Yogurt in Combination with Inactivated Pediococcus lactis Modulated Feline Lipid Metabolism, Anti-Inflammation and Fecal Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
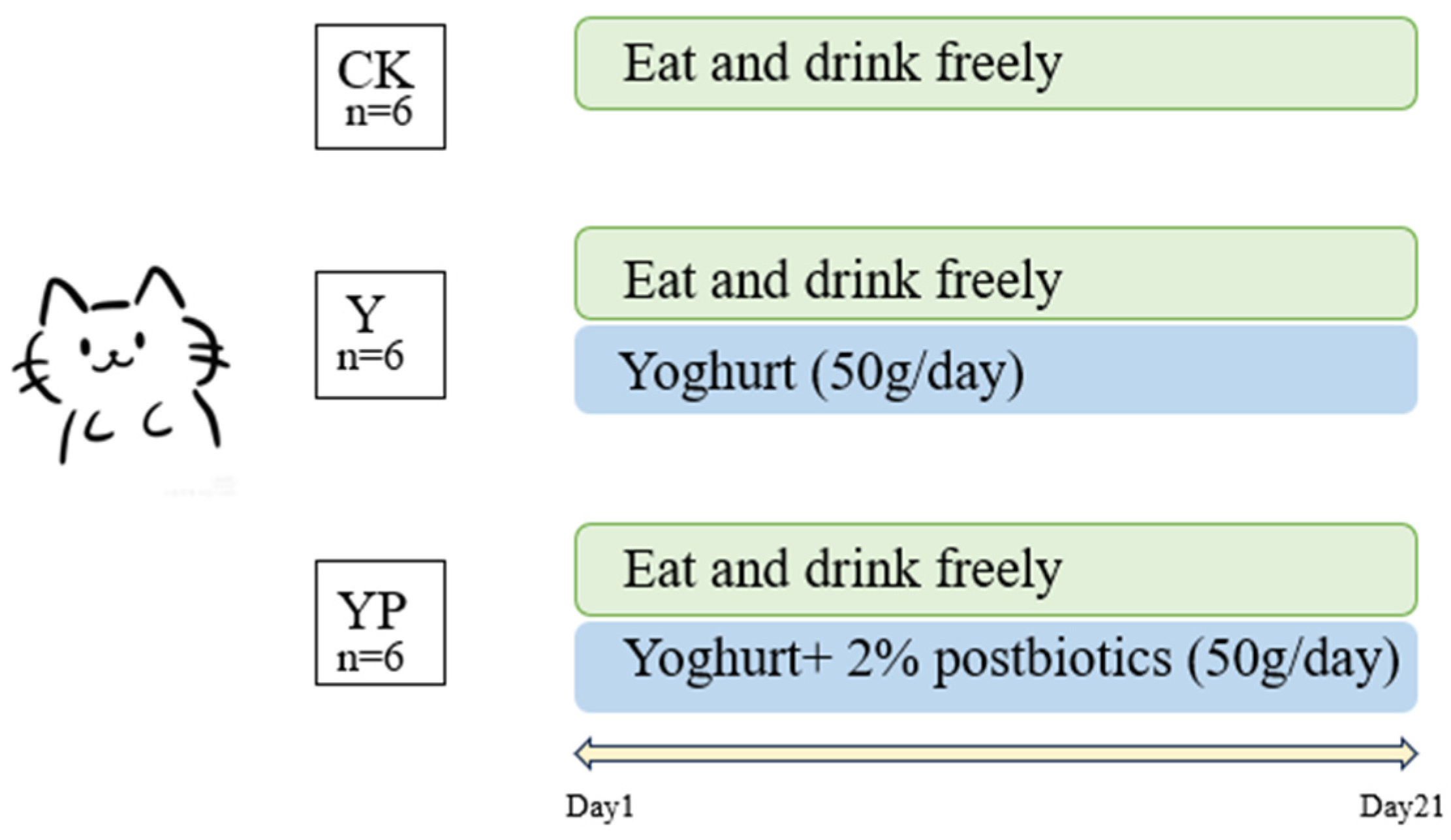
2.1. Experimental Design and Sample Collection
2.2. Yogurt Fermentation
2.3. Blood Biochemical Parameters
2.4. Analysis of Blood Immunological Parameters and Cellular Inflammatory Factors
2.5. Fecal DNA Extraction
2.6. PCR Amplification of Amplicons
2.7. Statistical Analysis
3. Results
3.1. Blood Biochemical Parameters Results
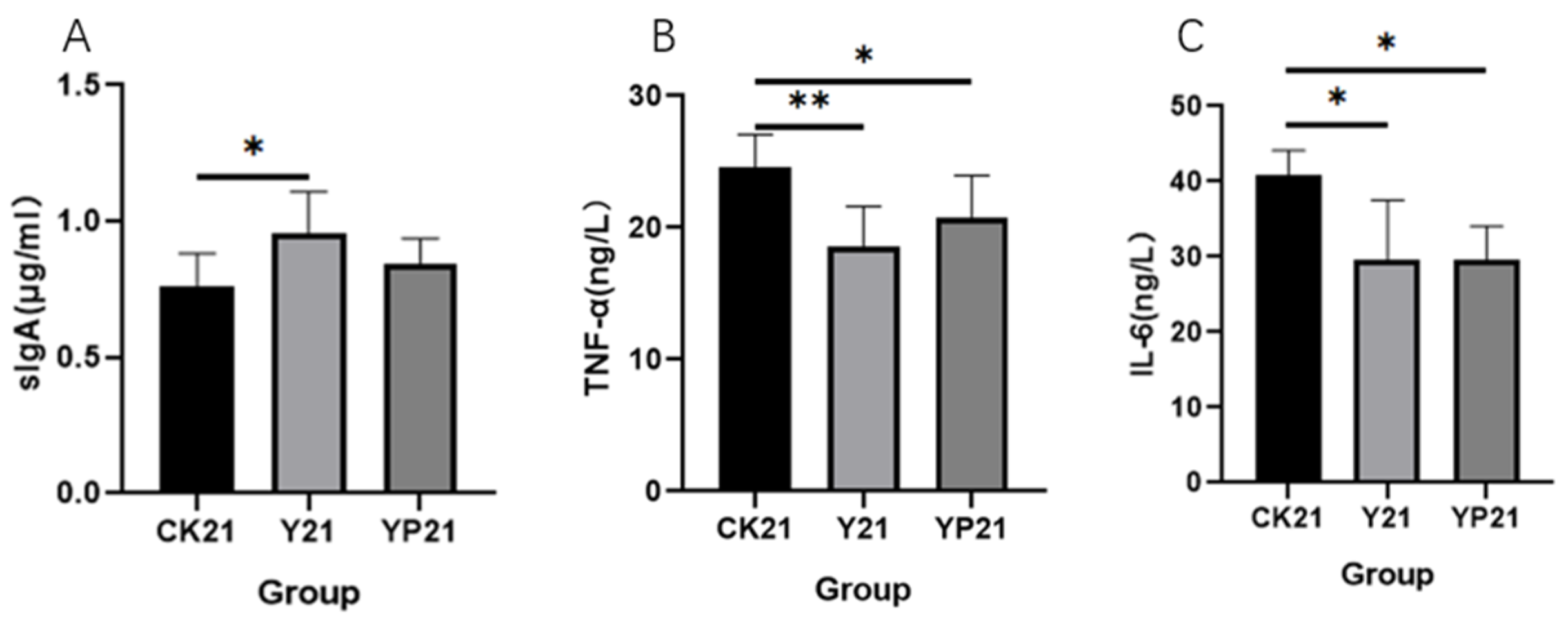
3.2. Blood Immune Parameters and Cytokine Results
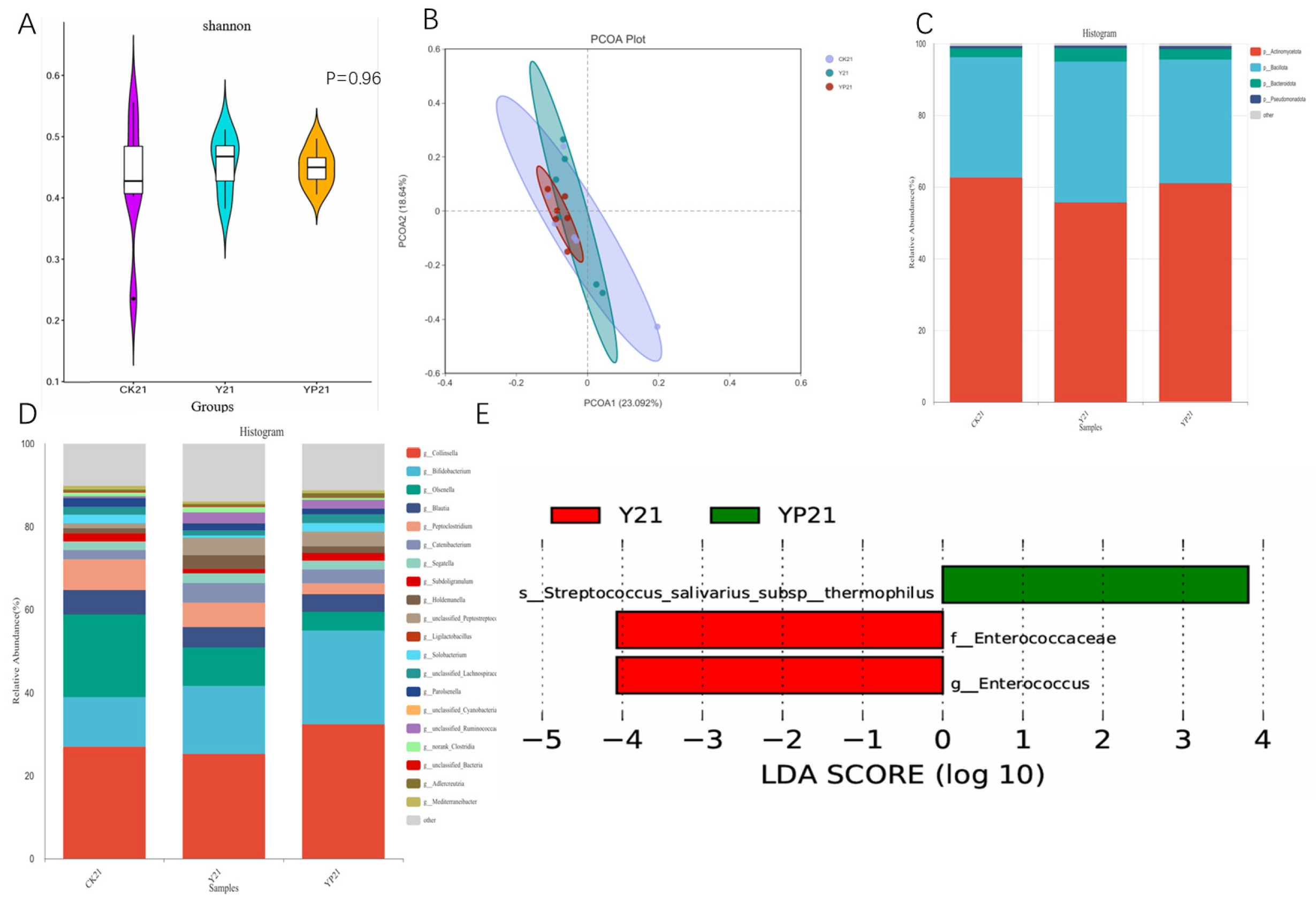
3.3. Fecal Microbiota Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Khorshidian, N.; Yousefi, M.; Mortazavian, A.M. Fermented milk: The most popular probiotic food carrier. Adv. Food Nutr. Res. 2020, 94, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut-inflammation and colon-cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Chen, L.; Bagnicka, E.; Chen, H.; Shu, G. Health potential of fermented goat dairy products: Composition comparison with fermented cow milk, probiotics selection, health benefits and mechanisms. Food Funct. 2023, 14, 3423–3436. [Google Scholar] [CrossRef]
- Pei, R.; Martin, D.A.; DiMarco, D.M.; Bolling, B.W. Evidence for the effects of yogurt on gut health and obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 1569–1583. [Google Scholar] [CrossRef]
- Gaschen, F.P.; Merchant, S.R. Adverse food reactions in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 361–379. [Google Scholar] [CrossRef]
- Wills, J.; Harvey, R. Diagnosis and management of food allergy and intolerance in dogs and cats. Aust. Vet. J. 1994, 71, 322–326. [Google Scholar] [CrossRef]
