonics, Billerica, MA, USA), following manufacturer’s instructions (Bruker Daltonik, Bremen, Germany). With this method, an ID score > 2 (green—high accuracy) or >1.8 (for Staphylococcus spp. isolates) was defined as positive for species identification. If the same bacterial species were isolated from different specimens from the same patient at the same time, they were considered as one.
According to the ISCAID guidelines [11] and based on the signalment, history, clinical signs and clinicopathological and imaging findings of the included patients, each positive sample was classified as sporadic bacterial cystitis (SBC), recurrent bacterial cystitis (RBC), upper urinary tract infection (uUTI), bacterial prostatitis (BP), subclinical bacteriuria (SUB) or catheter-associated urinary tract infection (CAUTI). Data about previous hospitalization/surgery in the past 30 days (yes/no), hospitalization at the time of sampling (yes/no), hospitalization in intensive care unit (yes/no) and surgery at the time of sampling (yes/no) were recorded in addition to medical data above reported. Moreover, antimicrobial use in the past 90 days (yes/no, number of antimicrobials, type of drugs used) and current antimicrobial use (yes/no, number of antimicrobials, type of drugs used) were also registered.
Antimicrobial susceptibility testing (AST) of all isolates was performed using the Kirby–Bauer disc diffusion method, according to the Clinical and Laboratory Standard Institute (CLSI) guidelines [24]. Overall, 12 antimicrobials from eight antimicrobial classes were included in the final analysis (Table A2 in Appendix A). All the discs were purchased from a commercial supplier (Oxoid, Milan, Italy). For every tested drug, each isolate was classified as susceptible (S), intermediate (I), or resistant (R) based on the 2020 CLSI veterinary breakpoints or, when not specifically present, human ones [25]. Subsequently, they were updated according to the 2024 version of the document [26]. For Gram-negative bacteria, clindamycin and erythromycin were not tested due to their known low-activity rates [27]. For some species, antimicrobials known to exhibit expected resistance phenotypes (intrinsic resistance), according to the National Reference Laboratory for AMR [28], were not tested and excluded from the analysis. Isolates identified as the same bacterial species, in the same patient, with the same AST profile results at different time points were considered as duplicates, and only the first one chronologically identified was included in the study. For AST interpretation, the strains were divided into “susceptible” and “non-susceptible,” as previously suggested by Sweeney et al. [29], where the “non-susceptible” category included resistant and intermediate isolates. Isolates that were non-susceptible to at least one antimicrobial drug were considered as AMR isolates, whereas isolates that were not susceptible to at least one antimicrobial drug in three or more antimicrobial classes were considered as MDR, according with the definition given by Magiorakos et al. [30].
Descriptive statistics were performed to evaluate patients’ clinical data, type of specimen, ISCAID UTI classification, patients’ hospitalization data, bacterial species identified, mixed or single-species infection, previous and current antimicrobial use, non-susceptibility percentages towards each tested drug and AMR/MDR percentages. Data regarding age were presented as median and range, while all the non-susceptibility percentages were shown with the 95% confidence interval (CI). Differences between dogs and cats in terms of prevalence of bacterial species, non-susceptibility rates and antibiotic usage patterns were statistically evaluated using the Fisher exact test. In a similar way, considering a previous manuscript by Marques et al. [7], a statistical analysis of temporal trends of MDR percentage and single non-susceptibility was performed with a logistic regression (stepwise approach) considering the semester of isolation (from 1st to 5th) as a continuous variable. The same statistical analysis was used to describe the temporal trend for previous and current antimicrobial use. Odds ratios (ORs) were calculated considering the baseline category the first semester of observation. The associations between MDR isolates and the antimicrobial drug previously used in both dogs and cats were calculated using a univariable logistic regression analysis. Statistically significant results were included in the multivariate analysis model, built up with a stepwise selection. A p value < 0.05 was used to determine statistically significant results. The normality and heteroskedasticity of data were assessed with the Shapiro–Wilk test and Levene’s test. A statistical analysis was performed with the commercially available MedCalc® statistical software package version 22.009 (MedCalc Software Ltd., Ostend, Belgium).
3. Results
3.1. Bacterial Identification and Data Collection Results
From 2049 specimens collected from 1311 patients, 670/2049 (32.7%) samples were positive for bacterial growth from 495/1311 patients (37.8%). A total of 729 isolates were obtained, including 602 (82.6%) from 555 specimens of 411 dogs (182 males and 229 females), and 127 isolates (17.3%) from 116 specimens of 84 cats (37 males and 47 females). The age distribution of sampled patients ranged from 1 year (≤1 year) to 17 years in dogs and from 1 year (≤1 year) to 19 years in cats, with a median age of 10 years for both species (interquartile range: 6 years).
The most frequently isolated bacterial species (Table 1) was Escherichia coli in both dogs (52.8%) and cats (45.7%). Staphylococcus felis was found only in cat specimens (5.5%). In cats, a higher proportion of Enterococcus faecalis (15%), compared with dogs (5.5%, p < 0.001) was recorded. Significant higher proportions in cats were also recorded for Corynebacterium urealyticum (p = 0.039), Staphylococcus aureus (p = 0.039) and Coagulase-negative Staphylococci (CoNS), other than Staphylococcus felis (p = 0.010). On the other hand, Streptococcus canis was isolated more frequently in dogs (5.8%) than in cats (0.8%, p = 0.012).

Table 1.
Distribution of the bacterial species isolated from positive specimens included in the study, considering both dogs and cats. p values comparing bacterial species between canine and feline isolates are shown, and values considered statistically significant are shown in bold.
Considering the 670 specimens, 662 (98.8%) were urine samples collected by cystocentesis, 5 (0.7%) were urinary bladder biopsies, 1 (0.2%) was a portion of an ureteral stent and 2 (0.3%) were bladder stones collected during endoscopy or surgical procedures. In 612 specimens (91.3%), bacteria were found in monoculture, while in 58 specimens (8.7%), mixed infections were identified, including 57 with two bacterial species and one with three species. The distribution of patients’ hospitalization data is shown in Table 2. Overall, 25% of the patients were hospitalized when specimens were sampled.

Table 2.
Descriptive statistics of patients’ hospitalization data considering all the 670 specimens collected from dogs and cats.
Considering only dogs, 39.5% (n = 219) of the submitted specimens reported an antimicrobial use in the previous 90 days; specifically, 89.5% (n = 196) were specimens with a previous one-drug treatment, 8.7% (n = 19) with a two-drug treatment and 1.8% (n = 4) with a three-drug administration, respectively. The most used antimicrobial was amoxicillin-clavulanate (Figure 1).
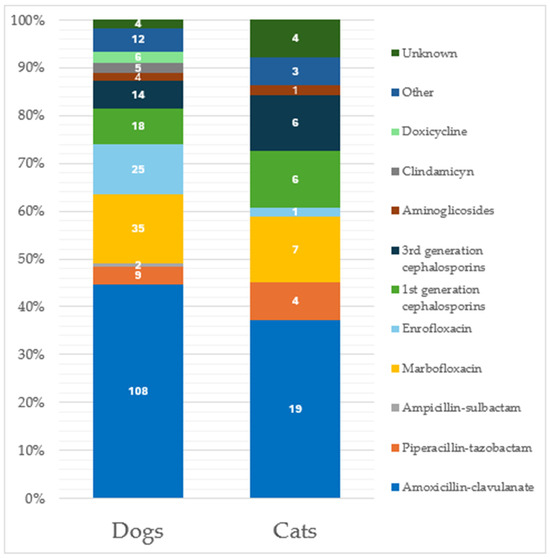
Figure 1.
