Heritability and Genetic Correlation of Age at First Egg and Egg Number up to 40 Weeks of Age After Long-Term Selection in Taiwan Indigenous Chicken
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inbreeding Coefficient Estimation
2.2. Heritability and Correlation Estimation
3. Results
3.1. Laying Traits of Chicken Lines
3.2. Effect of Inbreeding on Body Weight at 16 Weeks of Age
3.3. Estimates of Heritability and Correlation
4. Discussion
4.1. Age at First Egg
4.2. Effect of Inbreeding on W16
4.3. Heritability and Genetic Correlation After Long-Term Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tai, C.; Chung, H.C.; Huang, H.C.; Chang, H.L.; Huang, Y.C.; Tai, J.J.L. The breeding of inbred lines in Taiwanese native chicekns I. The effects of inbreeding under full-sib mating on body weights. J. Chin. Soc. Anim. Sci. 1995, 24, 421–433. [Google Scholar]
- Tai, C.; Huang, H.C.; Chung, H.C.; Chang, H.L.; Cheng, Y.S.; Tai, J.J.L. Breeding of inbred lines in Taiwanese native chickens II. Effects of inbreeding under Full-sib mating on laying traits. J. Chin. Soc. Anim. Sci. 1996, 25, 287–295. [Google Scholar]
- Lin, D.Y.; Lai, Y.Y.; Liu, H.L.; Tsai, M.Y.; Hung, C.M.; Chu, C.T.; Chang, H.L.; Wu, M.C.; Tzeng, S.J. Selection of high egg production in Taiwan native chicken inbreeding lines Taisui No. 1. Taiwan Livest. Res. 2022, 55, 180–192. [Google Scholar]
- Jambui, M.; Honaker, C.F.; Siegel, P.B. Correlated responses to long-term divergent selection for 8-week body weight in female White Plymouth Rock chickens: Sexual maturity. Poult. Sci. 2017, 96, 3844–3851. [Google Scholar] [CrossRef]
- Tan, Y.G.; Xu, X.L.; Cao, H.Y.; Zhou, W.; Yin, Z.Z. Effect of age at first egg on reproduction performance and characterization of the hypothalamo–pituitary–gonadal axis in chickens. Poult. Sci. 2021, 100, 1013125. [Google Scholar] [CrossRef] [PubMed]
- Dunnington, E.A.; Siegel, P.B. Age and body weight at sexual maturity in female White Leghorn chickens. Poult. Sci. 1984, 63, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Chandan, P.; Prince, L.L.L.; Bhattacharya, T.K.; Rajkumar, U.; Chatterjee, R.N. Estimation of heritability and genetic correlation of egg production traits using animal model in commercial layer. Indian J. Anim. Sci. 2019, 89, 1269–1273. [Google Scholar] [CrossRef]
- Kaneko, K.; Okano, K.; Fujihara, N. Studies on egg laying performance of the Gifu native fowl. Jpn. Poult. Sci. 1997, 34, 200–204. [Google Scholar] [CrossRef]
- Wezyk, S.; Rawczyk, J.; Calik, J.; Poltowicz, K. Relationship between hen age, body weight, laying rate, egg weight and rearing system. In Proceedings of the EPC 2006—12th European Poultry Conference, Verona, Italy, 10–14 September 2006. [Google Scholar]
- McMillan, I.; Fairfull, R.W.; Quinton, M.; Friars, G.W. The effect of simultaneous selection on the genetic correlation. Theor. Appl. Genet. 1995, 91, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics; Pearson Education Ltd.: Harlow, UK, 1996. [Google Scholar]
- Walsh, B.; Lynch, M. Evolution and Selection of Quantitative Traits; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Sosa-Madrid, B.S.; Maniatis, G.; Ibáñez-Escriche, N.; Avendaño, S.; Kranis, A. Genetic variance estimation over time in broiler breeding programmes for growth and reproductive traits. Animals 2023, 13, 3306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- SAS Institute Inc. The SAS System for Windows, Release 9.4; SAS Institute: Cary, NC, USA, 2015. [Google Scholar]
- Groeneveld, E.; Kovac, M.; Mielenz, N. VCE User’s Guide and Reference Manual Version 6.0; Corpus ID: 208560239; Institute of Farm Animal Genetics: Neustadt, Germany, 2010; Available online: https://www.semanticscholar.org/paper/VCE-User%27s-Guide-and-Reference-Manual-Version-6.0-Groeneveld/9e2ccfd80e2dd449a231a5aba79a2e55913ccb90 (accessed on 17 January 2024).