- Han, B.; Sun, J.; Gu, X.; Wang, J.; Wang, X.; Tao, H.; Wang, Z.; Liu, J. The prebiotic potential of lactobin and glucans combined with goat’s milk in cats. Can. J. Vet. Res. 2025, 89, 36–42. [Google Scholar]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Falchi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Fusi, J. Feline neonatology: From birth to commencement of weaning—What to know for successful management. J. Feline Med. Surg. 2022, 24, 232–242. [Google Scholar] [CrossRef]
- Ahola, M.K.; Vapalahti, K.; Lohi, H. Early weaning increases aggression and stereotypic behaviour in cats. Sci. Rep. 2017, 7, 10412. [Google Scholar] [CrossRef]
- Lin, A.; Yan, X.; Wang, H.; Su, Y.; Zhu, W. Effects of lactic acid bacteria-fermented formula milk supplementation on ileal microbiota, transcriptomic profile, and mucosal immunity in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 113. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The concept of postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary supplementation of postbiotics mitigates adverse impacts of heat stress on antioxidant enzyme activity, total antioxidant, lipid peroxidation, physiological stress indicators, lipid profile and meat quality in broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef]
- Duysburgh, C.; Nicolas, C.; Van den Broeck, M.; Lloret, F.; Monginoux, P.; Rème, C.; Marzorati, M. A specific blend of prebiotics and postbiotics improved gut microbiome of dogs with soft stools in the in vitro Simulator of the Canine Intestinal Microbial Ecosystem (SCIME). J. Anim. Sci. 2025, 103, skaf056. [Google Scholar] [CrossRef]
- Vandenplas, Y.; de Halleux, V.; Arciszewska, M.; Lach, P.; Pokhylko, V.; Klymenko, V.; Schoen, S.; Abrahamse-Berkeveld, M.; Mulder, K.A.; Porcel Rubio, R.; et al. A partly fermented infant formula with postbiotics including 3′-GL, specific oligosaccharides, 2′-FL, and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants: A double-blind, randomised, controlled, multi-country trial. Nutrients 2020, 12, 3560. [Google Scholar] [CrossRef]