Distribution of antimicrobial administration considering the 265 specimens from patients treated in the previous 90 days. Classification is made considering separately the two species (219 from dogs and 46 from cats). Data labels are shown within the bars.
Additionally, 13.2% of these previously cited specimens (n = 73) were collected from patients under antibiotic treatment at the time of sampling, including 66 specimens (90.4%) with one drug reported and 7 (9.6%) with double antibiotic treatment, respectively. Marbofloxacin was the most used antibiotic (Figure 2) at the time of specimen sampling.
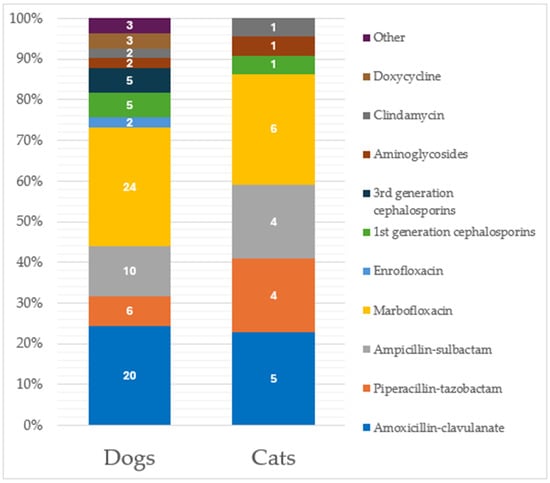
Figure 2.
Distribution of antimicrobial administration considering the 92 specimens from patients under antibiotic treatment at the time of sampling. Classification is made considering separately the two species (73 from dogs and 19 from cats). Data labels are shown within the bars.
In cats, similar percentages were recorded, with 39.7% (n = 46) of the total specimens obtained from animals previously treated with antimicrobials; specifically, 39 (84.8%) were treated with one antibiotic, 6 (13.0%) with two antibiotics, and 1 (2.2%) with three antibiotics. Amoxicillin-clavulanate represented the most previously used drug (Figure 1). Considering specimens from cats under antibiotic treatment at the time of sampling (n = 19, 16.4%), 16 (84.2%) were under treatment with one antibiotic, while 3 specimens (15.8%) reported a two-antibiotic administration, respectively. Again, marbofloxacin was the most used antibiotic at the time of specimen sampling (Figure 2). No significant differences were recorded in the antimicrobial drugs used between dogs and cats.
Considering ISCAID UTI classification, specimens from both dogs and cats were classified based on the type of UTI as follows: 190 (28.4%) SBC, 166 (24.9%) RBC, 167 (24.9%) uUTI, 114 (17%) as SUB, 16 (2.4%) as BP and 12 (1.8%) as CAUTI, respectively. Five cases (0.7%) were not classifiable. No significant differences in incidence of infections were recorded between dogs and cats (Table 3).

Table 3.
Distribution of the 670 specimens according to the ISCAID classification, considering animal species (dogs/cats), single or mixed infection and the use of antibiotics (previously or at the time of sampling).
Recurrent bacterial cystitis was the most prevalent ISCAID type of UTI among mixed infections (13.8%), while CAUTI (n = 8, 66.7%) and RBC (n = 110, 65.9%) were the types of infections with the highest frequency with previous antibiotic use; CAUTI (n = 5, 41.7%) and uUTI cases (n = 38, 22.8%) were the type of UTIs with the highest proportion regarding specimens for which the antibiotics were administered at the time of sampling.
3.2. AST Results
Overall, the AMR percentage was 75.5% (n = 550, 95% CI 71.8 to 78.1), while MDR percentage was 37.3% (n = 272, 95% CI 33.5 to 40.1). In dogs, the MDR percentage was 37.2% (n = 224, 95% CI 33.2 to 40.8), while in cats, it was 37.8% (n = 48, 95% CI 29.7 to 46.3). The non-susceptibility percentages for the tested drugs are shown in Table 4.

Table 4.
Non-susceptibility percentages of the bacterial isolates included in the study for each tested antibiotic. p values comparing non-susceptibility percentages between canine and feline isolates are shown, and the ones considered statistically significant are in bold.
In dogs, higher rates were recorded for clindamycin (65.1%), erythromycin (61.5%), ampicillin (54.5%) and enrofloxacin (44.4%); in cats, clindamycin (53.5%), enrofloxacin (50%), tetracycline (45%) and ampicillin (44.4%) were the antimicrobials tested with more associated non-susceptibility. Compared with cats, in dogs, a statistically significant higher non-susceptibility percentage was recorded for ampicillin (p = 0.048); on the other hand, in cats, a statistically significant higher non-susceptibility percentage for ceftiofur was recorded (p = 0.028).
Distribution of MDR isolates considering bacterial species is shown in Table 5. E. coli (44.1%) and Staphylococci part of the SIG (18.4%) were the most frequent MDR species. Additionally, Enterococcus faecium (80%) and the SIG members (74.6%) were the bacterial species with the highest MDR proportion within the species.

Table 5.
Distribution of the multidrug-resistant (MDR) isolates per bacterial species. The percentage of MDR isolates over the total number of MDR isolates (n = 272) is shown, as well as the proportion of MDR isolates to total number of isolates of the same species.
3.3. Temporal Trend and Multivariate Analysis Results
Temporal trend analysis for non-susceptibility rates showed a significant decrease during the five semesters regarding MDR percentage (Figure 3) (p = 0.001, OR 0.811, 95% CI 0.728 to 0.904) and MDR E coli percentage (p = 0.013, OR 0.824, 95% CI 0.707 to 0.960. Additionally, significant decreases were registered for non-susceptibility percentages towards amoxicillin-clavulanate (p < 0.001, OR 0.763, 95% CI 0.671 to 0.869), amikacin (p = 0.004, OR 0.712, 95% CI 0.588 to 0.862), gentamicin (p = 0.001, OR 0.756, 95% CI 0.655 to 0.874), piperacillin-tazobactam (p = 0.002, OR 0.772, 95% CI 0.671 to 0.888), cephazolin/cephalothin (p = 0.019, OR 0.861, 95% CI 0.759 to 0.976), tetracycline (p = 0.005, OR 0.858, 95% CI 0.770 to 0.956), enrofloxacin (p = 0.002, OR 0.850, 95% CI 0.766 to 0.944) and trimethoprim-sulfamethoxazole (p < 0.001, OR 0.787, 95% CI 0.687 to 0.902).