- Byrne, B.M. Structural Equation Modeling with AMOS: Basic Concepts, Applications, and Programming; Routledge: New York, NY, USA, 2010. [Google Scholar]
- Hair, J.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Educational International: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Wang, T.; Fernando, R.L.; Kachman, S.D. Matvec Users’ Guide. Version 1.03. 2003. Available online: https://www.yumpu.com/en/document/read/53822742/matvec-users-guide (accessed on 17 January 2024).
- Chomchuen, K.; Tuntiyasawasdikul, V.; Chankitisakul, V.; Boonkum, W. Genetic evaluation of body weights and egg production traits using a multi-trait animal model and selection index in Thai native synthetic chickens (Kaimook e-san2). Animals 2022, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Liu, X.; Xie, X.; Wang, K.; Yu, D. Identification of chicken FSHR gene promoter and the correlations between polymorphisms and egg production in Chinese native hens. Reprod. Domest. Anim. 2019, 54, 702–711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Sun, Y.; Ni, A.; Li, Y.; Yuan, J.; Ma, H.; Wang, P.; Shi, L.; Zong, Y.; Zhao, J.; et al. Research Note: Heterosis for egg production and oviposition pattern in reciprocal crossbreeds of indigenous and elite laying chickens. Poult Sci. 2022, 101, 102201. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Yuan, J.; Ni, A.; Ma, H.; Wang, Y.; Zong, Y.; Zhao, J.; Jin, S.; Sun, Y.; et al. Research Note: Genetic parameters for egg production and clutch-related traits in indigenous Beijing-You chickens. Poult. Sci. 2023, 102, 102904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, H.; Zeng, H.; Luo, C.; Zhang, D.; Wang, Q.; Sun, L.; Yang, L.; Zhou, M.; Nie, Q.; Zhang, X. Genetic effects of polymorphisms in candidate genes and the QTL region on chicken age at first egg. BMC Genet. 2011, 12, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolc, A.; Jankowski, T.; Arango, J.; Settar, P.; Fulton, J.E.; O’Sullivan, N.P.; Dekkers, J.C.M. Investigating the genetic determination of clutch traits in laying hens. Poult. Sci. 2019, 98, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, Y.; Isa, A.M.; Ma, H.; Yuan, J.; Wang, P.; Ge, P.; Gong, Y.; Chen, J.; Sun, Y. Characterization of clutch traits and egg production in six chicken breeds. Anim. Biosci. 