Characteristics of Amino Acid and Glucose Digestion and Metabolism in Energy and Protein Feedstuffs for Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstuff Sources
2.2. Nutritional Composition Analysis of Feedstuffs
2.3. In Vitro Digestion Experiment
2.4. Determination of Total Amino Acid (TAA) and Glucose Contents
2.5. Determination of Serum Free Amino Acids
2.6. In Vivo Amino Acid Release Kinetics Experiment
2.6.1. Animal Ethics Statement
2.6.2. Experimental Design
2.7. Statistical Analysis
3. Results
3.1. Nutritional Composition Analysis of Energy and Protein Feedstuffs
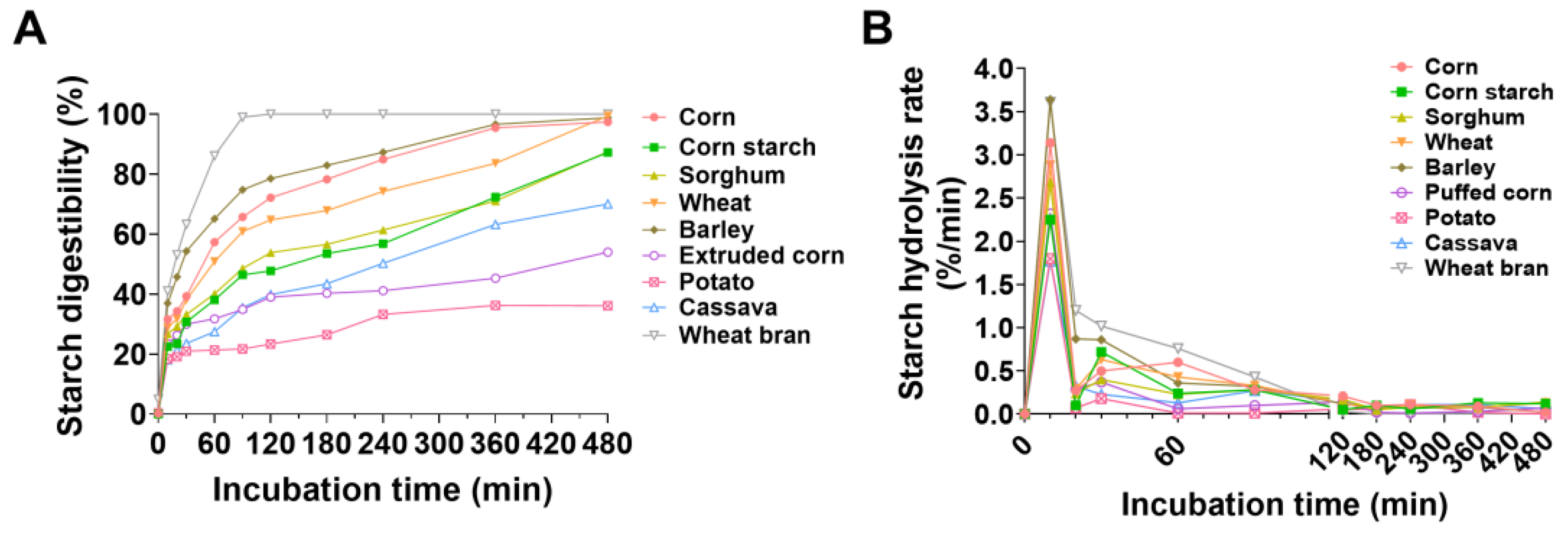
3.2. In Vitro Enzyme Hydrolysis Curve and Characteristics of Energy Feedstuffs
3.3. Multiple Linear Regression Model of Starch Digestibility on the Basis of Nutrient Composition of Energy Feedstuffs
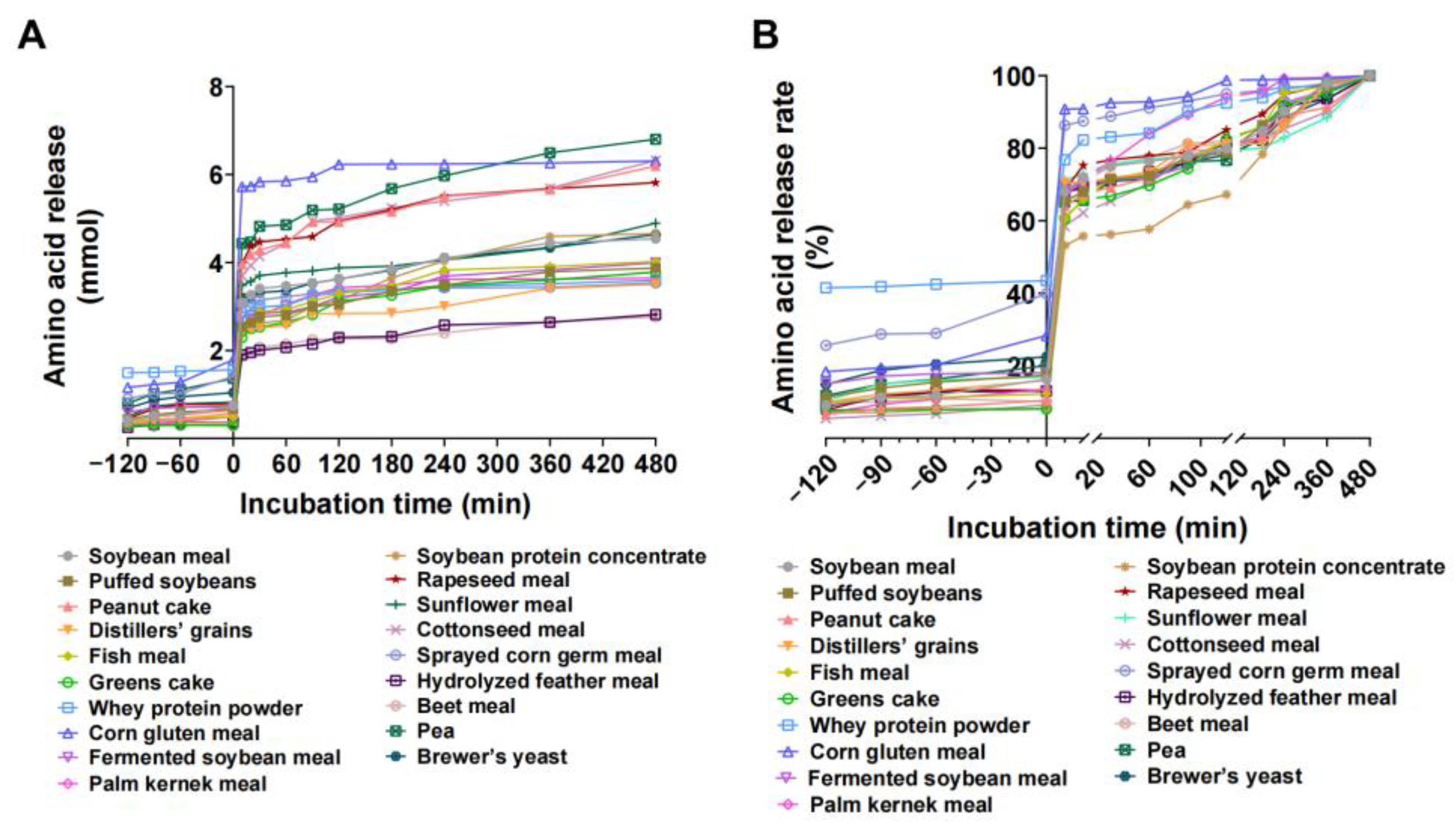
3.4. In Vitro Enzyme Hydrolysis and Amino Acid Release Characteristics of Protein Feedstuffs
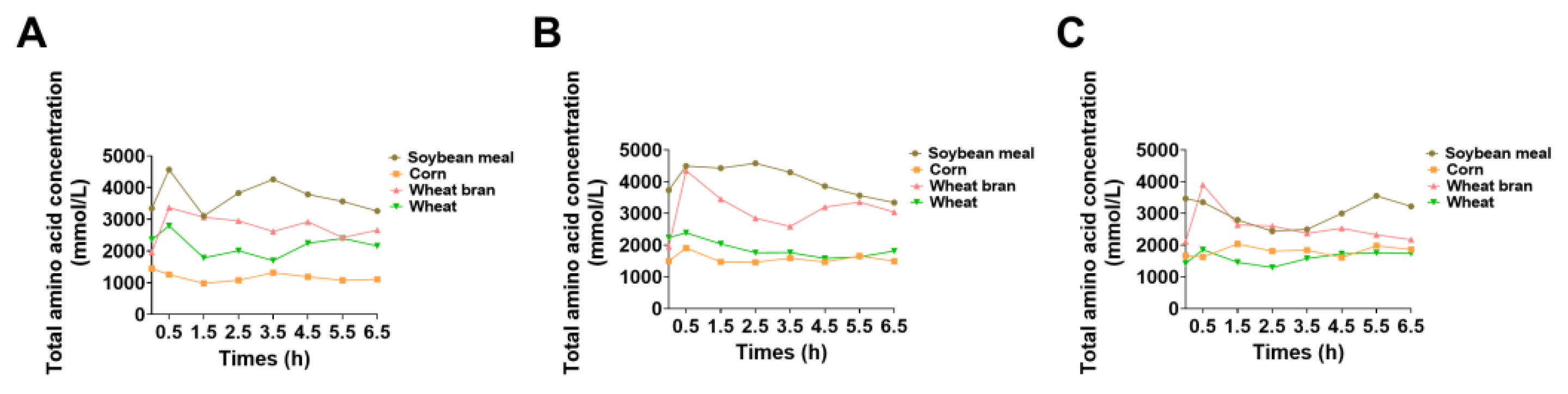
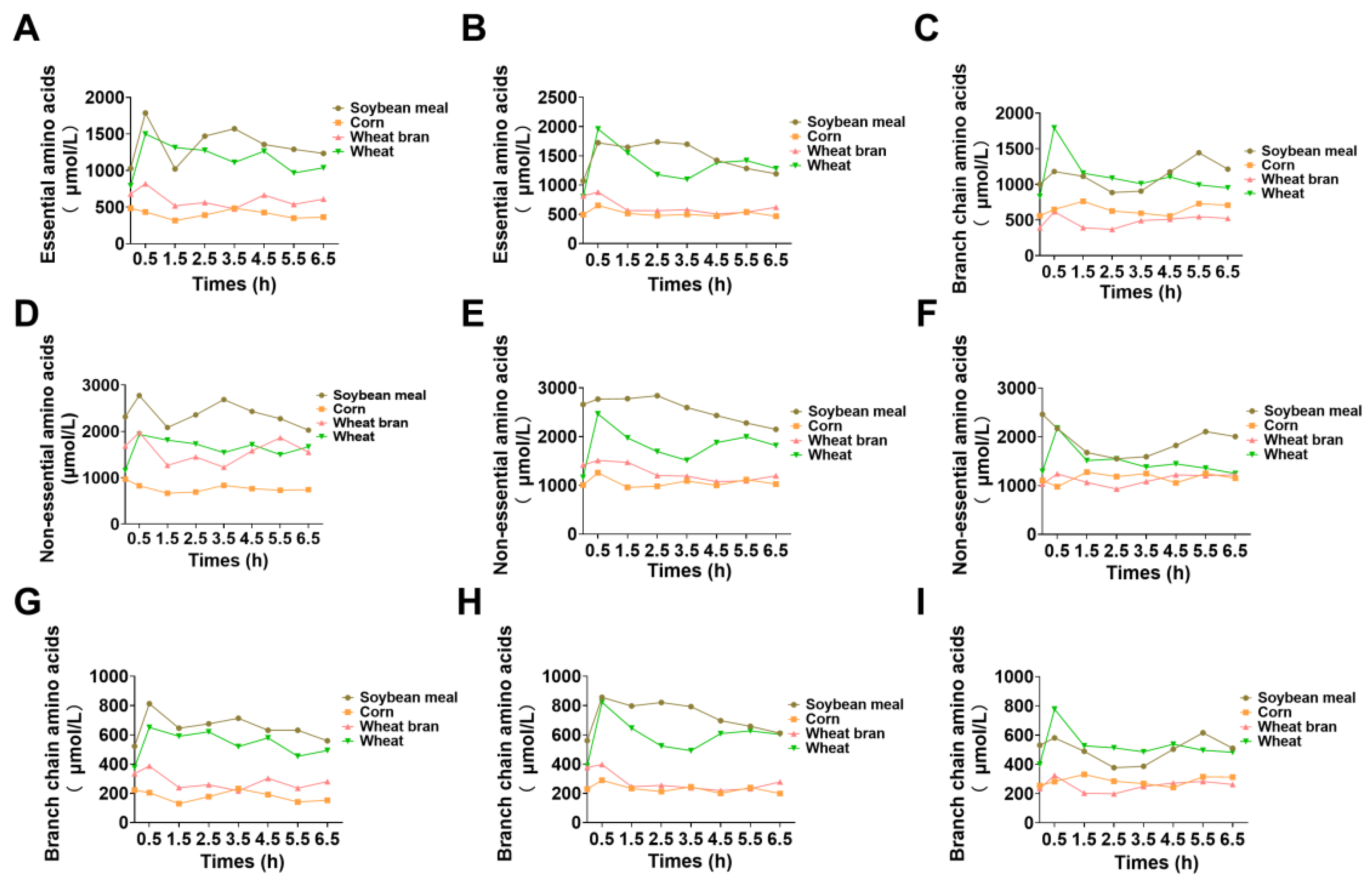
3.5. In Vivo Amino Acid Metabolism and Deposition Characteristics
3.6. Portal Vein Amino Acid Release Fitting Model Based on Chemical Components and In Vitro Digestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knudsen, K.E.B.; Lærke, H.N.; Ingerslev, A.K.; Hedemann, M.S.; Nielsen, T.S.; Theil, P.K. Carbohydrates in pig nutrition-recent advances. J. Anim. Sci. 2016, 94, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, S.; Huang, F.; Mo, C.; Wu, Y.; Cao, J.; Chen, S.; Wen, Z.; Liao, X. Antibiotic resistance profile of nitrogenmetabolizing microbes in anoxic-oxic processes for swine wastewater treatment. Npj Clean Water 2025, 8, 31. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, S.; Mo, C.; Dong, J.; Chen, S.; Wen, Z. Profiles of antibiotic resistome risk in diverse water environments. Commun. Earth Environ. 2025, 6, 158. [Google Scholar] [CrossRef]
- Martens, B.M.J.; Flecher, T.; de Vries, S.; Schols, H.A.; Bruininx, E.M.A.M.; Gerrits, W.J.J. Starch digestion kinetics and mechanisms of hydrolysing enzymes in growing pigs fed processed and native cereal-based diets. Br. J. Nutr. 2019, 121, 1124–1136. [Google Scholar] [CrossRef]
- Nadia, J.; Bronlund, J.; Singh, R.P.; Singh, H.; Bornhorst, G.M. Structural breakdown of starch-based foods during gastric digestion and its link to glycemic response: In vivo and in vitro considerations. Comp. Rev. Food Sci. Food Safe 2021, 20, 2660–2698. [Google Scholar] [CrossRef]