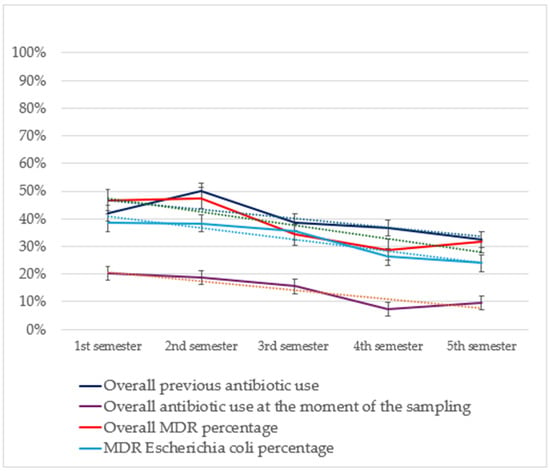
Figure 3.
Results from the temporal trend analysis of MDR percentages of the isolates included in the study (in orange) and of the MDR percentage of E. coli (in light blue) relating it with antibiotic use both in the previous 90 days (in dark blue) and at the time of sampling (in violet). Tendency lines (dashed) and 95% confidence intervals are also shown. MDR, multi-drug resistance bacteria.
A temporal trend analysis for antimicrobial use in patients with positive specimens included in the study (Figure 3) showed a significant decrease in the frequency of overall antimicrobial administration at the moment of sampling (p < 0.001, OR 0.752, 95% CI 0.641 to 0.882) considering the frequency of overall previous treatment (p = 0.018, OR 0.876, 95% CI 0.784 to 0.9775) and the previous use of marbofloxacin (p = 0.002, OR 0.700, 95% CI 0.555 to 0.882) and piperacillin-tazobactam (p = 0.013, OR 0.563, 95% CI 0.360 to 0.887).
The multivariate analysis between MDR and previous antimicrobial use showed that isolates from patients previously treated with amoxicillin-clavulanate (p = 0.001), marbofloxacin (p < 0.001), enrofloxacin (p < 0.001) and piperacillin-tazobactam (p = 0.015) were significantly associated with higher MDR rates.
4. Discussion
In our study, we analyzed specimens from dogs and cats with UTIs evaluated at an Italian VUH to obtain a complete overview on local epidemiological data and to better explore the relationship between antimicrobial use and the onset of antimicrobial resistance in these bacterial infections. Despite the increased focus on this topic, similar regional studies from Southern Europe about AMR and antibiotic administration are lacking. Indeed, the majority of the veterinary literature comes from Northern Europe, Oceania and North America [31,32,33,34,35,36,37], and few recent manuscripts from countries such as Italy or Spain [18,22,38,39] are focused only on AMR and MDR rates. Our study combined information about AMR and antibiotic administration evaluating also their trend over time with the application of the ISCAID guidelines. The knowledge of regional bacterial variations is an essential step to better manage infections, adapting empirical treatments to local AMR results [32]. We investigated the in vitro AMR by using the Kirby–Bauer disc diffusion method, still considered an accurate and reproducible method for AST, in line with the EUCAST guidelines [40].
Specimens from dogs were consistently more frequent, but this could be due to the larger number of dogs attending at the VTH. Nevertheless, the literature on this field reports that dogs, specifically females, tend to have a higher frequency of UTIs compared with cats [8]; on the other hand, cats are described having a higher prevalence of idiopathic cystitis without concurrent bacterial involvement [7,41]. The median age at the moment of sampling (10 years) was in line with previous reports [4,7,42].
In both dogs and cats, the most common bacterial species was E. coli, representing around half of the total isolates. This finding is in accordance with previous large studies in Europe, in which the E. coli prevalence in UTIs from dogs and cats varies from 34.5% to 59.8% [7,18,19,32,39,43,44,45]. Similarly to humans, its high prevalence as urinary pathogen in pets is mainly due to its plasticity and capacity to share and acquire antimicrobial resistance and virulence genes, which help its persistence [4]. Other frequently isolated species in our study were staphylococci members of the Staphylococcus intermedius group (SIG), K. pneumoniae, P. mirabilis, E. faecalis and S. canis. Specifically, S. canis was associated with a higher frequency in dogs, in line with similar studies from Europe [4,43,46]. On the other hand, bacterial species such as E. faecalis, C. urealyticum, S. aureus and S. felis were significantly more prevalent in cats. This differences are supported by other authors [4,43,46,47,48,49] and could reflect a species-specific propensity for some bacterial species causing UTIs. Notably, we did not observe any statistically significant difference about the distribution of species members of the SIG, unlike other studies, which described a lower incidence in cats, mainly due to the absence of S. pseudintermedius (the main SIG species in companion animals) in their skin microbiota [50,51].
Regarding the type of specimens, most of them were urine samples (98.8%), and bacterial growth in monoculture was observed in 91.3% of samples. The frequency of mixed infections (8.7%) was lower compared with other reports, describing a frequency from 15.8% to 28.9% [4,5,47]; on the other hand, our results are in line with a large European multicenter study by Marques et al. [7], in which the reported frequency was 5.36%, and also another Italian report by Rampacci et al. [19], in which the frequency was 5% and 6.3% in dogs and cats, respectively. In this latter manuscript, animals were previously treated with antibiotics. In our study as well, a significant proportion of patients—approximately 40%—had received prior antibiotic treatment, which may have contributed to the selection of single-species growth in the specimens.
The application of the ISCAID guidelines to categorize UTIs, highlights that almost half of the specimens came from cases classified as RBC or uUTI. The high proportion of these types of UTIs reflects the fact that our VTH is a tertiary-care facility, including a substantial number of referred, second-opinion patients with complicated infections. When compared with a previous study from the USA [12] that analyzed antimicrobial prescriptions in dogs with suspected UTIs from 2016 to 2018, and with a study conducted in Germany [33] on urinary isolates from cats in a 7-year period, we recorded a considerably higher proportion of RBC and uUTI cases. This discrepancy can be related to the different methodologies used, but also to the geographical and structural variability. Our high proportion of complicated cases is linked with the percentage of specimens (almost 40% in both dogs and cats) taken from patients previously treated with antibiotics, especially in cases classified as CAUTI (66.7%), RBC (65.9%) and uUTI (42.5%). On the other hand, antimicrobial treatment at the time of sampling involved 13.7% of the specimens, indicating that the empirical treatment (antibiotic started before the results from the AST based on clinical evaluation, laboratory and diagnostic imaging data) was infrequent, especially for cases classified as SUB (5.3%) and SBC (10%).