2023, 36, 899–907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, L.S. Balanced vs. slightly unbalanced selection. Heredity 2000, 84, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.; Pérez-Enciso, M. Optimization of selection response under restricted inbreeding. Genet. Sel. Evol. 1990, 22, 93–107. [Google Scholar] [CrossRef] [PubMed Central]
- Mtileni, B.; Dzama, K.; Nephawe, K.; Rhode, C. Estimates of effective population size and inbreeding in South African indigenous chicken populations: Implications for the conservation of unique genetic resources. Trop. Anim. Health Prod. 2016, 48, 943–950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- King, H.D.I. The effects of inbreeding on the growth and variability in body weight of the albino rat. J. Exp. Zool. 1918, 26, 1–54. [Google Scholar] [CrossRef]
- Waters, N.F.; Lambert, W.V. Inbreeding in the White Leghorn fowl. Iowa Agric. Home Econ. Exp. Stn. Res. Bull. 1936, 202, 1–55. [Google Scholar]
- Waters, N. Body Weight of Different Inbred Lines of Chickens. Poult. Sci. 1951, 30, 615–620. [Google Scholar] [CrossRef]
- Wright, S. The effects of inbreeding and crossbreeding on guinea pigs. USDA Dept. Bull. 1922, 1090, 1121. [Google Scholar]
- Singh, V.; Singh, D.; Singh, R.P. Genetic studies of growth and reproduction traits of broiler dam line. Indian J. Anim. Res. 2015, 49, 26–30. [Google Scholar] [CrossRef]
- Bulmer, M.G. The effect of selection on genetic variability: A simulation study. Genet Res. 1976, 28, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.G. Is continued genetic improvement of livestock sustainable? Genetics 2016, 202, 877–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Yang, N.; Yan, Y.Y.; Li, G.Q.; Liu, A.Q.; Wu, G.Q.; Sun, C.J. Genome-wide association analysis of egg production performance in chickens across the whole laying period. BMC Genet. 2019, 20, 67. [Google Scholar] [CrossRef]