- Li, J.; Tan, B.; Tang, Y.; Liao, P.; Yao, K.; Ji, P.; Yin, Y. Extraction and identification of the chyme proteins in the digestive tract of growing pigs. Sci. China Life Sci. 2018, 61, 1396–1406. [Google Scholar] [CrossRef]
- Wenderlein, J.; Kienzle, E.; Straubinger, R.K.; Schöl, H.; Ulrich, S.; Böswald, L.F. Morphology of starch particles along the passage through the gastrointestinal tract in laboratory mice fed extruded and pelleted diets. Animals 2022, 12, 952. [Google Scholar] [CrossRef]
- Ai, Y.; Hasjim, J.; Jane, J. Effects of lipids on enzymatic hydrolysis and physical properties of starch. Carbohydr. Polym. 2013, 92, 120–127. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z. Study on the release kinetics of amino acids of different protein sourcesin growing pigs diets. J. Anim. Sci. 2018, 54, 59–65. [Google Scholar]
- Yuan, L.; Tang, Y.; Liu, X. Research on the factors affecting digestibility of protein. Food Sci. Technol. Econ. 2015, 40, 43–46. [Google Scholar]
- Lee, T.; Huang, Y.; Chiang, C.; Chung, T.; Chiou, P.W.; Yu, B. Starch characteristics and their influences on in vitro and pig prececal starch digestion. J. Agric. Food Chem. 2011, 59, 7353–7359. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wu, X.; Bin, S.; Li, T.J.; Huang, R.; Liu, Z.; Liu, Y.; Ruan, Z.; Deng, Z.; Hou, Y.; et al. Dietary amylose and amylopectin ratio and resistant starch content affects plasma glucose, lactic acid, hormone levels and protein synthesis in splanchnic tissues. J. Anim. Physiol. Anim. Nutr. 2010, 94, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Donner, E.; Yada, R.Y.; Liu, Q. Physicochemical properties and in vitro starch digestibility of potato starch/protein blends. Carbohydr. Polym. 2016, 154, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Damiran, D.; Yu, P. Molecular basis of structural makeup of hulless barley in relation to rumen degradation kinetics and intestinal availability in dairy cattle: A novel approach. J. Dairy Sci. 2011, 94, 5151–5159. [Google Scholar] [CrossRef]
- Damiran, D.; Yu, P. Structural makeup, biopolymer conformation, and biodegradation characteristics of a newly developed super genotype of oats (CDC SO-I versus conventional varieties): A novel approach. J. Agric. Food Chem. 2010, 58, 2377–2387. [Google Scholar] [CrossRef]