In contrast with the majority of the studies on this topic, focusing on the analysis of quantitative consumption or antibiotic prescriptions [6,52], in our study we analyzed the antibiotic use only in patients with positive specimens; consequently, patients who did not have positive bacteriological samples but still received antibiotic treatment were excluded from the analysis. This could have led to an underestimation of the overall antibiotic consumption for UTIs; nevertheless, data can still be considered a reflection of the local habits of antibiotics prescription. Our results showed that amoxicillin-clavulanate was, not surprisingly, the most used antibiotic. In fact, it is considered a first-line antibiotic for SBC [6,16,53,54,55,56]. Compared with non-potentiated penicillins, amoxicillin-clavulanic acid has similar effects on antimicrobial resistance, although it could negatively impact on the beneficial microbiota [57]. Nevertheless, its efficacy for uUTI (e.g., pyelonephritis) is considered controversial due to the inability to reach adequate therapeutic concentration in renal tissue [16]. A Finnish study by Rantala et al. [58] described trimethoprim-sulfamethoxazole as the most commonly used drug to treat acute UTIs (52%). This antibiotic is considered by the ISCAID a first-line option for UTIs [16], but its use in our study was not reported. The reason behind this discrepancy could be mainly due to the possible adverse effects when used for long periods, such as immune-mediated reactions [16,59] and the absence of a specific veterinary oral formulation in Italy for cats and dogs. Fluoroquinolones, especially marbofloxacin, were the second most used antibiotic class, with higher proportions compared to other studies from the United States [6], but similar to other manuscripts from Denmark, United States and New Zealand [56,60,61]. A Swiss study from Schmitt et al. [62] on cats showed that fluoroquinolones prescriptions for lower UTIs were less frequent in university hospitals compared to private practices. On the other hand, they are still considered a first-line drug to treat pyelonephritis and prostatitis [16], the frequency of which tends to be higher in specialized facilities such as VTHs. In our case, marbofloxacin was more frequently used than enrofloxacin, whose use is reported to have side effects in cats [16]. In cats, several studies also highlight the frequent use of third-generation cephalosporins, such as cefovecin, to treat SBC [13,62,63], mainly for its single-injection use and, therefore, its use as an easier option in animals difficult to medicate such as cats [64]. In our study, only a small proportion of the specimens from cats was treated with third-generation cephalosporins; this finding agrees with the ISCAID guidelines, on which these drugs are not recommended for routine use [16]: also, our data could be partially explained by the fact that in VTHs there are fewer difficulties to administer antibiotics. Notably, in our study, we also reported the sporadic use of piperacillin-tazobactam (with higher proportions in cats), an ureidopenicillin with beta-lactamases inhibitor belonging to the antibiotic class “A” (“Avoid”) from the European Medicine Agency (EMA) [65]. Its use was allowed for critical patients in veterinary hospitals until the official prohibition with the 2022/1255 EU regulation, which entered into force in March 2023.
The non-susceptibility rates highlighted in this study can be considered only a partial reflection of the local epidemiological scenario, mainly influenced by the selective pressure exerted by antimicrobial administration [66]. Indeed, facilities such as the VTH of this study are referral centers with a higher frequency of patients with comorbidities, previously treated with antimicrobials, who need to be checked periodically and with the same pathogen isolated multiple times. Despite perfect duplicates not being included in the study, this could have led to an overassessment of resistance rates. Additionally, the choice to consider intermediate isolates as resistant ones could have been another source of overestimation, given that many antimicrobials considered (such as cephalosporins) can be highly concentrated in urine. In our study, E. coli was not surprisingly the most frequent MDR species (n = 120), given that it was also the most common. Notably, E. faecium showed the highest MDR proportion (80% and 74.6%, respectively), a result that aligns with the previous literature on companion animals [67,68]. In 2016, Marques et al. [7] described that frequencies in uropathogenic bacteria resistance collected in Southern Europe countries were considerably higher compared with Northern Europe: in Italy, 28.99% of E. coli isolates were considered MDR, similarly to our results, in which the MDR proportion of E. coli was 31.1%. This result took into consideration isolates collected between 2008 and 2013; given the temporal distance with our results, and the speed of AMR spread, comparisons should be made with caution. In our study, the overall non-susceptibility percentage recorded for amoxicillin-clavulanate (21.1%) was relatively low if compared with the other drugs tested, confirming its use as a first-line drug for SBC [16]. Although its use was not reported in our analysis, trimethoprim-sulfamethoxazole showed a similar non-susceptibility percentage (23.4%), suggesting that this antibiotic could be considered as an alternative option for short-term treatment, in order to avoid side effects [69]. On the other hand, non-potentiated penicillin such as amoxicillin, reported to be another first-line drug for SBC by the ISCAID guidelines [16], showed a non-susceptibility percentage higher than 50% especially in dogs, which is the reason why it should be used with caution in these kinds of patients. Such discrepancy between amoxicillin and amoxicillin-clavulanate percentages is similar to a study by KuKanich et al. [70] reporting susceptibility rates of 53% and 92%, respectively. Non-susceptibility towards third-generation cephalosporins was reported to be significantly higher in cats. Again, this could be due to a major tendency to use cefovecin in this species for ease of use, or also to the differences between population size. The percentage of non-susceptibility recorded for enrofloxacin (45.6%) is alarming, but in line with other similar studies from Italy [7,19,46]; this result underlines the need to limit fluoroquinolone use in small animal practice, or in any case its reasoned use in selected patients. Indeed, although they have been classified as Highest Priority Critically Important Antimicrobials (HPCIAs) by the WHO [17], in Italy, they are still frequently used in first-opinion practices due to their broad-spectrum effect and ease of administration [71,72,73,74].
The temporal analysis of the non-susceptibility rates reveals a general decreasing trend over the five semesters for most of the tested drugs; also, the overall MDR rate shows a similar trend. Our result is in line with data obtained in a 2021 study from Thailand by Amphaiphan et al. [75] regarding amoxicillin-clavulanate resistance rates, in which a decrease MDR trend over a 3-year period (from 50% to 15%) was highlighted in uropathogenic bacteria isolated from cats. Our results go together with the decreasing trend of specimens from patients with previous antibiotic treatment, including important ones such as marbofloxacin and piperacillin-tazobactam (whose use was completely stopped at the end of the last semester of observation). Furthermore, our results highlighted a decreasing trend of MDR for specimens from patients under antibiotic treatment at the time of sampling, suggesting a more prudent use as empirical antibiotic therapy. Applying ISCAID guidelines, especially in patients already on antibiotic therapy at the time of inclusion or with previous antibiotic therapy, using targeted molecules and reported timing of therapy, may have decreased the trend of antibiotic resistance selecting non-MDR bacterial populations. Moreover, the significant decrease over time in the proportion of MDR E. coli is worth mentioning for two reasons. First, as previously stated, this species was the most commonly found, and also there is evidence that uropathogenic E. coli can be shared between humans and pets [76,77,78], so a reduction in MDR over time is important also from a One Health perspective. Additionally, the results from the multivariate analysis confirmed the strict relationship between the previous use of antibiotics (specifically, fluoroquinolones, amoxicillin-clavulanate, piperacillin-tazobactam and third-generation cephalosporins) and multidrug-resistance. Although a clear cause–effect relationship cannot be demonstrated and the timeframe was relatively short, these results are probably linked with the application of the ISCAID guidelines for managing UTIs and should be considered regarding a correct antimicrobial stewardship to reduce the spread of antibiotic resistance. These guidelines provide an evidence-based approach about the diagnosis and management of UTIs and help veterinarians in the decision-making process. Antibiotic use is not only the major driving force for the onset of AMR, but also the most manageable action, so a proper intervention in this field can reduce the related risks. Additionally, this study was conducted in Italy, one of the European countries with the most concerning AMR situations in both human and veterinary medicine [79,80]. Given this geographical context, this significant decreasing trend in both MDR rates and antibiotic administration gives further importance to our results.