- Tanihiro, R.; Yuki, M.; Sakano, K.; Sasai, M.; Sawada, D.; Ebihara, S.; Hirota, T. Effects of heat-treated Lactobacillus helveticus CP790-fermented milk on gastrointestinal health in healthy adults: A randomized double-blind placebo-controlled trial. Nutrients 2024, 16, 2191. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Kang, Y.; Zhao, Y.; Sun, J.; Wang, X.; Tao, H.; Wang, Z.; Wang, J.; Zhong, Y.; Han, B. Characterization and potential lipid-lowering effects of lactic acid bacteria isolated from cats. Front. Microbiol. 2024, 15, 1392864. [Google Scholar] [CrossRef]
- Sugawara, T.; Sawada, D.; Yanagihara, S.; Aoki, Y.; Takehara, I.; Sugahara, H.; Hirota, T.; Nakamura, Y.; Ishikawa, S. Daily intake of paraprobiotic Lactobacillus amylovorus CP1563 improves pre-obese conditions and affects the gut microbial community in healthy pre-obese subjects: A double-blind, randomized, placebo-controlled study. Microorganisms 2020, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int. J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 582–587. [Google Scholar] [CrossRef]
- Eslami, M.; Pakmehr, A.; Pourghazi, F.; Kami, A.; Ejtahed, H.S.; Mohajeri-Tehrani, M.; Hasani-Ranjbar, S.; Larijani, B. The anti-obesity effects of postbiotics: A systematic review of pre-clinical and clinical studies. Clin. Nutr. ESPEN 2024, 64, 370–389. [Google Scholar] [CrossRef]