- Manikpuri, S.; Kheto, A.; Sehrawat, R.; Gul, K.; Routray, W.; Kumar, L. Microwave irradiation of guar seed flour: Effect on anti-nutritional factors, phytochemicals, in vitro protein digestibility, thermo-pasting, structural, and functional attributes. J. Food Sci. 2024, 89, 2188–2201. [Google Scholar] [CrossRef]
- Cao, H.; Huang, Q.; Wang, C.; Guan, X.; Huang, K.; Zhang, Y. Effect of compositional interaction on in vitro digestion of starch during the milling process of quinoa. Food Chem. 2023, 403, 134372. [Google Scholar] [CrossRef]
- Axentii, M.; Codină, G.G. Exploring the nutritional potential and functionality of hemp and rapeseed proteins: A review on unveiling anti-nutritional factors, bioactive compounds, and functional attributes. Plants. 2024, 13, 1195. [Google Scholar] [CrossRef]
- Berrocoso, J.D.; García-Ruiz, A.; Page, G.; Jaworski, N.W. The effect of added oat hulls or sugar beet pulp to diets containing rapidly or slowly digestible protein sources on broiler growth performance from 0 to 36 days of age. Poult Sci. 2020, 99, 6859–6866. [Google Scholar] [CrossRef]
- Zhou, J.; Tu, J.; Wang, L.; Yang, L.; Yang, G.; Zhao, S.; Zeng, X.; Qiao, S. Free amino acid enriched diets containing rapidly but not slowly digested carbohydrate promote amino acid absorption from intestine and net fluxes across skeletal muscle of pigs. J. Nutr. 2022, 152, 2471–2482. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, Z.; Huang, J.; Yin, Y. Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. Br. J. Nutr. 2010, 103, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, J.; Wang, X.; Jiao, H.; Lin, H. Use of encapsulated L-lysine-HCl and DL-methionine improves postprandial amino acid balance in laying hens. J. Anim. Sci. 2020, 98, skaa315. [Google Scholar] [CrossRef]
- Luo, C.; Yu, Y.; Meng, G.; Yuan, J. Slowly digestible starch impairs growth performance of broiler chickens offered low-protein diet supplemental higher amino acid densities by inhibiting the utilization of intestinal amino acid. J Anim Sci Biotechnol. 2025, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yan, F.; Huang, F.; Xiao, Y.; Xie, L. Determination of amylose and amylopectin contents in yam and taros by dual-wavelength spectrophotometry. Sci. Technol. Food Ind. 2022, 43, 303–309. [Google Scholar]