This study has some limitations. First, the time lapse of the observation period (30 months) was relatively short for a comprehensive evaluation of the MDR temporal trends. Second, the use of laboratory data could not reflect the overall overview over antibiotic consumption and resistances in our VUH, since uncomplicated cases (for which urine culture was not performed) may have been overlooked [81,82]. However, we anecdotally report that the empirical treatment of UTIs, even in SBC, is a very rare event at our VUH, so our findings could be quite representative of the internal situation. Third, because not all cases were followed directly by the clinicians in the nephrology and urology unit who authored this study, we cannot be sure that the ISCAID guidelines were always applied accurately; however, we consider this unlikely given the percentages reported above.
5. Conclusions
This study highlights the impact of AMR in UTIs from companion animals in an Italian VUH, and designed how it could be reduced over time. Our results add novelty into the Italian epidemiological scene and reinforce the role of clinicians through a judicious and rational UTIs management with antibiotics administration oriented by scientific evidence-based guidelines, linked to the local situation. Additionally, our work underlines how a well-defined monitoring system can help in detecting correlations between antibiotics consumption and resistance rates, prioritizing antibiotics use following antimicrobial stewardship. Given that pets are a potential reservoir of resistance towards several antibiotics and are in contact with people, such findings also represent an important challenge from a One Health point of view, especially regarding HCPIAs such as fluoroquinolones and third-generation cephalosporins.
Author Contributions
Conceptualization, R.S. and S.P.; data curation, R.S. and S.P.; formal analysis, R.S., S.P., E.M. (Elisabetta Mondo) and E.E.; investigation, R.S., S.P., E.M. (Erika Monari), K.V., E.M. (Elisabetta Mondo)., E.E. and F.D.; methodology, R.S., S.P., E.M. (Erika Monari), K.V. and F.D.; project administration, S.P. and F.D.; resources, S.P.; supervision, S.P. and F.D.; validation, R.S. and S.P.; visualization, E.M. (Elisabetta Mondo), K.V., F.T. and F.D.; writing—original draft, R.S., S.P., E.M. (Erika Monari) and E.E.; writing—review and editing, E.M. (Erika Monari), F.T. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it was part of a locally conducted surveillance program based on international guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the database of the Veterinary university Hospital.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI | Multidisciplinary Digital Publishing Institute |
| UTIs | Urinary tract infections |
| AMR | Antimicrobial resistance |
| ISCAID | International Society for Companion Animals Infectious Disease |
| MDR | Multi-drug resistance |
| VUH | Veterinary University Hospital |
| MALDI-TOF | Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry |
| ID | Identification |
| SBC | Sporadic bacterial cystitis |
| RBC | Recurrent bacterial cystitis |
| uUTI | Upper urinary tract infection |
| BP | Bacterial prostatitis |
| SUB | Subclinical bacteriuria |
| CAUTI | Catheter-associated urinary tract infection |
| AST | Antimicrobial susceptibility testing |
| CLSI | Clinical and Laboratory Standard Institute |
| CI | Confidence Interval |
| OR | Odds Ratio |
| CoNS | Coagulase-negative Staphylococci other than Staphylococcus felis |
| EUCAST | European Committee for Antimicrobial Susceptibility Testing |
| SIG | Staphylococcus intermedius group |
| EMA | European Medicine Agency |
| WHO | World Health Organization |
| HPCIAs | Highest priority critically important antimicrobials |
Appendix A

Table A1.
List of culture media, condition and temperature used.
Table A1.
List of culture media, condition and temperature used.
| Specimen Type | Media a | Incubation |
|---|---|---|
| Urine b, bladder stone, ureteral stent | Blood Agar, Cled, Mac Conkey | Aerobic conditions |
| Bladder biopsy | Blood Agar, Cled, Mac Conkey Columbia Agar Columbia Agar | Aerobic conditions |
| Capnophilic conditions | ||
| Anaerobic conditions |
a All the culture media were purchased from Oxoid, Germany, and prepared following manufacturer’s instructions; b specimen obtained by cystocentesis or catheterization.

Table A2.
List of tested antimicrobials divided for antimicrobial class.
Table A2.
List of tested antimicrobials divided for antimicrobial class.
| Antimicrobial Class | Antimicrobial Drug |
|---|---|
| Aminoglycosides | Amikacin 30 μg Gentamicin 10 μg (120 μg for Enterococcus spp. Isolates) |
| Penicillins ± beta-lactamase inhibitors | Ampicillin 10 μg Amoxicillin-clavulanate 30 μg Piperacillin-tazobactam 110 μg |
| Cephalosporins | Cephazolin/cephalotin 30 μg Ceftiofur 30 μg |
| Tetracyclines | Tetracycline 30 μg |
| Macrolides | Erythromicin 15 μg |
| Lincosamides | Clindamycin 2 μg |
| Fluoroquinolones | Enrofloxacin 5 μg |
| Sulfonamides + dihydrofolate reductase inhibitors | Trimethoprim-sulfamethoxazole 1.25/23.7 μg |
References
- WHO. WHO List of Critically Important Antimicrobials for Human Medicine (WHO CIA List); World Health Organization: Geneve, Switzerland, 2019.
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public Health Risk of Antimicrobial Resistance Transfer from Companion Animals. J. Antimicrob. Chemother. 2016, 72, 957–968. [Google Scholar] [CrossRef] [PubMed]
- De Briyne, N.; Atkinson, J.; Borriello, S.P.; Pokludová, L. Antibiotics Used Most Commonly to Treat Animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef] [PubMed]
- Aurich, S.; Prenger-Berninghoff, E.; Ewers, C. Prevalence and Antimicrobial Resistance of Bacterial Uropathogens Isolated from Dogs and Cats. Antibiotics 2022, 11, 1730. [Google Scholar] [CrossRef]
- Wong, C.; Epstein, S.E.; Westropp, J.L. Antimicrobial Susceptibility Patterns in Urinary Tract Infections in Dogs (2010–2013). Vet. Intern. Medicne 2015, 29, 1045–1052. [Google Scholar] [CrossRef]
- Bloch, R.A.; Papich, M.G.; Stürmer, T. Veterinary Antimicrobial Prescribing Practices for Treatment of Presumptive Sporadic Urinary Tract Infections in Dogs Examined at Primary Care Practices in the United States (2010–2019). J. Am. Vet. Med. Assoc. 2022, 260, S21–S27. [Google Scholar] [CrossRef]
- Marques, C.; Gama, L.T.; Belas, A.; Bergström, K.; Beurlet, S.; Briend-Marchal, A.; Broens, E.M.; Costa, M.; Criel, D.; Damborg, P.; et al. European Multicenter Study on Antimicrobial Resistance in Bacteria Isolated from Companion Animal Urinary Tract Infections. BMC Vet. Res. 2016, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Teh, H. A Review of the Current Concepts in Canine Urinary Tract Infections. Aust. Vet. J. 2022, 100, 56–62. [Google Scholar] [CrossRef]
- Tompson, A.C.; Mateus, A.L.P.; Brodbelt, D.C.; Chandler, C.I.R. Understanding Antibiotic Use in Companion Animals: A Literature Review Identifying Avenues for Future Efforts. Front. Vet. Sci. 2021, 8, 719547. [Google Scholar] [CrossRef]
- Pujades-Rodriguez, M.; West, R.M.; Wilcox, M.H.; Sandoe, J. Lower Urinary Tract Infections: Management, Outcomes and Risk Factors for Antibiotic Re-Prescription in Primary Care. eClinicalMedicine 2019, 14, 23–31. [Google Scholar] [CrossRef]
- Schmiemann, G.; Kniehl, E.; Gebhardt, K.; Matejczyk, M.M.; Hummers-Pradier, E. The Diagnosis of Urinary Tract Infection. Dtsch. Ärzteblatt Int. 2010, 107, 361–367. [Google Scholar] [CrossRef]
- Weese, J.S.; Webb, J.; Ballance, D.; McKee, T.; Stull, J.W.; Bergman, P.J. Evaluation of Antimicrobial Prescriptions in Dogs with Suspected Bacterial Urinary Tract Disease. Vet. Intern. Med. 2021, 35, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Stull, J.W.; Evason, M.; Webb, J.; Ballance, D.; McKee, T.; Bergman, P.J. A Multicenter Study of Antimicrobial Prescriptions for Cats Diagnosed with Bacterial Urinary Tract Disease. J. Feline Med. Surg. 2022, 24, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Compri, M.; Mader, R.; Mazzolini, E.; De Angelis, G.; Mutters, N.T.; Babu Rajendran, N.; Galia, L.; Tacconelli, E.; Schrijver, R.; the ARCH working group; et al. White Paper: Bridging the Gap between Surveillance Data and Antimicrobial Stewardship in the Animal Sector—Practical Guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net Networks. J. Antimicrob. Chemother. 2020, 75, ii52–ii66. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H.; Page, S.W. Antimicrobial Stewardship in Veterinary Medicine. Microbiol. Spectr. 2018, 6, 1–22. [Google Scholar] [CrossRef]
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) Guidelines for the Diagnosis and Management of Bacterial Urinary Tract Infections in Dogs and Cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef]
- World Health Organization. WHO’s List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneve, Switzerland, 2024.