- Gao, J.; Xu, W.; Zeng, T.; Tian, Y.; Wu, C.; Liu, S.; Zhao, Y.; Zhou, S.; Lin, X.; Cao, H.; et al. Genome-Wide Association Study of Egg-Laying Traits and Egg Quality in LingKun Chickens. Front. Vet. Sci. 2022, 9, 877739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Q.; Lu, X.; Li, G.; Zhang, H.; Zhou, C.; Yin, J.; Han, W.; Yang, H. Genetic analysis of egg production traits in Luhua chickens: Insights from a multi-trait animal model and a genome-wide association study. Genes 2024, 15, 796. [Google Scholar] [CrossRef]
- Roff, D.A. Trade-offs between growth and reproduction: An analysis of the quantitative genetic evidence. J. Evol. Biol. 2000, 13, 434–445. [Google Scholar] [CrossRef]
- Ghazikhani Shad, A.; Nejati Javaremi, A.; Mehrabani Yeganeh, H. Animal Model Estimation of Genetic Parameters for Most Important Economic Traits in Iranian Native Fowls. Pak. J. Biol. Sci. 2007, 10, 2787–2789. [Google Scholar] [PubMed]
- Niknafs, S.; Nejati-Javaremi, A.; Mehrabani-Yeganeh, H.; Fatemi, S.A. Estimation of genetic parameters for body weight and egg production traits in Mazandaran native chicken. Trop. Anim. Health Prod. 2012, 44, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.A.; Savegnago, R.P.; Grupioni, N.V.; Ramos, S.B.; Stafuzza, N.B.; de Figueiredo, E.A.P.; Schmidt, G.S.; Ledur, M.C.; Munari, D.P. Reduced-rank estimation of genetic parameters for egg production traits and cluster analyses with predicted breeding values. Acta Agric. Scand. Sect. A—Anim. Sci. 2019, 68, 81–86. [Google Scholar] [CrossRef]
- Lwelamira, J.; Kifaro, G.C.; Gwakisa, P.S. Genetic parameters for body weights, egg traits and antibody response against Newcastle Disease Virus (NDV) vaccine among two Tanzania chicken ecotypes. Trop. Anim. Health Prod. 2009, 41, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Oleforuh-Okoleh, V.U. Estimation of genetic parameters and selection for egg production traits in a Nigerian local chicken ecotype. J. Agri. Biol. Sci. 2011, 6, 54–57. [Google Scholar]
- Jafarnejad, A.; Kamali, M.A.; Fatemi, S.J.; Aminafshar, M. Genetic evaluation of laying traits in Iranian indigenous hens using univariate and bivariate animal models. J. Anim. Plant Sci. 2017, 27, 20–27. [Google Scholar]