- van Kempen, T.A.T.G.; Regmi, P.R.; Matte, J.J.; Zijlstra, R.T. In vitro starch digestion kinetics, corrected for estimated gastric emptying, predict portal glucose appearance in pigs. J. Nutr. 2010, 140, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Yang, L.; Yang, G.; Zeng, X.; Qiao, S. Different dietary starch patterns in low-protein diets: Effect on nitrogen efficiency, nutrient metabolism, and intestinal flora in growing pigs. J. Anim. Sci. Biotechnol. 2022, 13, 78. [Google Scholar] [CrossRef]
- Yin, D.; Selle, P.H.; Moss, A.F.; Wang, Y.; Dong, X.; Xiao, Z.; Guo, Y.; Yuan, J. Influence of starch sources and dietary protein levels on intestinal functionality and intestinal mucosal amino acids catabolism in broiler chickens. J. Anim. Sci. Biotechnol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Drew, M.D.; Schafer, T.C.; Zijlstra, R.T. Glycemic index of starch affects nitrogen retention in grower pigs. J. Anim. Sci. 2012, 90, 1233–1241. [Google Scholar] [CrossRef]
- Gong, X.; Hui, X.; Wu, G.; Morton, J.D.; Brennan, M.A.; Brennan, C.S. In vitro digestion characteristics of cereal protein concentrates as assessed using a pepsin-pancreatin digestion model. Food Res. Int. 2022, 152, 110715. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Singh, R.; de Campo, L.; Gilbert, E.P.; Wu, Z.; Hemar, Y. Effect of amyloglucosidase hydrolysis on the multi-scale supramolecular structure of corn starch. Carbohyd. Polym. 2019, 212, 40–50. [Google Scholar] [CrossRef]
- Weurding, R.E.; Veldman, A.; Veen, W.A.G.; Van Der Aar, P.J.; Verstegen, M.W.A. Starch digestion rate in the small intestine of broiler chickens differs among feedstuffs. J. Nutr. 2001, 131, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Cochrane, M.P. An evaluation of confocal microscopy for the study of starch granule enzymic digestion. Starch Stärke 1997, 49, 106–110. [Google Scholar] [CrossRef]
- Wang, Y.; Saulnier, L.; Ral, J.-P.; Falourd, X.; Kansou, K. Determining whether granule structural or surface features govern the wheat starch digestion, a kinetic analysis. Carbohydr. Polym. 2023, 315, 120966. [Google Scholar] [CrossRef]
- Ramadoss, B.R.; Gangola, M.P.; Agasimani, S.; Jaiswal, S.; Venkatesan, T.; Sundaram, G.R.; Chibbar, R.N. Starch granule size and amylopectin chain length influence starch in vitro enzymatic digestibility in selected rice mutants with similar amylose concentration. J. Food Sci. Technol. 2019, 56, 391–400. [Google Scholar] [CrossRef]