- Smoglica, C.; Evangelisti, G.; Fani, C.; Marsilio, F.; Trotta, M.; Messina, F.; Di Francesco, C.E. Antimicrobial Resistance Profile of Bacterial Isolates from Urinary Tract Infections in Companion Animals in Central Italy. Antibiotics 2022, 11, 1363. [Google Scholar] [CrossRef]
- Rampacci, E.; Bottinelli, M.; Stefanetti, V.; Hyatt, D.R.; Sgariglia, E.; Coletti, M.; Passamonti, F. Antimicrobial Susceptibility Survey on Bacterial Agents of Canine and Feline Urinary Tract Infections: Weight of the Empirical Treatment. J. Glob. Antimicrob. Resist. 2018, 13, 192–196. [Google Scholar] [CrossRef]
- Bellato, A.; Robino, P.; Stella, M.C.; Scalas, D.; Savarino, P.; Zanatta, R.; Re, G.; Nebbia, P. Ten-Year Antimicrobial Resistance Trend in Uropathogenic Escherichia Coli (UPEC) Isolated from Dogs and Cats Admitted to a Veterinary Teaching Hospital in Italy. Microorganisms 2024, 12, 2175. [Google Scholar] [CrossRef]
- Leet-Otley, K.; Fellman, C.L.; Wayne, A.S.; Beaulac, K.; DeStefano, I.M.; Chambers, K.; Marino, K.B.; Doron, S. Demonstrating the Importance of Local Culture and Susceptibility Data: Antibiograms from Dogs at a Veterinary Tertiary Care Center. J. Am. Vet. Med. Assoc. 2023, 261, 1–7. [Google Scholar] [CrossRef]
- Scarpellini, R.; Assirelli, G.; Giunti, M.; Esposito, E.; Mondo, E.; Piva, S. Monitoring the Prevalence of Antimicrobial Resistance in Companion Animals: Results from Clinical Isolates in an Italian University Veterinary Hospital. Transbound. Emerg. Dis. 2023, 2023, 6695493. [Google Scholar] [CrossRef]
- Markey, B.; Quinn, P.J.; Carter, M.E.G.R. Carter Section 1: General Procedures in Microbiology. In Clinical Veterinary Microbiology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 21–67. ISBN 0-7234-1711-3. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilutions Susceptibility Test for Bacteria Isolated from Animals, 4th ed.; CLSI Supplement Vet08; CLSI: Malvern, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), CLSI Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; CLSI: Malvern, PA, USA, 2020.
- Clinical and Laboratory Standards Institute CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; CLSI Supplement VET01S; CLSI: Malvern, PA, USA, 2024. [Google Scholar]
- Kuriyama, T.; Karasawa, T.; Williams, D.W. Antimicrobial Chemotherapy. In Biofilms in Infection Prevention and Control; Elsevier: Amsterdam, The Netherlands, 2014; pp. 209–244. ISBN 978-0-12-397043-5. [Google Scholar]
- National Reference Center for Antimicrobial Resistance, Tecnhical Report, Tabelle Resistenze Intrinseche Dei Batteri Di Interesse Veterinario [Intrinsic Resistance Tables of Bacteria of Veterinary Interest]; CRAB: Rome, Italy, 2018.