- Dana, N.; vander Waaij, E.H.; van Arendonk, J.A.M. Genetic and phenotypic parameter estimates for body weights and egg production in Horro chicken of Ethiopia. Trop. Anim. Health Prod. 2011, 43, 21–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ekinci, O.; Esenbuga, N.; Dagdemir, V. The effects of body weight and age on performance, egg quality, blood parameters, and economic production of laying hens. J. Hell. Vet. Med. Soc. 2023, 74, 5799–5806. [Google Scholar] [CrossRef]
- Kuhnlein, U.; Ni, L.; Weigend, S.; Gavora, J.S.; Fairfull, W.; Zadworny, D. DNA polymorphisms in the chicken growth hormone gene: Response to selection for disease resistance and association with egg production. Anim. Genet. 1997, 28, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.P.; Kuhnlein, U.; Fairfull, W.R.; Aggrey, S.E.; Yao, J.; Zadworny, D. A genetic marker in the growth hormone receptor gene associated with body weight in chickens. J. Hered. 1998, 89, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Zhu, W.Q.; Chen, K.W.; Wu, X.; Tang, Q.P.; Gao, Y.S. Associations between GHR and IGF-1 gene polymorphisms, and reproductive traits in Wenchang chickens. Turk. J. Vet. Anim. Sci. 2008, 32, 281–285. [Google Scholar]
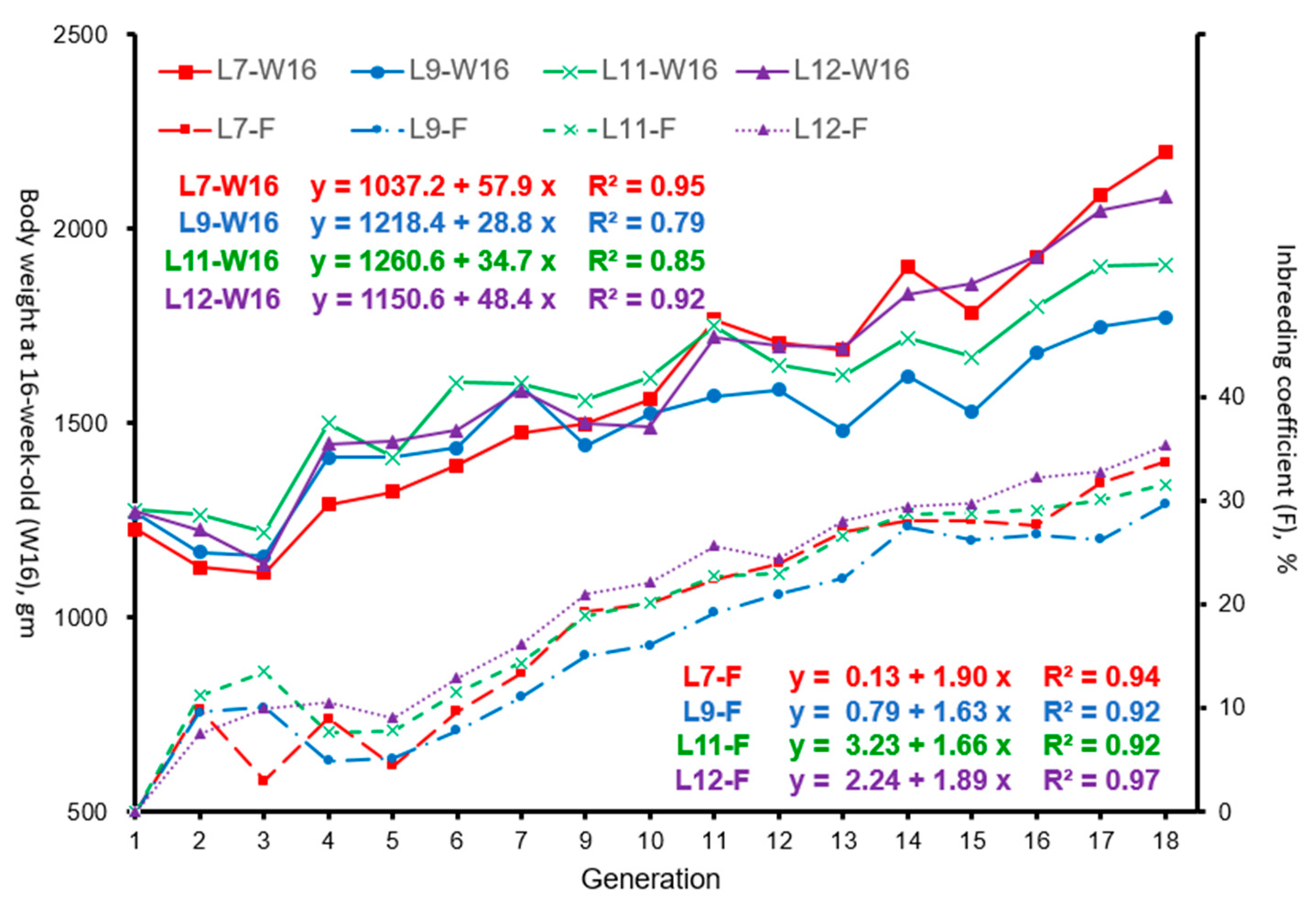

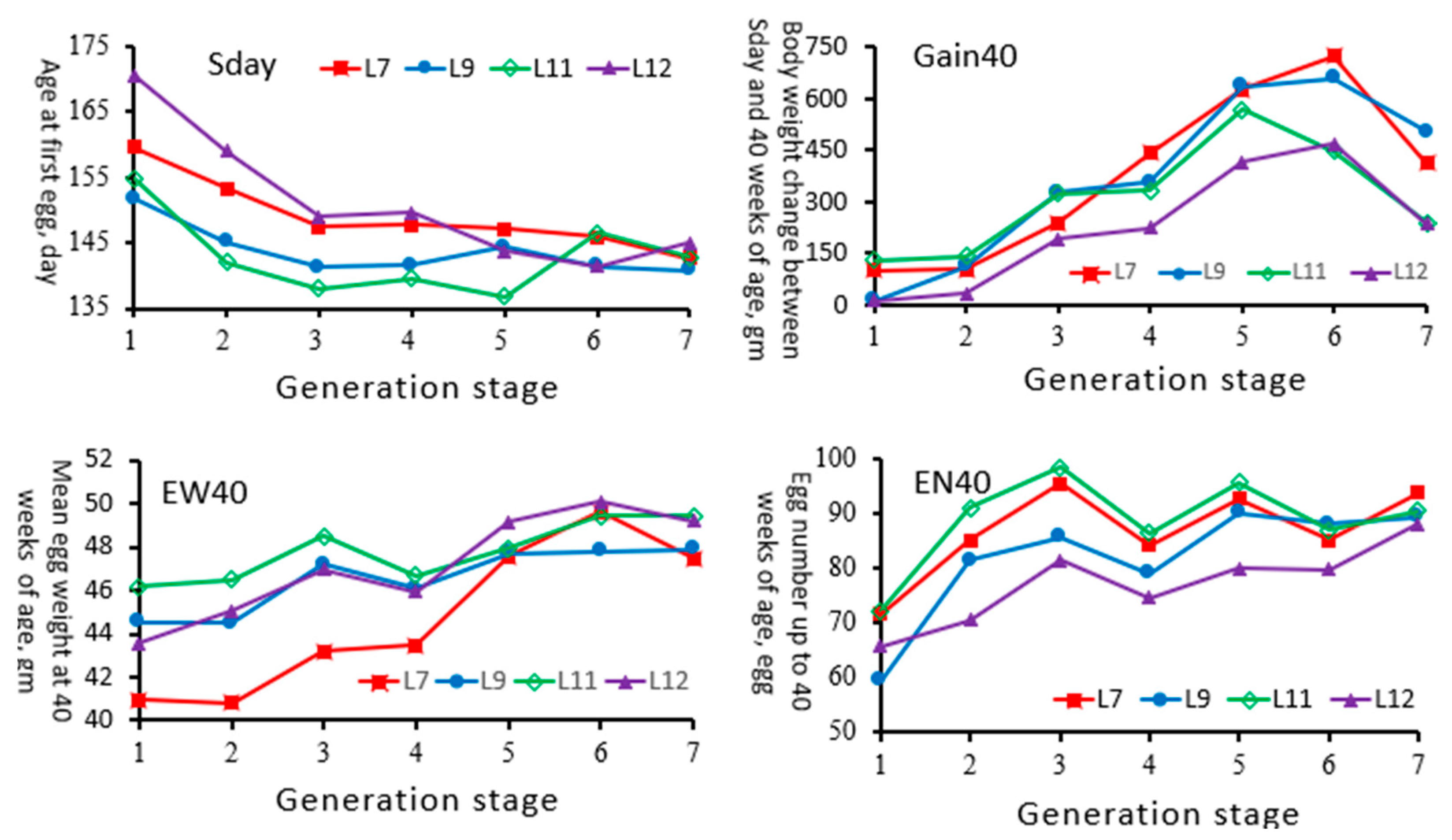
| Line | No of Laid Hen Recorded | Paternal Lineage | Maternal Lineage | ||||
|---|---|---|---|---|---|---|---|
| Sire | PGS | PGD | Dam | MGS | MGD | ||
| L7 | 2390 | 190 | 116 | 166 | 678 | 219 | 424 |
| L9 | 2417 | 186 | 116 | 157 | 735 | 213 | 459 |
| L11 | 2982 | 204 | 136 | 174 | 826 | 270 | 540 |
| L12 | 2045 | 182 | 113 | 148 | 651 | 221 | 406 |
| Line | Trait | N | Mean | Min | Max | Quantile | CV | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 | 0.95 | (%) | ||||||||
| L7 | W16, gm | 2265 | 1627.4 | 580 | 2630 | 1112 | 2198 | 20.9 | 0.13 | −0.65 |
| Sday, day | 2390 | 148.5 | 116 | 209 | 131 | 169 | 7.9 | 0.71 | 1.38 | |
| Gain40, gm | 2379 | 439.0 | −857 | 1747 | −33 | 1005 | 74.8 | 0.31 | −0.23 | |
| EW40, gm | 2169 | 45.5 | 27 | 66 | 37.6 | 53.2 | 10.7 | 0.02 | 0.20 | |
| EN40, egg | 2389 | 86.9 | 3 | 133 | 50 | 114 | 22.6 | −0.75 | 0.85 | |