- Khatun, M.A.; Razzak, M.; Hossain, M.A.; Rahman, M.A.; Khan, R.A.; Huque, R. Gamma radiation application to rice: Reduced glycemic index in relation to modified carbohydrate observed in FTIR spectra. Curr. Res. Food Sci. 2020, 4, 11–17. [Google Scholar] [CrossRef]
- Ye, Y.; Du, H.; Liu, X.; Jia, G.; Lu, Z.; Wang, Y. Effects of different exogenous proteins combined with dry heat treatment on physicochemical properties and digestive characteristics of puffed corn flour. J. Sci. Food Agric. 2025, 105, 4209–4216. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Yang, Y.; Jiao, A.; Jin, Z. Isothermal retrogradation preparation of type iii resistant starch from extruded-debranched starch: Structure and in vitro digestibility. Int. J. Biol. Macromol. 2024, 280, 135216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, A.R.; Luo, H.F.; Wei, H.; Zhou, Z.; Peng, J.; Ru, Y.J. In vitro and in vivo digestibility of corn starch for weaned pigs: Effects of amylose:amylopectin ratio, extrusion, storage duration, and enzyme supplementation. J. Anim. Sci. 2015, 93, 3512–3520. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Zhang, H.; Wu, G.; Cheng, L.; Zhang, Y. A review of endogenous non-starch components in cereal matrix: Spatial distribution and mechanisms for inhibiting starch digestion. Crit. Rev. Food Sci. Nutr. 2024, 1–16. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef]
- Jennings, J.S.; Meyer, B.E.; Guiroy, P.J.; Cole, N.A. Energy costs of feeding excess protein from corn-based by-products to finishing cattle. J. Anim. Sci. 2018, 96, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, H.; Zhang, S.; Zhang, Y. Amino acid composition analysis and in vitro dynamic digestion of proteins from three different sources. J. Henan Univ. Technol. Nat. Sci. Ed. 2019, 40, 62–68. [Google Scholar]
- Zhao, F.; Guo, X.; Zhao, S.; Zhang, M.; Yang, Y. Effects of processing conditions on the solubility and structure of alcoholeached soybean protein concentrate. China Oils Fats 2023, 48, 57–63. [Google Scholar]
- Li, Y.; Wang, L.; Wang, H.; Li, Z.; Qiu, J.; Wang, L. Correlation of microstructure, pore characteristics and hydration properties of wheat bran modified by airflow impact mill. Innov. Food Sci. Emerg. 2022, 77, 102977. [Google Scholar] [CrossRef]