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying Definitions for Multidrug Resistance, Extensive Drug Resistance and Pandrug Resistance to Clinically Significant Livestock and Companion Animal Bacterial Pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Dorsch, R.; Vopelius-Feldt, C.V.; Wolf, G.; Mueller, R.S.; Straubinger, R.K.; Hartmann, K. Bakterielle Harnwegsinfektionen bei Katzen: Prävalenz prädisponierender Erkrankungen und bakterieller Isolate sowie Ermittlung der antimikrobiellen Resistenz gegenüber häufig eingesetzten Antibiotika. Tierarztl. Prax. Ausg. K. 2016, 44, 227–236. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Mavrides, D.E.; Graham, P.A.; McHugh, T.D. Results of Urinary Bacterial Cultures and Antibiotic Susceptibility Testing of Dogs and Cats in the UK. J. Small Anim. Pract. 2021, 62, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Koontz, C.W.; Epstein, S.E.; Westropp, J.L. Antimicrobial Susceptibility Patterns from Urinary Isolates Obtained from Cats (2013-2020). Vet. Intern. Medicne 2023, 37, 1077–1087. [Google Scholar] [CrossRef]
- Mack, C.; Gibson, J.; Meler, E.; Woldeyohannes, S.; Yuen, N.; Herndon, A. Antimicrobial Susceptibility Patterns of Aerobic Bacteria Isolated from Canine Urinary Samples in South East Queensland, 2013 to 2018. Aust. Vet. J. 2024, 102, 362–368. [Google Scholar] [CrossRef]
- Roberts, M.; White, J.; Lam, A. Prevalence of Bacteria and Changes in Trends in Antimicrobial Resistance of Escherichia Coli Isolated from Positive Canine Urinary Samples from an Australian Referral Hospital over a 5-year Period (2013–2017). Vet. Rec. Open 2019, 6, e000345. [Google Scholar] [CrossRef]
- Scarborough, R.; Bailey, K.; Galgut, B.; Williamson, A.; Hardefeldt, L.; Gilkerson, J.; Browning, G. Use of Local Antibiogram Data and Antimicrobial Importance Ratings to Select Optimal Empirical Therapies for Urinary Tract Infections in Dogs and Cats. Antibiotics 2020, 9, 924. [Google Scholar] [CrossRef]
- Yudhanto, S.; Hung, C.-C.; Maddox, C.W.; Varga, C. Antimicrobial Resistance in Bacteria Isolated from Canine Urine Samples Submitted to a Veterinary Diagnostic Laboratory, Illinois, United States. Front. Vet. Sci. 2022, 9, 867784. [Google Scholar] [CrossRef]
- Darwich, L.; Seminati, C.; Burballa, A.; Nieto, A.; Durán, I.; Tarradas, N.; Molina-López, R.A. Antimicrobial Susceptibility of Bacterial Isolates from Urinary Tract Infections in Companion Animals in Spain. Vet. Rec. 2021, 188, e60. [Google Scholar] [CrossRef]
- Hernando, E.; Vila, A.; D’Ippolito, P.; Rico, A.J.; Rodon, J.; Roura, X. Prevalence and Characterization of Urinary Tract Infection in Owned Dogs and Cats From Spain. Top. Companion Anim. Med. 2021, 43, 100512. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST Disk Diffusion Antimicrobial Susceptibility Testing Method and Its Implementation in Routine Microbiology Laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Franzo, G.; Bano, L.; Urbani, L.; Segatore, S.; Rizzardi, A.; Cordioli, B.; Cornaggia, M.; Terrusi, A.; Vasylyeva, K.; et al. No viable bacterial communities reside in the urinary bladder of cats with feline idiopathic cystitis. Res. Vet. Sci. 2024, 168, 105137. [Google Scholar] [CrossRef]
- Kocúreková, T.; Koščová, J.; Hajdučková, V. Infections of the Urinary Tract of Bacterial Origin in Dogs and Cats. Folia Vet. 2021, 65, 59–66. [Google Scholar] [CrossRef]
- Moyaert, H.; Morrissey, I.; De Jong, A.; El Garch, F.; Klein, U.; Ludwig, C.; Thiry, J.; Youala, M. Antimicrobial Susceptibility Monitoring of Bacterial Pathogens Isolated from Urinary Tract Infections in Dogs and Cats Across Europe: ComPath Results. Microb. Drug Resist. 2017, 23, 391–403. [Google Scholar] [CrossRef]
- Marques, C.; Belas, A.; Franco, A.; Aboim, C.; Gama, L.T.; Pomba, C. Increase in Antimicrobial Resistance and Emergence of Major International High-Risk Clonal Lineages in Dogs and Cats with Urinary Tract Infection: 16 Year Retrospective Study. J. Antimicrob. Chemother. 2018, 73, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Lopes, R.; Silva, A.; Sampaio, F.; Duque, D.; Brilhante-Simões, P. Bacterial Isolates from Urinary Tract Infection in Dogs and Cats in Portugal, and Their Antibiotic Susceptibility Pattern: A Retrospective Study of 5 Years (2017–2021). Antibiotics 2022, 11, 1520. [Google Scholar] [CrossRef]
- Vercelli, C.; Della Ricca, M.; Re, M.; Gambino, G.; Re, G. Antibiotic Stewardship for Canine and Feline Acute Urinary Tract Infection: An Observational Study in a Small Animal Hospital in Northwest Italy. Antibiotics 2021, 10, 562. [Google Scholar] [CrossRef]
- Litster, A.; Moss, S.M.; Honnery, M.; Rees, B.; Trott, D.J. Prevalence of Bacterial Species in Cats with Clinical Signs of Lower Urinary Tract Disease: Recognition of Staphylococcus Felis as a Possible Feline Urinary Tract Pathogen. Vet. Microbiol. 2007, 121, 182–188. [Google Scholar] [CrossRef]
- Torre, M.; Furrow, E.; Foster, J.D. Effect of Urine-specific Gravity on Performance of Bacteriuria in Predicting Urine Culture Results. J. Small Anim. Pract. 2022, 63, 286–292. [Google Scholar] [CrossRef]
- KuKanich, K.S.; Lubbers, B.V. Review of Enterococci Isolated from Canine and Feline Urine Specimens from 2006 to 2011. J. Am. Anim. Hosp. Assoc. 2015, 51, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bierowiec, K.; Korzeniowska-Kowal, A.; Wzorek, A.; Rypuła, K.; Gamian, A. Prevalence of Staphylococcus Species Colonization in Healthy and Sick Cats. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Lu, Y.; McEwan, N.A. Staphylococcal and Micrococcal Adherence to Canine and Feline Corneocytes: Quantification Using a Simple Adhesion Assay. Vet. Dermatol. 2007, 18, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Investigation of Antimicrobial Use and the Impact of Antimicrobial Use Guidelines in a Small Animal Veterinary Teaching Hospital: 1995–2004. J. Am. Vet. Med. Assoc. 2006, 228, 553–558. [Google Scholar] [CrossRef]
- Robbins, S.N.; Goggs, R.; Lhermie, G.; Lalonde-Paul, D.F.; Menard, J. Antimicrobial Prescribing Practices in Small Animal Emergency and Critical Care. Front. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.L.P.; Brodbelt, D.C.; Barber, N.; Stärk, K.D.C. Qualitative Study of Factors Associated with Antimicrobial Usage in Seven Small Animal Veterinary Practices in the UK. Prev. Vet. Med. 2014, 117, 68–78. [Google Scholar] [CrossRef]
- Jessen, L.R.; Sørensen, T.M.; Lilja, Z.L.; Kristensen, M.; Hald, T.; Damborg, P. Cross-Sectional Survey on the Use and Impact of the Danish National Antibiotic Use Guidelines for Companion Animal Practice. Acta Vet. Scand. 2017, 59, 81. [Google Scholar] [CrossRef]
- Sørensen, T.M.; Bjørnvad, C.R.; Cordoba, G.; Damborg, P.; Guardabassi, L.; Siersma, V.; Bjerrum, L.; Jessen, L.R. Effects of Diagnostic Work-Up on Medical Decision-Making for Canine Urinary Tract Infection: An Observational Study in Danish Small Animal Practices. Vet. Intern. Medicne 2018, 32, 743–751. [Google Scholar] [CrossRef]
- Espinosa-Gongora, C.; Jessen, L.R.; Kieler, I.N.; Damborg, P.; Bjørnvad, C.R.; Gudeta, D.D.; Pires Dos Santos, T.; Sablier-Gallis, F.; Sayah-Jeanne, S.; Corbel, T.; et al. Impact of Oral Amoxicillin and Amoxicillin/Clavulanic Acid Treatment on Bacterial Diversity and β-Lactam Resistance in the Canine Faecal Microbiota. J. Antimicrob. Chemother. 2020, 75, 351–361. [Google Scholar] [CrossRef]
- Rantala, M.; Huovinen, P.; Hölsö, K.; Lilas, A.; Kaartinen, L. Survey of Condition-based Prescribing of Antimicrobial Drugs for Dogs at a Veterinary Teaching Hospital. Vet. Rec. 2004, 155, 259–262. [Google Scholar] [CrossRef]
- Trepanier, L.A. Idiosyncratic Toxicity Associated with Potentiated Sulfonamides in the Dog. Vet. Pharm. Ther. 2004, 27, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Fowler, H.; Davis, M.A.; Perkins, A.; Trufan, S.; Joy, C.; Buswell, M.; McElwain, T.F.; Moore, D.; Worhle, R.; Rabinowitz, P.M. Survey of Veterinary Antimicrobial Prescribing Practices, Washington State 2015. Vet. Rec. 2016, 179, 651. [Google Scholar] [CrossRef] [PubMed]
- Pleydell, E.; Souphavanh, K.; Hill, K.; French, N.; Prattley, D. Descriptive Epidemiological Study of the Use of Antimicrobial Drugs by Companion Animal Veterinarians in New Zealand. N. Z. Vet. J. 2012, 60, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Lehner, C.; Schuller, S.; Schüpbach-Regula, G.; Mevissen, M.; Peter, R.; Müntener, C.R.; Naegeli, H.; Willi, B. Antimicrobial Use for Selected Diseases in Cats in Switzerland. BMC Vet. Res. 2019, 15, 94. [Google Scholar] [CrossRef]
- Murphy, C.P.; Reid-Smith, R.J.; Boerlin, P.; Weese, J.S.; Prescott, J.F.; Janecko, N.; McEwen, S.A. Out-Patient Antimicrobial Drug Use in Dogs and Cats for New Disease Events from Community Companion Animal Practices in Ontario. Can. Vet. J. 2012, 53, 291–298. [Google Scholar]
- Frey, E. The Role of Companion Animal Veterinarians in One-Health Efforts to Combat Antimicrobial Resistance. J. Am. Vet. Med. Assoc. 2018, 253, 1396–1404. [Google Scholar] [CrossRef]
- European Medicines Agency. Categorisation of Antibiotics in the European Union; European Medicines Agency: Amsterdam, NY, USA, 2019.