| L9 | W16, gm | 2312 | 1493.7 | 815 | 2324 | 1093 | 1866 | 15.7 | −0.07 | −0.13 |
| Sday, day | 2418 | 143.4 | 116 | 210 | 126 | 164 | 8.1 | 0.56 | 0.74 | |
| Gain40, gm | 2413 | 397.7 | −870 | 1897 | −122 | 1017 | 86.0 | 0.37 | 0.42 | |
| EW40, gm | 2139 | 46.7 | 26 | 76 | 40.6 | 53.0 | 8.4 | −0.07 | 2.60 | |
| EN40, egg | 2413 | 83.3 | 1 | 143 | 38 | 114 | 27.5 | −0.87 | 0.87 | |
| L11 | W16, gm | 2815 | 1564.7 | 824 | 2382 | 1136 | 1973 | 16.5 | −0.03 | −0.49 |
| Sday, day | 2982 | 143.5 | 116 | 222 | 127 | 168 | 9.4 | 1.50 | 4.07 | |
| Gain40, gm | 2980 | 303.3 | −702 | 1545 | −62.5 | 748.5 | 84.9 | 0.63 | 1.27 | |
| EW40, gm | 2700 | 47.8 | 26 | 69 | 41.8 | 53.8 | 8.0 | 0.12 | 1.71 | |
| EN40, egg | 2979 | 88.2 | 1 | 145 | 41 | 119 | 26.8 | −0.95 | 0.96 | |
| L12 | W16, gm | 1942 | 1616.4 | 650 | 2785 | 1095 | 2148 | 20.4 | 0.06 | −0.66 |
| Sday, day | 2045 | 151.1 | 121 | 211 | 130 | 180 | 10.2 | 0.75 | 0.42 | |
| Gain40, gm | 2043 | 234.3 | −714 | 1599 | −147 | 725 | 113.8 | 0.45 | 0.88 | |
| EW40, gm | 1829 | 47.3 | 32 | 72 | 40.5 | 54.2 | 8.8 | 0.12 | 1.09 | |
| EN40, egg | 2044 | 77.1 | 2 | 132 | 34 | 106 | 28.0 | −0.90 | 0.89 | |
| Generation Stage | Generation | L7 | L9 | L11 | L12 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | |||
| GS1 | G1 | 0 | - | 0 | - | 0 | - | 0 | - | - |
| GS2 | G2–G4 | 0.07 | 0–0.31 | 0.09 | 0–0.38 | 0.10 | 0–0.38 | 0.09 | 0–0.34 | 0.043 |
| GS3 | G5–G7 | 0.09 | 0.02–0.15 | 0.08 | 0.02–0.15 | 0.11 | 0–0.26 | 0.13 | 0–0.28 | <0.0001 |
| GS4 | G8–G10 | 0.18 | 0.11–0.34 | 0.15 | 0.10–0.33 | 0.19 | 0.11–0.37 | 0.21 | 0.16–0.37 | <0.0001 |
| GS5 | G11–G13 | 0.25 | 0.17–0.41 | 0.21 | 0.14–0.35 | 0.24 | 0.19–0.34 | 0.26 | 0.20–0.43 | <0.0001 |
| GS6 | G14–G16 | 0.28 | 0.23–0.49 | 0.27 | 0.22–0.43 | 0.29 | 0.24–0.49 | 0.31 | 0.27–0.48 | <0.0001 |
| GS7 | G17–G18 | 0.33 | 0.28–0.52 | 0.28 | 0.24–0.44 | 0.31 | 0.28–0.47 | 0.34 | 0.30–0.50 | <0.0001 |
| Line | Trait | Generational Stage | ||||||
|---|---|---|---|---|---|---|---|---|
| GS1 | GS2 | GS3 | GS4 | GS5 | GS6 | GS7 | ||
| L7 | Sday | 0.31 (0.11) | 0.61 (0.13) | 0.25 (0.11) | 0.44 (0.12) | 0.20 (0.10) | 0.46 (0.10) | 0.28 (0.15) NS |