- Heyer, C.M.E.; Wang, L.F.; Beltranena, E.; Zijlstra, R.T. Nutrient digestibility of extruded canola meal in ileal-cannulated growing pigs and effects of its feeding on diet nutrient digestibility and growth performance in weaned pigs. J. Anim. Sci. 2021, 99, skab135. [Google Scholar] [CrossRef] [PubMed]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F.J. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Yang, X.; Darko, K.O.; Huang, Y.; He, C.; Yang, H.; He, S.; Li, J.; Li, J.; Hocher, B.; Yin, Y. Resistant starch regulates gut microbiota: Structure, biochemistry and cell signalling. Cell Physiol. Biochem. 2017, 42, 306–318. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.; He, J.; Ni, Y.; Kovatcheva-Datchary, P.; Yuan, R.; Liu, S.; et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024, 6, 578–597. [Google Scholar] [CrossRef]
| Time (min) | Flow Rate (mL/min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|---|
| 0.0 | 0.3 | 97 | 3 |
| 3.0 | 0.3 | 93 | 7 |
| 3.8 | 0.3 | 88 | 12 |
| 8.0 | 0.3 | 66 | 34 |
| 8.9 | 0.3 | 30 | 70 |
| 12.0 | 0.3 | 97 | 3 |
| 15.0 | 0.3 | 97 | 3 |
| Items | Diets (as Fed Basis) | |||
|---|---|---|---|---|
| Corn | Soybean Meal | Wheat | Wheat Bran | |
| Corn (%) | 96.70 | 0.00 | 0.00 | 0.00 |
| Soybean meal (%) | 0.00 | 33.00 | 0.00 | 0.00 |
| Wheat (%) | 0.00 | 0.00 | 96.70 | 0.00 |
| Wheat bran (%) | 0.00 | 0.00 | 0.00 | 30.00 |
| Corn starch (%) | 0.00 | 47.00 | 0.00 | 50.00 |
| Soybean oil (%) | 0.00 | 3.00 | 0.00 | 3.00 |
| Sucrose (%) | 0.00 | 15.00 | 0.00 | 15.00 |
| Dicalcium phosphate (%) | 1.50 | 0.90 | 1.50 | 0.90 |
| Salt (%) | 0.30 | 0.30 | 0.30 | 0.30 |
| Limestone (%) | 1.00 | 0.30 | 1.00 | 0.30 |
| Premix (%) | 0.50 | 0.50 | 0.50 | 0.50 |
| Total (%) | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels | ||||
| Net Energy (MJ/kg) | 2676.66 | 3216.29 | 2483.26 | 3126.50 |
| Dry matter (%) | 87.79 | 89.99 | 89.97 | 89.93 |
| Crude protein (%) | 7.75 | 14.95 | 12.79 | 5.68 |
| Lys | 0.17 | 0.87 | 0.31 | 0.17 |
| Met | 0.16 | 0.19 | 0.18 | 0.06 |
| Thr | 0.19 | 0.52 | 0.34 | 0.10 |
| Trp | 0.04 | 0.15 | 0.13 | 0.06 |
| Val | 0.31 | 0.61 | 0.55 | 0.17 |
| Ile | 0.19 | 0.57 | 0.43 | 0.13 |
| Leu | 0.78 | 0.98 | 0.80 | 0.28 |
| Arg | 0.26 | 1.05 | 0.55 | 0.29 |
| His | 0.18 | 0.38 | 0.30 | 0.12 |
| Phe | 0.26 | 0.60 | 0.65 | 0.15 |
| Ala | 0.42 | 0.57 | 0.41 | 0.17 |
| Asp | 0.35 | 1.48 | 0.56 | 0.26 |
| Cys | 0.16 | 0.17 | 0.28 | 0.09 |
| Glu | 1.05 | 2.13 | 4.10 | 0.85 |
| Gly | 0.19 | 0.51 | 0.47 | 0.17 |
| Pro | 0.66 | 0.63 | 1.33 | 0.32 |
| Ser | 0.28 | 0.66 | 0.50 | 0.17 |
| Tyr | 0.16 | 0.50 | 0.39 | 0.13 |
| Items | GE (MJ/kg) | DM (%) | CP (%) | EE (%) | CF (%) | NDF (%) | ADF (%) | Total Starch (%) | Amylopectin/Amylose |