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2021; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2022.
- Da Silva, L.; Grecellé, C.Z.; Frazzon, A.P.G.; Streck, A.F.; Kipper, D.; Fonseca, A.S.K.; Ikuta, N.; Lunge, V.R. Multidrug-Resistant Enterococcus Faecium and Enterococcus Faecalis Isolated from Dogs and Cats in Southern Brazil. Microbiol. Res. 2024, 15, 1083–1090. [Google Scholar] [CrossRef]
- Tumpa, A.; Štritof, Z.; Pintarić, S. Prevalence and Antimicrobial Susceptibility of Enterococcus Spp. from Urine of Dogs and Cats in Northwestern Croatia. Res. Vet. Sci. 2022, 151, 42–46. [Google Scholar] [CrossRef]
- Clare, S.; Hartmann, F.A.; Jooss, M.; Bachar, E.; Wong, Y.Y.; Trepanier, L.A.; Viviano, K.R. Short- and Long-Term Cure Rates of Short-Duration Trimethoprim-Sulfamethoxazole Treatment in Female Dogs with Uncomplicated Bacterial Cystitis. Vet. Intern. Medicne 2014, 28, 818–826. [Google Scholar] [CrossRef]
- KuKanich, K.; Lubbers, B.; Salgado, B. Amoxicillin and Amoxicillin-clavulanate Resistance in Urinary ESCHERICHIA COLI Antibiograms of Cats and Dogs from the Midwestern United States. Vet. Intern. Medicne 2020, 34, 227–231. [Google Scholar] [CrossRef]
- Escher, M.; Vanni, M.; Intorre, L.; Caprioli, A.; Tognetti, R.; Scavia, G. Use of Antimicrobials in Companion Animal Practice: A Retrospective Study in a Veterinary Teaching Hospital in Italy. J. Antimicrob. Chemother. 2011, 66, 920–927. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Vernaccini, M.; Meucci, V.; Briganti, A.; Lippi, I.; Marchetti, V.; Intorre, L. Six-Year Prescription Pattern of Antimicrobial Use in Cats at the Veterinary Teaching Hospital of the University of Pisa. Animals 2024, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Foglia Manzillo, V.; Peruzy, M.F.; Gizzarelli, M.; Izzo, B.; Sarnelli, P.; Carrella, A.; Vinciguerra, G.; Chirollo, C.; Ben Fayala, N.E.H.; Balestrino, I.; et al. Examining the Veterinary Electronic Antimicrobial Prescriptions for Dogs and Cats in the Campania Region, Italy: Corrective Strategies Are Imperative. Animals 2023, 13, 2869. [Google Scholar] [CrossRef]
- Chirollo, C.; Nocera, F.P.; Piantedosi, D.; Fatone, G.; Della Valle, G.; De Martino, L.; Cortese, L. Data on before and after the Traceability System of Veterinary Antimicrobial Prescriptions in Small Animals at the University Veterinary Teaching Hospital of Naples. Animals 2021, 11, 913. [Google Scholar] [CrossRef]
- Amphaiphan, C.; Yano, T.; Som-in, M.; Kungwong, P.; Wongsawan, K.; Pusoonthornthum, R.; Salman, M.D.; Tangtrongsup, S. Antimicrobial Drug Resistance Profile of Isolated Bacteria in Dogs and Cats with Urologic Problems at Chiang Mai University Veterinary Teaching Hospital, Thailand (2012–2016). Zoonoses Public Health 2021, 68, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Owens, K.; Gajewski, A.; Clabots, C. Escherichia Coli Colonization Patterns among Human Household Members and Pets, with Attention to Acute Urinary Tract Infection. J. Infect. Dis. 2008, 197, 218–224. [Google Scholar] [CrossRef]
- Aurich, S.; Wolf, S.A.; Prenger-Berninghoff, E.; Thrukonda, L.; Semmler, T.; Ewers, C. Genotypic Characterization of Uropathogenic Escherichia Coli from Companion Animals: Predominance of ST372 in Dogs and Human-Related ST73 in Cats. Antibiotics 2023, 13, 38. [Google Scholar] [CrossRef]
- Damborg, P.; Pirolo, M.; Schøn Poulsen, L.; Frimodt-Møller, N.; Guardabassi, L. Dogs Can Be Reservoirs of Escherichia Coli Strains Causing Urinary Tract Infection in Human Household Contacts. Antibiotics 2023, 12, 1269. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2022; ECDC: Stockholm, Sweden, 2023.
- EFSA; ECDC. European Food Safety Authority and European Centre for Disease Prevention and Control The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2020/2021. EFSA J. 2023, 21, 7867. [Google Scholar]
- Ball, K.R.; Rubin, J.E.; Chirino-Trejo, M.; Dowling, P.M. Antimicrobial Resistance and Prevalence of Canine Uropathogens at the Western College of Veterinary Medicine Veterinary Teaching Hospital, 2002-2007. Can. Vet. J. 2008, 49, 985–990. [Google Scholar]
- Hall, J.L.; Holmes, M.A.; Baines, S.J. Prevalence and Antimicrobial Resistance of Canine Urinary Tract Pathogens. Vet. Rec. 2013, 173, 549. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).