| Gain40 | 0.35 (0.07) | 0.44 (0.14) | 0.47 (0.12) | 0.41 (0.10) | 0.34 (0.09) | 0.42 (0.07) | 0.51 (0.12) | |
| EW40 | 0.54 (0.10) | 0.75 (0.14) | 0.74 (0.08) | 0.80 (0.11) | 0.56 (0.05) | 0.42 (0.07) | 0.32 (0.07) | |
| EN40 | 0.35 (0.10) | 0.38 (0.14) | 0.19 (0.10) NS | 0.20 (0.09) | 0.15 (0.09) NS | 0.58 (0.05) | 0.27 (0.16) NS | |
| L9 | Sday | 0.38 (0.13) | 0.09 (0.08) NS | 0.17 (0.09) NS | 0.10 (0.13) NS | 0.29 (0.13) | 0.42 (0.12) | 0.23 (0.12) NS |
| Gain40 | 0.38 (0.14) | 0.29 (0.09) | 0.23 (0.09) | 0.34 (0.14) | 0.70 (0.12) | 0.66 (0.06) | 0.60 (0.07) | |
| EW40 | 0.42 (0.13) | 0.38 (0.10) | 0.44 (0.09) | 0.57 (0.14) | 0.60 (0.12) | 0.51 (0.05) | 0.74 (0.10) | |
| EN40 | 0.48 (0.12) | 0.15 (0.07) | 0.25 (0.10) | 0.27 (0.14) NS | 0.52 (0.06) | 0.12 (0.06) | 0.00 (0.01) NS | |
| L11 | Sday | 0.36 (0.10) | 0.24 (0.14) | 0.38 (0.12) | 0.32 (0.13) | 0.22 (0.11) | 0.28 (0.09) | 0.37 (0.15) |
| Gain40 | 0.31 (0.10) | 0.17 (0.07) | 0.57 (0.07) | 0.52 (0.07) | 0.45 (0.12) | 0.36 (0.05) | 0.30 (0.10) | |
| EW40 | 0.63 (0.11) | 0.54 (0.08) | 0.61 (0.09) | 0.53 (0.04) | 0.57 (0.07) | 0.63 (0.07) | 0.65 (0.11) | |
| EN40 | 0.49 (0.12) | 0.31 (0.11) | 0.17 (0.08) | 0.09 (0.06) NS | 0.13 (0.06) | 0.10 (0.06) NS | 0.52 (0.08) | |
| L12 | Sday | 0.49 (0.11) | 0.27 (0.10) | 0.36 (0.11) | 0.48 (0.14) | 0.27 (0.14) NS | 0.32 (0.11) | 0.28 (0.14) |
| Gain40 | 0.34 (0.09) | 0.15 (0.08) NS | 0.39 (0.10) | 0.03 (0.05) NS | 0.47 (0.12) | 0.48 (0.15) | 0.34 (0.11) | |
| EW40 | 0.69 (0.09) | 0.32 (0.06) | 0.52 (0.15) | 0.57 (0.11) | 0.64 (0.09) | 0.83 (0.08) | 0.48 (0.14) | |
| EN40 | 0.65 (0.09) | 0.25 (0.09) | 0.18 (0.09) | 0.19 (0.10) NS | 0.44 (0.15) | 0.39 (0.18) | 0.16 (0.10) NS | |
| Line | Trait | Generational Stage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GS1 (G1) | GS7 (G17–G18) | ||||||||
| Sday | Gain40 | EW40 | EN40 | Sday | Gain40 | EW40 | EN40 | ||
| L7 | Sday | - | −0.54 * | −0.09 | −0.27 | - | −0.09 | −0.38 * | −0.81 * |
| Gain40 | −0.18 * | - | 0.07 | 0.23 | −0.19 * | - | 0.45 | 0.20 | |
| EW40 | −0.09 | −0.03 | - | −0.47 * | −0.13 * | 0.13 * | - | −0.11 | |
| EN40 | −0.33 * | 0.07 | −0.03 | - | −0.27 * | −0.03 | 0.04 | - | |