|---|---|---|---|---|---|---|---|---|---|
| Corn | 16.01 | 88.11 | 8.23 | 3.62 | 1.81 | 6.56 | 1.62 | 71.64 | 1.71 |
| Corn starch | 14.48 | 86.38 | 1.05 | ND | ND | 0.14 | ND | 87.15 | 1.53 |
| Sorghum | 16.31 | 87.83 | 9.75 | 4.35 | 2.33 | 8.85 | 2.31 | 65.03 | 0.81 |
| Wheat | 16.06 | 88.31 | 15.31 | 1.99 | 1.84 | 10.78 | 1.79 | 62.58 | 1.77 |
| Barley | 16.63 | 90.36 | 12.19 | 2.23 | 3.80 | 20.10 | 6.26 | 53.14 | 2.31 |
| Extruded corn | 16.45 | 88.93 | 8.34 | 1.77 | 1.57 | 7.18 | 1.41 | 73.31 | 4.16 |
| Potato | 14.58 | 90.68 | 10.18 | 1.11 | 2.00 | 2.49 | 1.44 | 63.25 | 0.89 |
| Wheat bran | 16.55 | 89.03 | 17.15 | 2.67 | 10.73 | 38.37 | 10.66 | 18.35 | 5.82 |
| Cassava | 14.55 | 89.27 | 2.07 | 1.04 | 2.52 | 2.40 | 1.18 | 77.81 | 0.38 |
| SEM | 0.31 | 0.44 | 1.79 | 0.41 | 1.09 | 3.97 | 1.20 | 6.54 | 0.59 |
| Items | GE (MJ/kg) | DM (%) | CP (%) | EE (%) | CF (%) | NDF (%) | ADF (%) |
|---|---|---|---|---|---|---|---|
| Soybean meal | 17.47 | 92.11 | 45.53 | 2.05 | 8.78 | 14.28 | 6.70 |
| Extruded soybeans | 21.48 | 93.55 | 36.57 | 16.49 | 17.91 | 21.55 | 10.45 |
| Peanut cake | 18.17 | 90.78 | 29.13 | 4.99 | 8.41 | 13.73 | 7.93 |
| Distillers’ grain | 18.66 | 87.79 | 27.94 | 7.42 | 8.92 | 24.38 | 7.20 |
| Fish meal | 17.94 | 91.84 | 68.73 | 5.52 | 1.67 | 18.96 | 2.53 |
| Greens cake | 18.36 | 91.08 | 37.15 | 7.56 | 13.55 | 23.20 | 16.18 |
| Whey protein powder | 14.00 | 95.27 | 3.81 | 2.48 | ND | 0.31 | ND |
| Corn gluten meal | 12.07 | 92.68 | 48.14 | 1.70 | 10.00 | 33.96 | 12.04 |
| Fermented soybean meal | 17.56 | 91.81 | 52.72 | 1.34 | 7.45 | 21.62 | 8.52 |
| Palm kernel meal | 17.49 | 90.51 | 17.89 | 5.47 | 16.63 | 57.71 | 32.00 |
| Soybean protein concentrate | 18.81 | 93.27 | 63.99 | 1.18 | 3.95 | 14.52 | 4.09 |
| Rapeseed meal | 17.97 | 89.38 | 41.62 | 3.47 | 10.13 | 25.43 | 13.68 |
| Sunflower meal | 17.29 | 90.70 | 41.08 | 1.16 | 12.65 | 23.25 | 14.25 |
| Cottonseed meal | 17.11 | 88.87 | 58.14 | 1.74 | 4.77 | 12.90 | 5.52 |
| Sprayed corn germ meal | 17.28 | 92.62 | 29.77 | 1.77 | 7.36 | 30.15 | 7.46 |
| Hydrolyzed feather meal | 22.22 | 92.38 | 86.47 | 4.60 | 1.32 | 29.21 | 4.90 |
| Beet meal | 14.99 | 90.71 | 8.65 | 0.57 | 15.47 | 34.23 | 19.76 |
| Pea | 17.69 | 93.43 | 31.66 | 3.99 | 6.36 | 24.11 | 12.22 |
| Brewer’s yeast | 17.45 | 92.26 | 43.69 | 0.55 | ND | 8.98 | 3.24 |
| SEM | 0.52 | 0.41 | 4.65 | 0.86 | 1.19 | 2.77 | 1.68 |