- Clark, M.; Hoenig, M. Metabolic Effects of Obesity and Its Interaction with Endocrine Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Ren, Y.; Cui, Y.; Li, R.; Wang, C.; Zhang, Y. Obeticholic acid protects mice against lipopolysaccharide-induced liver injury and inflammation. Biomed Pharmacother. 2017, 96, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Zeng, Y.; Zhang, Z.C.; Lin, Z.H.; Chen, L.C.; Ye, Z.S. Incidence and clinical characteristics of hypertriglyceridemic acute pancreatitis: A retrospective single-center study. World J Gastroenterol. 2022, 28, 3946–3959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Wen, Y.; Wang, L.; Chen, B.; Chen, J.; Wang, H.; Chen, L. Hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Exp. Mol. Med. 2021, 53, 1735–1747, Erratum in Exp. Mol. Med. 2022, 54, 862–865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ban, Q.; Sun, X.; Jiang, Y.; Cheng, J.; Guo, M. Effect of synbiotic yogurt fortified with monk fruit extract on hepatic lipid biomarkers and metabolism in rats with type 2 diabetes. J. Dairy Sci. 2022, 105, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Q.; Zhang, L.; Liu, J.; Wang, G. Serum bilirubin level is increased in metabolically healthy obesity. Front. Endocrinol. 2022, 12, 792795. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Joung, M.; Park, J.H.; Ha, S.K.; Park, H.Y. Role of postbiotics in diet-induced metabolic disorders. Nutrients 2022, 14, 3701. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, J.; Qin, Y.; Huang, W.; You, Y.; Zhan, J. Dietary regulation of the SIgA-gut microbiota interaction. Crit. Rev. Food Sci. Nutr. 2023, 63, 6379–6392. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Cao, Y.; Yan, J.K.; Cai, W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine 2016, 83, 189–192. [Google Scholar] [CrossRef]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef]