| L9 | Sday | - | −0.33 * | −0.22 | −0.46 * | - | 0.31 | 0.52 | −0.31 |
| Gain40 | −0.26 * | - | 0.30 | 0.72 * | −0.07 | - | 0.49 * | −0.30 | |
| EW40 | −0.14 | 0.07 | - | −0.22 | 0.18 * | 0.20 * | - | −0.96 | |
| EN40 | −0.27 * | 0.36 * | 0.13 | - | −0.19 * | −0.11 | 0.02 | - | |
| L11 | Sday | - | −0.12 | −0.31 | −0.72 * | - | −0.22 | 0.02 | −0.70 * |
| Gain40 | −0.28 * | - | 0.34 * | 0.30 | −0.09 | - | −0.05 | 0.20 | |
| EW40 | −0.19 * | 0.20 * | - | 0.18 | −0.03 | −0.01 | - | −0.24 * | |
| EN40 | −0.35 * | 0.04 | 0.17 * | - | −0.24 * | −0.12 * | −0.11 | - | |
| L12 | Sday | - | −0.19 | −0.26 | −0.57 * | - | 0.86 * | −0.50 | −0.81 * |
| Gain40 | −0.19 * | - | 0.01 | 0.13 | 0.08 | - | −0.42 | −0.40 | |
| EW40 | −0.11 | −0.02 | - | 0.30 | −0.09 | −0.09 | - | 0.42 | |
| EN40 | −0.46 * | 0.17 * | 0.12 * | - | −0.29 * | −0.17 * | 0.18 * | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.-Y.; Chu, C.-T.; Lin, M.-Y.; Tsai, M.-Y.; Tzeng, S.-J.; Wu, M.-C.; Chang, H.-L. Heritability and Genetic Correlation of Age at First Egg and Egg Number up to 40 Weeks of Age After Long-Term Selection in Taiwan Indigenous Chicken. Animals 2025, 15, 1534. https://doi.org/10.3390/ani15111534
Lin D-Y, Chu C-T, Lin M-Y, Tsai M-Y, Tzeng S-J, Wu M-C, Chang H-L. Heritability and Genetic Correlation of Age at First Egg and Egg Number up to 40 Weeks of Age After Long-Term Selection in Taiwan Indigenous Chicken. Animals. 2025; 15(11):1534. https://doi.org/10.3390/ani15111534
Chicago/Turabian StyleLin, Der-Yuh, Chia-Te Chu, Mu-Yao Lin, Ming-Yang Tsai, Shwu-Jen Tzeng, Ming-Che Wu, and Hsiu-Luan Chang. 2025. "Heritability and Genetic Correlation of Age at First Egg and Egg Number up to 40 Weeks of Age After Long-Term Selection in Taiwan Indigenous Chicken" Animals 15, no. 11: 1534. https://doi.org/10.3390/ani15111534
APA StyleLin, D.-Y., Chu, C.-T., Lin, M.-Y., Tsai, M.-Y., Tzeng, S.-J., Wu, M.-C., & Chang, H.-L. (2025). Heritability and Genetic Correlation of Age at First Egg and Egg Number up to 40 Weeks of Age After Long-Term Selection in Taiwan Indigenous Chicken. Animals, 15(11), 1534. https://doi.org/10.3390/ani15111534