| Items | Modeling | R2 |
|---|---|---|
| 0.5 h | SD0.5 = 3.969 × CF − 0.751 × TS + 3.242 × AA + 70.290 | 0.96 |
| 1 h | SD1.0 = 4.961 × CF − 0.632 × TS + 2.769 × AA + 67.815 | 0.87 |
| 2 h | SD2.0 = 6.889 × CF − 0.301 × TS + 1.922 × AA + 53.145 | 0.77 |
| 3 h | SD3.0 = 7.569 × CF − 0.205 × TS + 1.386 × AA + 49.319 | 0.74 |
| 4 h | SD4.0 = 9.084 × CF − 0.106 × TS + 0.117 × AA + 46.392 | 0.74 |
| 6 h | SD6.0 = 10.716 × CF − 0.175 × TS − 1.306 × AA + 34.560 | 0.67 |
| 8 h | SD8.0 = 11.425 × CF − 0.354 × TS − 1.308 × AA + 27.850 | 0.61 |
| Items | Modeling | R2 |
|---|---|---|
| 0.5 h | PR30min = 429.143 × DM − 10.833 × CP − 46.567 × NDF + 0.089 × R0 − 37,965.420 | 0.95 |
| 1.5 h | PR90min = 912.650 × DM + 1608.709 × EE − 129.991 × NDF + 0.067 × R1.5 − 88,252.98 | 0.99 |
| 2.5 h | PR150min = 7035.103 × GE − 282.173 × EE + 107.339 × TS + 0.028 × R2.0 − 119,520.792 | 0.99 |
| 3.5 h | PR210min = 511.211 × DM + 3979.605 × GE + 57.622 × TS + 0.004 × R3.0 − 111,495.633 | 0.93 |
| 4.5 h | PR270min = 4813.429 × GE + 73.348 × TS − 583.392 × EE + 0.022 × R4.0 − 80,244.804 | 0.99 |
| 5.5 h | PR330min = 2979.714 × GE − 1045.729 × DM + 24.195 × CP + 0.007 × R6.0 + 45,125.873 | 0.98 |
| 6.5 h | PR390min = 58.115 × DM − 945.941 × GE − 93.759 × TS − 0.059 × R8.0 + 25,526.378 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, J.; Chen, Q.; Zhou, J.; Fan, Y.; Li, Y.; Ma, Y.; Zeng, X.; Qiao, S.; Cai, S. Characteristics of Amino Acid and Glucose Digestion and Metabolism in Energy and Protein Feedstuffs for Pigs. Animals 2025, 15, 1510. https://doi.org/10.3390/ani15111510
Tu J, Chen Q, Zhou J, Fan Y, Li Y, Ma Y, Zeng X, Qiao S, Cai S. Characteristics of Amino Acid and Glucose Digestion and Metabolism in Energy and Protein Feedstuffs for Pigs. Animals. 2025; 15(11):1510. https://doi.org/10.3390/ani15111510
Chicago/Turabian StyleTu, Jiayu, Qingyun Chen, Junyan Zhou, Yuxin Fan, Yanlong Li, Yonghang Ma, Xiangfang Zeng, Shiyan Qiao, and Shuang Cai. 2025. "Characteristics of Amino Acid and Glucose Digestion and Metabolism in Energy and Protein Feedstuffs for Pigs" Animals 15, no. 11: 1510. https://doi.org/10.3390/ani15111510
APA StyleTu, J., Chen, Q., Zhou, J., Fan, Y., Li, Y., Ma, Y., Zeng, X., Qiao, S., & Cai, S. (2025). Characteristics of Amino Acid and Glucose Digestion and Metabolism in Energy and Protein Feedstuffs for Pigs. Animals, 15(11), 1510. https://doi.org/10.3390/ani15111510