- Duysburgh, C.; Miclotte, L.; Green, J.B.; Watts, K.T.; Sardi, M.I.; Chakrabarti, A.; Khafipour, E.; Marzorati, M. Saccharomyces cerevisiae derived postbiotic alters gut microbiome metabolism in the human distal colon resulting in immunomodulatory potential in vitro. Front. Microbiol. 2024, 15, 1358456. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Mining Lactobacillus and Bifidobacterium for organisms with long-term gut colonization potential. Clin. Nutr. 2020, 39, 1315–1323. [Google Scholar] [CrossRef]
- Lu, J.; Shataer, D.; Yan, H.; Dong, X.; Zhang, M.; Qin, Y.; Cui, J.; Wang, L. Probiotics and Non-Alcoholic Fatty Liver Disease: Unveiling the Mechanisms of Lactobacillus plantarum and Bifidobacterium bifidum in Modulating Lipid Metabolism, Inflammation, and Intestinal Barrier Integrity. Foods 2024, 13, 2992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Gu, X.; Wang, J.; Wang, X.; Wang, Z.; Tao, H.; Wang, J.; Han, B. Yogurt in Combination with Inactivated Pediococcus lactis Modulated Feline Lipid Metabolism, Anti-Inflammation and Fecal Microbiota. Animals 2025, 15, 1531. https://doi.org/10.3390/ani15111531
Sun J, Gu X, Wang J, Wang X, Wang Z, Tao H, Wang J, Han B. Yogurt in Combination with Inactivated Pediococcus lactis Modulated Feline Lipid Metabolism, Anti-Inflammation and Fecal Microbiota. Animals. 2025; 15(11):1531. https://doi.org/10.3390/ani15111531
Chicago/Turabian StyleSun, Jintao, Xinshu Gu, Jiaxue Wang, Xiumin Wang, Zhenlong Wang, Hui Tao, Jinquan Wang, and Bing Han. 2025. "Yogurt in Combination with Inactivated Pediococcus lactis Modulated Feline Lipid Metabolism, Anti-Inflammation and Fecal Microbiota" Animals 15, no. 11: 1531. https://doi.org/10.3390/ani15111531
APA StyleSun, J., Gu, X., Wang, J., Wang, X., Wang, Z., Tao, H., Wang, J., & Han, B. (2025). Yogurt in Combination with Inactivated Pediococcus lactis Modulated Feline Lipid Metabolism, Anti-Inflammation and Fecal Microbiota. Animals, 15(11), 1531. https://doi.org/10.3390/ani15111531






